Abstract
OBJECTIVE
To describe the clinical features of patients diagnosed with upper respiratory tract infections (URIs), and determine which clinical features are associated with antibiotic use.
DESIGN
Prospective cohort study.
SETTING
Three ambulatory care practices at a group-model HMO in the Denver metropolitan area.
PATIENTS
Adults (aged 18 years or older) seeking care for acute respiratory illnesses.
MEASUREMENTS
Clinical features were documented on standardized encounter forms. Clinician type, secondary diagnoses, and antibiotic treatment were extracted from administrative databases. Results are presented as adjusted odds ratios (ORs) with 95% confidence intervals (CIs).
MAIN RESULTS
Antibiotics were prescribed to 33% (95% CI 28%, 38%) of patients diagnosed with URI, after excluding patients with coexisting antibiotic-responsive conditions (e.g., sinusitis, pharyngitis) or a history of cardiopulmonary disease. Multivariate logistic regression analysis identified tobacco use (OR 2.8; 95% CI 1.5, 5.1), history of purulent nasal discharge (OR 2.0; 95% CI 1.1, 3.6) or green phlegm (OR 4.8; 95% CI 2.1, 11.1), and examination findings of purulent nasal discharge (OR 5.2; 95% CI 2.4, 11.2) or tonsillar exudate (OR 3.7; 95% CI 1.1, 12.1) to be independently associated with antibiotic use. The majority of patients treated with antibiotics (82%) had at least one of these factors present.
CONCLUSIONS
Antibiotic treatment of URIs is most common when purulent manifestations are present. Efforts to reduce antibiotic treatment of URIs should educate clinicians about the limited value of purulent manifestations in predicting antibiotic-responsive disease.
Keywords: upper respiratory tract infections, antimicrobial therapy, purulence, clinical decision making, physician practice patterns
Upper respiratory tract infections (URIs) are important targets for reducing unnecessary antibiotic use in ambulatory practice. Typically, URI refers to an acute respiratory illness that lacks a dominant clinical feature such as sore throat, runny nose, or cough, although these symptoms are frequently present to a lesser degree.1 In an evaluation of ambulatory care visits to physician offices, hospital outpatient departments, and emergency departments during 1995, URIs (including the common cold) ranked as the most frequent reason for seeking care in the United States, resulting in more than 37 million visits.2 Because URIs are viral infections, antibiotic therapy is not generally recommended.1 Yet antibiotics are frequently prescribed for URIs.3, 4 The National Ambulatory Medical Care Survey, which specifically instructs participating physicians and staff how to code their survey instruments, found antibiotic prescription rates of 52% for uncomplicated URIs.3 As a result of this practice, URIs are the second leading condition for which antibiotics are prescribed each year, and account for 10% of all antibiotics prescribed annually in ambulatory practice.5
The overuse of antibiotics for URIs has prompted attempts to better understand this practice. Physicians have reported that unrealistic patient expectations, pressure to prescribe antibiotics, and insufficient time to educate patients about the inefficacy of antibiotics are some of the reasons why antibiotics are prescribed for URIs.6–8 However, little is known about how the clinical presentation of patients diagnosed with URIs affects the decision to prescribe antibiotics for these conditions. Although the diagnosis of URI is usually one of exclusion, we hypothesize that clinicians may, in fact, utilize a more structured approach in deciding which patients to treat with antibiotics.
The aims of the present study are to describe the clinical features of adult patients diagnosed with URIs and to determine which clinical features of URIs are associated with antibiotic treatment.
METHODS
We have previously described the methods used for evaluating the clinical encounter for patients with acute respiratory illnesses (ARIs) in our study population.9 In brief, we conducted a prospective cohort study of adults presenting to Kaiser Permanente of Colorado medical facilities with ARIs. Kaiser Permanente of Colorado is a group-model HMO serving approximately 250,000 adult members in the Denver-Boulder metropolitan area. Members received medical care services solely from Kaiser Permanente clinicians, staff, laboratory, and pharmacy. Most patients with acute or urgent illnesses were given same-day appointments in these practices, rather than being seen in urgent care centers or emergency departments.
Eligible patients included consecutive adult patients presenting to one of the three study facilities between February and May 1996, with acute symptoms referable to the chest, throat, ears, or nasal passages. Patients less than 18 years old were excluded. Eligible clinicians included internal medicine board-certified physicians, nurse practitioners, physician assistants, and registered nurses (RNs). Health plan members seeking care for suspected URIs were often scheduled with RNs as a mechanism for providing same-day medical evaluation for nonemergent conditions. Physicians supervised all RN visits and were required to cosign all RN medical record notes. No specific guidelines or algorithms for URIs were in place for RN visits. Nurses discussed most patient cases with the supervising physician, and always discussed cases of patients who might need a prescripton. The ultimate decision to prescribe an antibiotic rested with the physician.
In order to standardize the documentation of duration of illness, past medical history, symptoms, and physical examination findings present at the office visit, a patient encounter form, called the ARI form, was designed to replace the traditional free-text medical record note.9 Our study cohort was taken from patient visits that resulted in a diagnosis of URI on the ARI form. If there was no written diagnosis on the ARI form, we also accepted visits of patients that resulted in a diagnosis of URI in the Kaiser Permanente administrative database using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code 465.90. The diagnoses entered into the administrative database were coded by the clinician who cared for the patient. We excluded visits of patients with other conditions that might have influenced the decision to prescribe antibiotic therapy for a diagnosis of URI, including past medical history of chronic heart or lung disease, or coexisting antibiotic-responsive illness. Antibiotic-responsive illnesses in our sample were defined as sinusitis (ICD-9-CM code 461.9), pharyngitis (ICD-9-CM code 462), otitis media (ICD-9-CM code 382), and pneumonia (ICD-9-CM 486). We also excluded visits of patients with the coexisting diagnosis of acute bronchitis, even though patients with acute bronchitis without underlying lung disease do not benefit from antibiotic therapy.10 These visits were excluded because physicians in our study cohort prescribed antibiotics for acute bronchitis at very high rates (85%), suggesting these clinicians perceive acute bronchitis to be an indication for antibiotics.9
We performed χ2tests to compare antibiotic prescription rates associated with the absence or presence of each clinical variable. Those factors significantly associated with antibiotic use (p < .05) were entered into a multivariate logistic regression model using stepwise selection to identify independent predictors of antibiotic use. The final model underwent further testing to evaluate whether clinician type (physician vs nonphysician) affected the conclusions. To test for confounding, the variable for clinician type was forced into the final model. To test for effect modification, interaction terms representing clinician type and each clinical predictor of antibiotic use were forced into the final model. We performed χ2tests and logistic regression analyses using the SAS statistical application program (release 6.12, copyright 1989–1998 by SAS Institute, Cary, NC). Results are presented as adjusted odds ratios (ORs) and likelihood ratio with 95% confidence intervals (CIs). A receiver operating charactistic (ROC) curve was constructed using the true-positive and false-positive ratios for points corresponding to increasing numbers of clinical predictors that were positive. The area under the ROC curve and its standard error were calculated for nonparametric data using ROC curve analysis software (copyright 1992, version 6.0, Robert M. Centor and Jerry Kneightly, Richmond, Va.).
RESULTS
Upper respiratory tract infections were diagnosed in 451 (30%) of 1,525 adult office visits for ARI in our study cohort. After excluding patients with underlying cardiopulmonary disease (15% of URI visits), as well as visits of patients with coexisting conditions for which clinicians might have intended antibiotic therapy (14% of URI visits), the final study cohort of URI visits represented 21% (n= 322) of ARI encounters.
Patient and clinician characteristics of these office visits are presented in Table 1). The majority of patients were aged 18 to 44 years, female, and nonsmokers. Seventy-five percent of patients sought care within 1 week of URI onset, and 47% had missed at least 1 day of work because of their illness. Eighty-one different clinicians were represented in our final study cohort. Nonphysicians (RNs, physician assistants, and nurse practitioners) were the primary clinicians of record in 81% of visits.
Table 1.
Patient, Clinician and Severity-of-Illness Characteristics in Clinical Encounters for Upper Respiratory Tract Infections
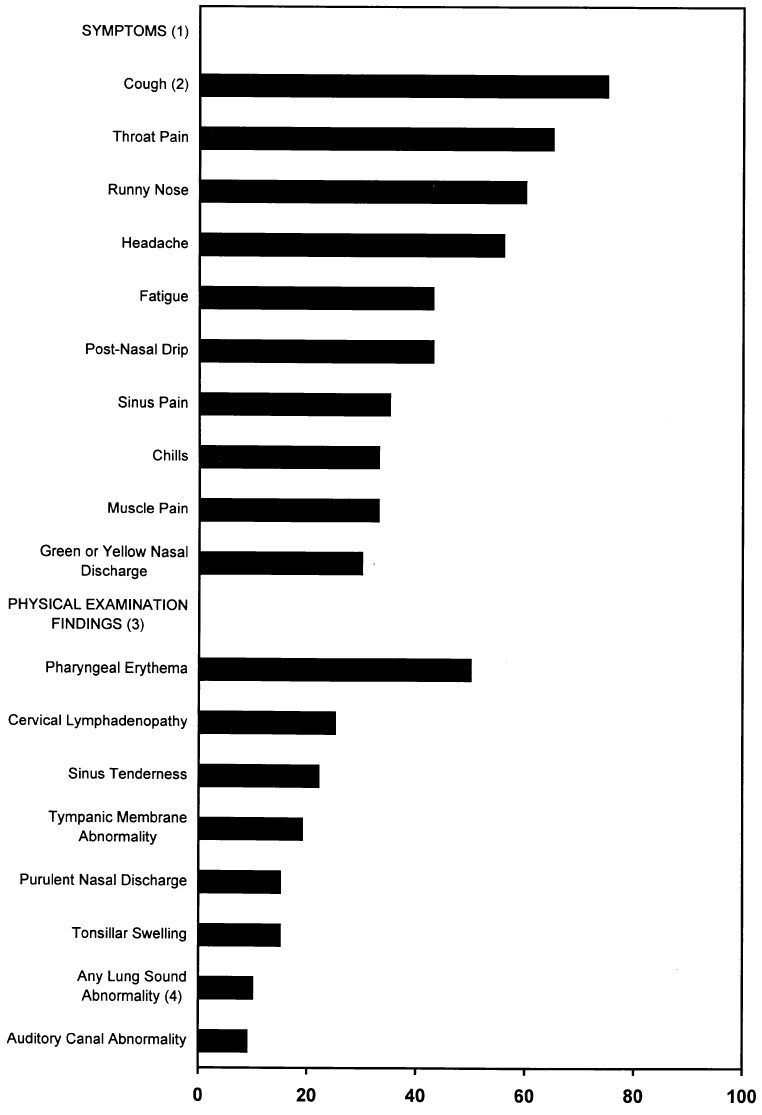
Patients reported a wide range of symptoms resulting from the URI (Fig. 1). The symptoms most frequently reported were cough (75%), throat pain (65%), runny nose (60%), headache (56%), fatigue (43%), and postnasal drip (43%). In contrast to symptoms reported, few abnormal findings were noted on physical examination (Fig. 1). The most common findings included pharyngeal erythema (50%), cervical lymphadenopathy (25%), sinus tenderness (22%), and tympanic membrane abnormality (19%). Thirty percent of visits yielded no abnormal physical examination findings.
Figure 1.
Symptoms and physical examination findings in patients diagnosed with upper respiratory tract infections (n= 322). 1Other symptoms reported by patients included fever (27%), sweats (26%), difficulty swallowing (23%), shortness of breath (16%), throat swelling (16%), wheezing (9%), chest pain (8%), and painful breathing (6%). 2Eleven percent of patients reported cough producing green phlegm, 25% producing white or yellow phlegm, and 39% without phlegm (dry cough). 3Other physical examination findings reported by clinicians included toxic appearance (6%), oral temperature>100°F (6%), tonsillar exudate (5%), and stridor (0.5%). 4Lung sound abnormalities included decreased breath sounds (5%), rhonchi (2%), wheezes (2%), and rales (1 %).
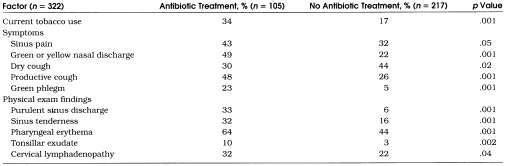
The crude antibiotic prescription rate for patients with URIs in our study cohort was 33% (95% CI 28%, 38%). To select factors for inclusion in a multivariate logistic regression model of antibiotic prescribing for URIs, we first compared antibiotic prescription rates by patient age, gender, smoking status, duration of illness, days of work missed, clinician type, symptoms reported, and physical examination findings present. Eleven variables were significantly associated with antibiotic use on bivariate analyses (Table 2 Dry cough had a strong inverse correlation with productive cough and was excluded from multivariate analysis. Because pharyngeal erythema and tonsillar exudate were strongly colinear, these variables were reclassified to allow for multivariate logistic regression analysis. We created a single variable representing the physical examination findings of the throat consisting of three levels: (1) presence of tonsillar exudate regardless of pharyngeal erythema, (2) presence of pharyngeal erythema without tonsillar exudate, and (3) absence of tonsillar exudate and pharyngeal erythema.
Table 2.
Factors Associated with Antibiotic Use for Upper Respiratory Tract Infections
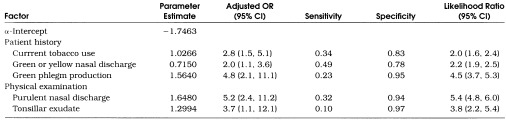
Multivariate logistic regression analysis identified five factors independently associated with antibiotic use for URIs: current tobacco use, symptoms of green or yellow nasal discharge, symptoms of green phlegm, physical examination findings of purulent nasal discharge, and physical examination findings of tonsillar exudate (Table 3) Purulent nasal discharge on examination and patient report of green phlegm production were the variables most strongly associated with antibiotic use (adjusted OR 5.2 and 4.8, respectively). Addition of interaction terms between these five variables did not have any major effects on the performance of this model.
Table 3.
Factors Independently Associated with Antibiotic Use for Upper Respiratory Tract Infections Using Multivariate Logistic Regression Analysis
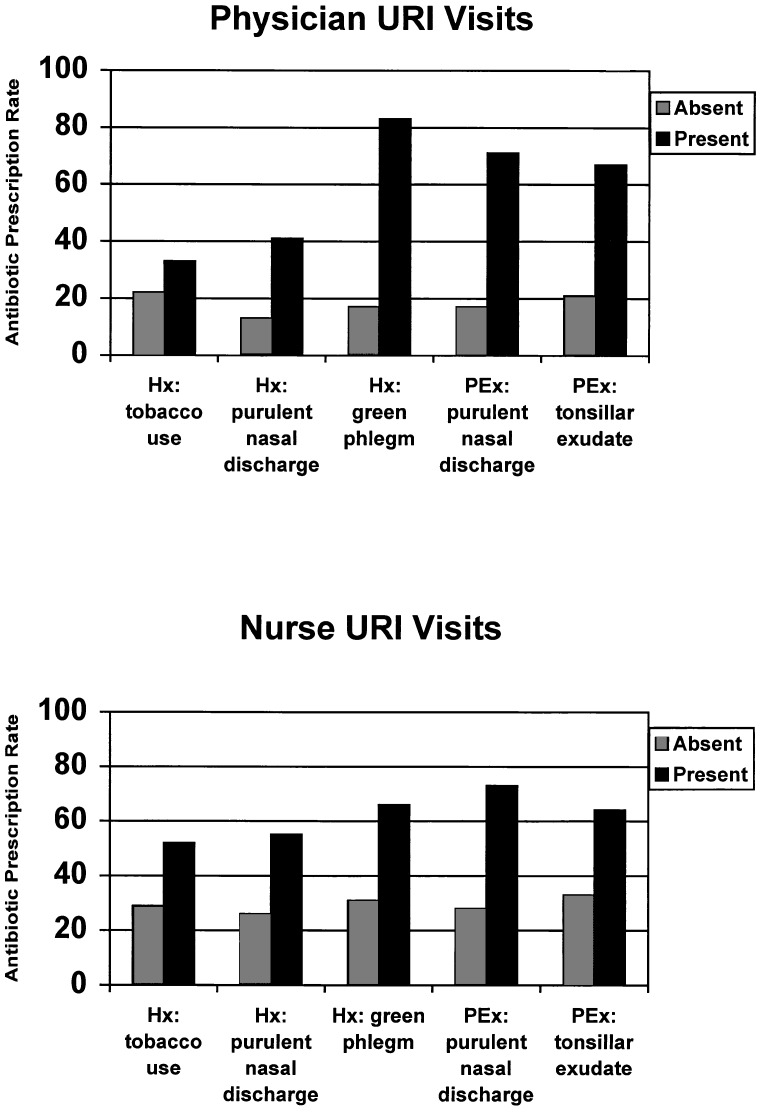
Because physicians and nurses might differ in their interpretation of the presence or absence of the clinical variables in our model, we also evaluated whether this model was consistent across clinician types. A stratified analysis of antibiotic prescription rates for URIs as a function of the presence or absence of the five clinical features is displayed in Figure 2. Addition of clinician type to the final regression model had a negligible effect (<5%) on the adjusted ORs of the clinical variables in the final model. The addition of interaction terms between clinician type and each clinical variable also did not add significantly to the main effects model (p= .47 to .80, data not shown).
Figure 2.
Factors independently associated with antibiotic use for upper respiratory tract infections (URIs) stratified by clinician type. Hx indicates findings (or symptoms) reported by patient from history of present illness; PEx, findings observed by clinician during physical examination. Total number of physician URI visits = 61, and total number of nurse URI visits = 2 45.
Table 3 also displays the sensitivity, specificity, and likelihood ratios for an abnormal finding for the five factors independently associated with antibiotic use for URIs. In general, any single factor was present in the minority of total URI cases for which antibiotics were prescribed (low sensitivity). However, any given factor was unlikely to exist when a patient was not prescribed antibiotics (high specificity). At least one of these factors was present in 82% of all antibiotic prescriptions for URIs.
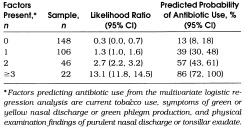
The probability of prescribing antibiotics for URIs based on this model increased with the number of clinical variables present (Table 4) The antibiotic prescription rate was 13% when no factors were present and increased in a linear manner to 100% when all five factors were present. The area under the ROC curve for this model was 0.76 ± 0.03 (see Methods section).
Table 4.
Predicted Probability of Antibiotic Use for Upper Respiratory Tract Infections
DISCUSSION
In this prospective cohort study of patients with ARIs, we found that antibiotics were prescribed to 33% of patients diagnosed with URI, after excluding URI patients with coexisting conditions for which the clinician might have intended antibiotic therapy. This prescription rate is consistent with previous studies of treatment of colds and URIs in the United States and Europe.3, 4, 7, 11
Classification of patients with ARIs has been traditionally based on the anatomic localization of the prominent clinical signs and symptoms accompanying the illness (e.g., sinusitis, pharyngitis, or bronchitis), while the diagnosis of URI has been reserved for cases with no prominent localizing features.1, 12 However, our findings suggest that clinicians also identify and treat a subset of URIs defined by the presence of purulent manifestations. We identified purulent nasal discharge (reported or observed), green phlegm production, tonsillar exudate, and current tobacco use as independent predictors of antibiotic treatment of URIs. Our practice-based study validates a physician survey in which respondents were more likely to prescribe antibiotics for hypothetical cases of URI when purulent nasal discharge was present.13
Considering the diversity of clinical findings in patients diagnosed with URIs, and the low prevalence of any single symptom or sign, it is not surprising that any single factor had poor sensitivity for predicting antibiotic use. Nonetheless, several observations suggest that the presence of these clinical factors does guide the treatment decision for patients with URIs. First, the vast majority (82%) of patients prescribed antibiotics had at least one of these factors present. Second, there was a linear relation between the number of factors present and the likelihood of prescribing an antibiotic. Third, the area under the ROC curve for predicting antibiotic treatment of URIs using this model was 0.76 ± 0.03. While the area under the ROC curve for our model confirms that these factors discriminate who receives antibiotics for URIs, it represents only moderate discriminatory power for a clinical prediction rule (where 1.0 represents a perfect test or rule, and 0.5 represents a worthless test or rule). This suggests that the clinical presentation of patients, alone (see below), does not explain all antibiotic prescriptions to patients with URIs.
Though not assessed in our study, the decision to prescribe antibiotics for URIs is also influenced by patient expectations for antibiotics.6, 7 Patient beliefs that antibiotics are effective for URIs, which may increase their expectations for antibiotics, appear to be greater when purulent findings are present.14, 15 Practice characteristics also appear to affect antibiotic prescribing practices for respiratory illnesses. For example, providers with increased patient workloads are more likely to prescribe antibiotics for patients with URIs.16 Unfortunately, the use of purulent manifestations is probably not a valid approach for identifying patients with URIs who are likely to benefit from antibiotic treatment. Purulent nasal discharge and purulent sputum are weak predictors of bacterial infection.17, 18 Purulence primarily occurs when inflammatory cells or sloughed mucosal epithelial cells are present, and can result from either viral or bacterial infection.19, 20 Similarly, tonsillar exudate can result from either viral or bacterial pharyngitis.21 Placebo-controlled trials of patients with acute nasopharyngitis have found no difference in outcomes between patients with and patients without purulent rhinorrhea, although Kaiser et al. did report a benefit in patients whose culture of nasal secretions subsequently grew pathogenic bacteria.17, 22 Other studies have also failed to find a major clinical benefit of antibiotic treatment of adults with cough who had purulent sputum.23, 24
The potential for misclassification threatens the validity of our findings and conclusions. If clinicians recorded a diagnosis of URI, but intended antibiotic treatment for a coexisting antibiotic-responsive infection, we would overestimate the antibiotic prescription rate for URIs. Our efforts to minimize misclassification included the use of administrative diagnosis forms to exclude the most common competing diagnoses for URIs (e.g., sinusitis, pharyngitis, bronchitis, and otitis media), and instructions to record up to five acute or chronic conditions per visit.
The generalizability of our findings may also be limited because we studied prescribing practices in a group-model HMO population of clinicians and patients. However, in a national survey of ambulatory care physicians, we found no difference in antibiotic prescription rates for colds, URIs and bronchitis based on insurance type.3 Other features of this health plan to consider in assessing the generalizability of our findings include readily available telephone advice by nurses, same-day office visits, and pharmacy benefits.
Because RN visits accounted for 76% of cases in our final study cohort, one concern is that the final model actually represents RN rather than physician behavior. The decision to prescribe antibiotics, however, should still reflect physician decision making, as physicians supervised all RN visits and signed all prescriptions. Other analyses also support the conclusion that physicians and nurses use the identified clinical predictors in a similar fashion. The direction and magnitude of associations between the clinical features of URIs and antibiotic use were similar when we added clinician type to our final model, suggesting that clinician type was not a major confounder of our final model. Interaction terms between clinician type and the clinical predictors to our final model were not statistically significant, suggesting that there is no major difference in how physicians and nurses used individual clinical features to decide whom to treat with antibiotics. Finally, it is also unlikely that our results are skewed by a few “outlier” clinicians, or represent the practice style of a minority of clinicians, as 81 different clinicians were represented in the final study cohort, and the median number of visits per clinician was 2 (range 1–21).
The forces that lead to the decision to prescribe antibiotics for patients with URIs are complex. Although past studies have focused on patient pressure and demand for antibiotics as the major reason physicians treat colds and URIs with antibiotics, our findings suggest that the clinical presentation of the illness is also important. Future efforts to reduce unnecessary antibiotic use for patients with URIs will need to include clinician education on the appropriate assessment and treatment of purulent manifestations of URIs.
Acknowledgments
The authors thank Mike Bodily, MBA, and Don Walker for their technical support in extracting information from the Kaiser Permanente administrative databases. This work was supported in part by a National Research Service Award (5 T32 PE10006) and a Robert Wood Johnson Minority Medical Faculty Development Program Grant (2532434) to Dr. Gonzales, and a Kaiser Permanente of Colorado Research and Development Grant to Drs. Gonzales and Barrett.
References
- 1.Evans AS. Clinical syndromes in adults caused by respiratory infection. Med Clin North Am. 1967;51:803–29. doi: 10.1016/s0025-7125(16)33048-6. [DOI] [PubMed] [Google Scholar]
- 2.Schappert SM. Ambulatory care visits to physician offices, hospital outpatient departments, and emergency departments: United States, 1995. National Center for Health Statistics. Vital Health Stat 13. 1997:129. [PubMed] [Google Scholar]
- 3.Gonzales R, Steiner JF, Sande MA. Antibiotic prescribing for adults with colds, upper respiratory infections and bronchitis by ambulatory physicians in the United States. JAMA. 1997;278:901–4. [PubMed] [Google Scholar]
- 4.Mainous AG, III, Hueston WJ, Clark JR. Antibiotics and upper respiratory infection: do some folks think there is a cure for the common cold? J Fam Pract. 1996;42:357–61. [PubMed] [Google Scholar]
- 5.McCaig LF, Hughes JM. Trends in antimicrobial drug prescribing among office-based physicians in the United States. JAMA. 1995;273:214–9. [PubMed] [Google Scholar]
- 6.Schwartz B, Bell DM, Hughes JM. Preventing the emergence of antimicrobial resistance. A call for action by clinicians, public health officials, and patients. JAMA. 1997;278:944–5. doi: 10.1001/jama.278.11.944. Editorial. [DOI] [PubMed] [Google Scholar]
- 7.Hamm RM, Hicks RJ, Bemben DA. Antibiotics and respiratory infections: are patients more satisfied when expectations are met? J Fam Pract. 1996;43:56–62. [PubMed] [Google Scholar]
- 8.Palmer DA, Bauchner HR. Parents’ and physicians’ views on antibiotics. Pediatrics. 1997;99:E6. doi: 10.1542/peds.99.6.e6. [DOI] [PubMed] [Google Scholar]
- 9.Gonzales R, Crane LA, Barrett PH, Jr, Steiner JF. The clinical encounter for acute bronchitis: factors associated with antibiotic use. J Gen Intern Med. 1998;13:541–8. doi: 10.1046/j.1525-1497.1998.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKay DN. Treatment of acute bronchitis in adults without underlying lung disease. J Gen Intern Med. 1996;11:557–62. doi: 10.1007/BF02599608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeMelker RA, Kuyvenhoven MM. Management of upper respiratory tract infections in Dutch family practice. J Fam Pract. 1994;38:353–7. [PubMed] [Google Scholar]
- 12.Hope-Simpson RE, Miller DL. The definition of acute respiratory illnesses in general practice. Postgrad Med J. 1973;49:763–70. doi: 10.1136/pgmj.49.577.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mainous AG, III, Hueston WJ, Eberlein C. Colour of respiratory discharge and antibiotic use. Lancet. 1997;350:1077. doi: 10.1016/S0140-6736(05)70457-8. [DOI] [PubMed] [Google Scholar]
- 14.Mainous AG, III, Zoorob RJ, Oler MJ, Haynes DM. Patient knowledge of upper respiratory infections: implications for antibiotic expectations and unnecessary utilization. J Fam Pract. 1997;45:75–83. [PubMed] [Google Scholar]
- 15.Brett AS, Mathieu AE. Perceptions and behaviors of patients with upper respiratory tract infections. J Fam Pract. 1982;15:277–9. [PubMed] [Google Scholar]
- 16.Hutchinson JM, Foley RN. Influence of nonmedical factors on antibiotic prescription rates. 37th Interscience Conference on Antimicrobial Agents and Chemotherapy; September 28; Toronto, Ont., Canada. 1997. Presented at the. [Google Scholar]
- 17.Kaiser L, Lew D, Hirschel B, et al. Effects of antibiotic treatment in the subset of common-cold patients who have bacteria in nasopharyngeal secretions. Lancet. 1996;347:1507–10. doi: 10.1016/s0140-6736(96)90670-4. [DOI] [PubMed] [Google Scholar]
- 18.Hays GC, Mullard JE. Can nasal bacterial flora be predicted from clinical findings? Pediatrics. 1973;49:596–9. [PubMed] [Google Scholar]
- 19.Robertson AJ. Green sputum. Lancet. 1952;1:12–5. doi: 10.1016/s0140-6736(52)90988-4. [DOI] [PubMed] [Google Scholar]
- 20.Heald A, Auckenthaler R, Borst F, et al. Adult bacterial nasopharyngitis: a clinical entity? J Gen Intern Med. 1993;8:667–73. doi: 10.1007/BF02598283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walsh TB, Bookheim WW, Johnson RC, Tompkins RK. Recognition of streptococcal pharyngitis in adults. Arch Intern Med. 1975;135:1493–7. [PubMed] [Google Scholar]
- 22.Todd JK, Todd N, Damato J, Todd WA. Bacteriology and treatment of purulent nasopharyngitis: a double blind, placebo-controlled evaluation. Pediatr Infect Dis J. 1984;3:226–32. doi: 10.1097/00006454-198405000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Stott NCH, West RR. Randomised controlled trial of antibiotics in patients with cough and purulent sputum. BMJ. 1976;2:556–9. doi: 10.1136/bmj.2.6035.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verheij TJM, Hermans J, Mulder JD. Effects of doxycycline in patients with acute cough and purulent sputum: a double blind placebo controlled trial. Br J Gen Pract. 1994;44:400–4. [PMC free article] [PubMed] [Google Scholar]