Abstract
Cases of porcine malignant catarrhal fever were analyzed by a combination of identification and quantitation of ovine gammaherpesvirus 2 DNA in a variety of paraffin-embedded tissues from diseased pigs, serology, and exclusion of primary porcine gammaherpesviruses. In spite of reduced signal due to fixation and paraffin embedding, ovine gammaherpesvirus 2 DNA in pig brains exceeded the amounts found in sheep brains by orders of magnitude.
Malignant catarrhal fever, an often lethal viral infection of ruminants, is caused by the ovine gammaherpesvirus 2 and is thought to be transmitted from sheep, which remain unaffected, to other animal species, which succumb to malignant catarrhal fever (2, 4, 5, 17, 20, 21, 24, 25).
Suspected cases of malignant catarrhal fever in pigs without identification of the agent have been reported from Italy (19), Germany (12), Switzerland (23), Norway (3, 22), and Sweden (10). Only in 1998 was ovine herpesvirus 2 DNA identified for the first time in tissues from pigs with suspected malignant catarrhal fever (16). Yet, quantitative aspects and the roles of phylogenetically related viruses, i.e., porcine lymphotropic herpesviruses 1, 2, and 3 (7, 8, 26) or the alcelaphine herpesvirus 1 (agent of the wildebeest-associated form of malignant catarrhal fever) (17), have not been analyzed. Probably, the disease is heavily underestimated because the clinical signs as well as the available diagnostic procedures are not generally known. A series of recent cases of porcine malignant catarrhal fever in Switzerland was taken as an opportunity to shed more light on the diagnosis of this fascinating disease.
In June 2000, on Swiss farm A, where specific-pathogen-free (1) pigs shared stables and meadows with sheep, gilts showed symptoms reminiscent of porcine malignant catarrhal fever, i.e., anorexia, high fever, and neurological symptoms such as ataxia, tremor, convulsions, and hyperesthesia (17, 20). Two animals were euthanized in extremis (pig 3 and pig 4). Pig 5, which survived for a longer time period than its mates, showed breathing problems, cyanotic ears and snout, and multiple reddish spots on the skin. A foul-smelling nasal discharge was noticed as well as small erosions in the nasal and oral mucosa. A similar disease had been observed previously (April 1999) on the same farm and on a second specific-pathogen-free farm, farm B, in May 1986. Stored samples from such pigs (pig 1, 1986, farm B; pig 2, 1999, farm A) could be included in this study.
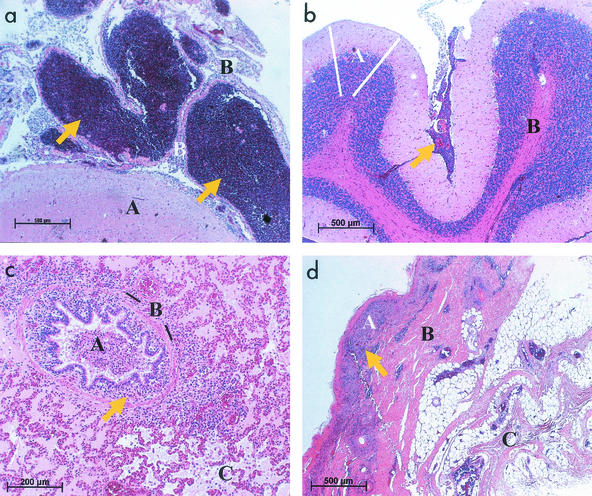
Histology (Fig. 1) revealed lesions reminiscent of malignant catarrhal fever. A high-grade nonpurulent meningoencephalitis with perivasculitis and mononuclear vasculitis was observed in the cerebrum and the cerebellum and other organs. Sometimes a few neutrophils or eosinophils were also seen in the perivascular cuffs. In the lung of pig 5, a catarrhalic bronchopneumonia with purulent bronchitis, bronchiolitis, edema, peribronchitis, and peribronchiolitis with round cell cuffs was apparent. The nasal mucosa of pig 1 showed purulent inflammation. Disseminated perivascular cuffs, round cell infiltrations, i.e., in the mucosa and submucosa of the skin (pig 3), as well as vasculitis were seen in the lung, the alimentary tract (gastritis, colitis), and the kidneys.
FIG. 1.
Histology of porcine malignant catarrhal fever (hematoxylin-eosin). (a) Cerebrum, pig 1, showing severe nonpurulent meningoencephalitis. Arrows point to massive accumulations of round cells in the meninges. A, grey matter (cortex cerebri); B, meninges (pia mater). Bar, 500 μm. (b) Cerebellum, pig 2. Arrow points to disseminated round cell infiltration in the meninges, indicating severe nonpurulent meningoencephalitis, although not as severe as in panel a. A, grey matter (cortex cerebri), spanned by white lines; B, white matter (corpus medullare); C, meninges (pia mater). Bar, 500 μm. (c) Lung, pig 5. Catarrhalic bronchopneumonia with purulent bronchitis, bronchiolitis, edema, peribronchitis, and peribronchiolitis with round cell cuffs. Arrow points to an accumulation of round cells in the area of a bronchiolus. A, lumen of bronchiolus; B, plain muscle cells (lamina muscularis mucosae), emphasized by black lines; C, destroyed alveoli. Bar, 200 μm. (d) Skin, pig 3. Alterations include hyperkeratosis, ulceration, and perivascular dermatitis with lymphocytes and plasma cells. Arrow points to accumulation of round cells. A, epidermis; B, cutis; C, subcutis. Bar, 500 μm.
Ovine herpesvirus 2 cannot be routinely propagated in cell culture to corroborate the suspicion of porcine malignant catarrhal fever. Therefore, DNA was extracted (QIAamp DNA minikit; Qiagen, Basel, Switzerland) from tissues of the diseased as well as control pigs before being subjected to fluorogenic PCR amplification specific for ovine herpesvirus 2 DNA with essentially the primers, probe, reaction mix, and thermal cycle conditions described previously (11). After lysis of the sample in order to reduce viscosity, it was transferred to a QIAshredder column (Qiagen) and centrifuged at room temperature for 2 min at 16,060 × g. DNA from fresh and paraffin-embedded ovine tissue was eluted in 100 μl of sterile water. Paraffin-embedded swine tissue was eluted in 100 μl of sterile water with 50 μg of salmon sperm DNA (Life Technologies AG, Basel, Switzerland) per ml as carrier DNA to prevent adhesion of sample DNA to tube walls (13).
Negative controls, originating from eight different farms (C through J) in five different regions of Switzerland, comprised 19 healthy pigs, ranging in age between 7 months and 4 years, with an average of 1 year. Six farms (including farm G with pig 8, Table 1) kept specific-pathogen-free pigs, while farms E and H (pig 7) were under conventional hygienic management.
TABLE 1.
Overview of animals, their origin, duration of disease, detection of herpesvirus DNA, and serological results
| Animal | Farm | Date | Duration of disease | Tissue | Viral DNA (copies per 25 mg of tissue) | Conventional PCRa | Serology |
|---|---|---|---|---|---|---|---|
| Pig 1 | B | May 1986 | >5 days | Cerebrum | 144,334 | Pan; OV | NA |
| Cerebellum | 443,233 | ND | |||||
| Kidney | 94,317 | ND | |||||
| Nasal mucosa | 10,961 | ND | |||||
| Pig 2 | A | April 1999 | ∼5 days | Cerebrum | 172,627 | Pan; OV | NA |
| Cerebellum | 29,843 | ND | |||||
| Pig 3 | A | June 2000 | ∼2 days | Cerebrum | 80,918 | Pan; OV | Negative |
| Cerebellum | 103,004 | ND | |||||
| Pharynx | 18,060,515 | ND | |||||
| Skin | 17,268,761 | ND | |||||
| Pig 4 | A | June 2000 | ∼2 days | Cerebrum | 4 | 0 | Negative |
| Cerebellum | 0 | 0 | |||||
| Pig 5 | A | June 2000 | >5 days | Cerebrum | 3,071,006 | Pan; OV | Positive |
| Cerebellum | 683,662 | ND | |||||
| Spinal cord | 622,910 | ND | |||||
| Lung | 633,163 | ND | |||||
| Pig 6 | A, 4339 | Unspec.b | Cerebrum | 0 | Pan PLHV-1/2c | Negative | |
| Pig 7 | H, B-179 | Healthy | Cerebrum | 0 | Pan PLHV-3 | NA | |
| Pig 8 | G, 965-1497 | Healthy | Cerebellum | 0 | Pan | NA |
With pan-herpesvirus (pan) or ovine herpesvirus 2 (OV) primers, as shown. ND, not determined; NA, samples not available. Animals presenting only negative results were not included in the table.
Unspecific disease signs, not typical for porcine MCF.
Sequence determination of the amplification product identified the virus as porcine lymphotropic herpesvirus 1 (PLHV-1).
All of the available samples from four diseased pigs reacted positively by PCR. Yet, ovine herpesvirus 2 DNA concentrations in available tissues from pig 4 ranged around the limit of detection. Thus, the area within the tissue selected for diagnostic analysis may influence the accuracy of the diagnosis. Four out of five diseased pigs but none of the control pigs contained high concentrations of ovine herpesvirus 2 DNA in various tissues (Table 1). Most frequently, viral DNA was detected in both the cerebrum and the cerebellum of those animals. Viral DNA was also found in the pharynx and the skin of one animal (pig 3).
Although no serum samples were available from pigs that had succumbed to the disease in the previous years, it was of interest to test by an improved competitive inhibition enzyme-linked immunosorbent assay (14, 15, 21) whether any of the diseased animals had raised antibodies against ovine herpesvirus 2. All serum samples from pigs with malignant catarrhal fever, their healthy penmates, or unrelated control pigs reacted negatively, with the following exception. Serum from pig 5, which had been euthanized more than 5 days after clinical onset of the disease, showed an inhibition of 77% in a 1:5 dilution and a 50% inhibition in a 1:25 dilution. These results suggested that seroconversion to ovine herpesvirus 2 may be detected but only in the context of prolonged disease progression. Therefore, serological analysis may be misleading for the diagnosis of malignant catarrhal fever in pigs.
To analyze possible contributions of other herpesviruses to porcine malignant catarrhal fever, samples from the cerebrums of the five diseased pigs as well as from five controls were subjected to conventional PCR, and the results were included in Table 1. The primers for these reactions had been selected on the basis of published sequences with the following GenBank accession numbers: AF005370 (alcelaphine herpesvirus 1); AF327830 (bovine lymphotropic herpesvirus); AF327831 (ovine herpesvirus 2); AF478169 (porcine lymphotropic herpesvirus 1); AY170317 (porcine lymphotropic herpesvirus 2); AY170316 (porcine lymphotropic herpesvirus 3); and M14336 (pseudorabies virus).
A nested PCR, which amplifies the DNA polymerase of various herpesviruses (6, 7), revealed seven pan-herpesvirus-positive samples, three from diseased animals and four from the controls. Four out of the five samples from diseased animals were positive in a conventional PCR for ovine herpesvirus 2 DNA (primer 721 sense [5′-ATG CTG CCC TGC CTC ATG ATA GCC-3′] and primer 721 antisense [5′-CTG TGA ATC TCG GGG TCG GGT GCT-3′]), while the others, including all of the controls, reacted negatively. This observation may be important with regard to the possible future use of pigs in xenotransplantation (18).
Product purification following conventional PCRs and direct sequencing were done as described before (26). Ovine herpesvirus 2-positive amplificates revealed ovine herpesvirus 2-specific sequences. Importantly, none of the ovine herpesvirus 2-positive samples reacted positively in PCRs specific for porcine cytomegalovirus (essentially as described in reference 9), alcelaphine herpesvirus 1 (primer 117 sense [5′-ACC AGG AGG GTC TTA TCA GAT GGA C-3′] and primer 117 antisense [5′-ATA CAC TGC TAG ATG AGG CAG GTT G-3′]), or bovine lymphotropic herpesvirus (primer 679 sense [5′-GCT ACT CCA CCA TGA TAG AGC ACG AC-3′] and primer 679 antisense [5′-AGC TGC TGC TTG TCC AAG ATT GTT T-3′]).
Interestingly, porcine lymphotropic herpesvirus DNA was detected for the first time in the brains of two healthy Swiss control animals, one originating from a specific-pathogen-free farm and the second from a conventional farm. Brain tissue from ovine herpesvirus 2-negative pig 6 was positive for porcine lymphotropic herpesvirus 1 and 2 (primer 747 sense [5′-CAY GGT AGT ATT TAT TCA GAC A-3′] and primer 747 antisense [5′-GAT ATC CTG GTA CAT TGG AAA G-3′]), while pig 7 was positive for porcine lymphotropic herpesvirus 3 (primer 886 sense [5′-CAA GAT TGC TGA GAC GGT GAC TAC-3′] and primer 886 antisense [5′-AAA TGG CAT GGT TAC ATC TTT AGG-3′]).
These results confirmed that the diseased pigs from farms A and B had been infected with ovine herpesvirus 2 but not with other gammaherpesviruses. Therefore, ovine herpesvirus 2 has to be considered a porcine pathogen that does not normally circulate among pigs.
The quantities of viral DNA in the different organs (25 mg, deparaffinized) were determined by using a standard plasmid method (11), and the results were consistent with the likelihood that the disease symptoms had been caused by ovine herpesvirus 2.
Since paraffin had to be removed from the samples prior to amplification and fresh samples from diseased pigs were unavailable, concern emerged over quantitation of ovine herpesvirus 2 DNA in formalin-fixed and paraffin-embedded tissue of the historic cases. Therefore, a comparison of the quantitative detection of ovine herpesvirus 2 DNA in either fresh or paraffin-embedded sheep tissues was performed. Twelve different tissues, which were available from a previous study (11a), i.e., epididymis, vesicular gland, testis, spinal cord, pars disseminata of prostate gland, palatine tonsil, urinary bladder, ampulla of deferent duct, pituitary gland, rhombencephalon, trigeminal ganglion, and cerebrum, were analyzed.
A net loss of the DNA quantity amounting to more than one order of magnitude compared to fresh tissue was observed in paraffin-embedded tissues (data not shown). The least losses (one order of magnitude) were observed in the pituitary gland and the cerebrum, the highest in the epididymis (two orders of magnitude). These results suggested that ovine herpesvirus 2 DNA may be detected in paraffin-embedded tissue but that the sensitivity was below that for fresh tissue. Therefore, negative results with this technique have to be interpreted with caution. The cerebrum seemed best suited for quantitative analysis of ovine herpesvirus 2 DNA in paraffin-embedded tissue.
In view of the loss of sensitivity caused by analyzing paraffin-embedded tissues, the amounts of ovine herpesvirus 2 DNA in the paraffin-embedded brain tissue from malignant catarrhal fever-diseased pigs were surprisingly high, although within the same range as those in either fresh or paraffin-embedded brain tissue of cattle with malignant catarrhal fever (Table 2). In contrast, available sheep brains, representative of an animal species that does not succumb to malignant catarrhal fever, contained low copy numbers of ovine herpesvirus 2 DNA, ranging from not detectable to 4,348 copies per 25 mg of tissue. The number of ovine herpesvirus 2 DNA copies measured in brains of cattle with malignant catarrhal fever exceeded the number found in sheep brains 4- to 27-fold in the case of paraffin-embedded organs and 140- to 400-fold in the case of fresh samples. The pig brains contained even higher amounts of ovine herpesvirus 2 DNA, exceeding the amounts found in sheep brains in a range from 40-fold to 1,500-fold.
TABLE 2.
Comparison of viral DNA load in the cerebrum of different animal species
| Species and animal no. | Ovine herpesvirus 2 (no. of copies/25 mg)
|
|
|---|---|---|
| Fresh samplesa | Embedded samples | |
| Pig 1 | NA | 144,334 |
| Pig 2 | NA | 172,627 |
| Pig 3 | NA | 80,918 |
| Pig 4 | NA | 4 |
| Pig 5 | NA | 3,071,006 |
| Sheep 1 | 0 | 0 |
| Sheep 2 | 276 | 0 |
| Sheep 3 | 4,348 | 0 |
| Sheep 4 | 273 | 0 |
| Sheep 5 | 149 | 382 |
| Cattle 1 | 833,559 | NA |
| Cattle 2 | 274,808 | NA |
| Cattle 3 | NA | 7,288 |
| Cattle 4 | NA | 54,868 |
NA, not available.
These results suggest that the observed neurologic symptoms correlate to the viral loads in the brains of affected animals. There was one pig (pig 4) that presented only small amounts of ovine herpesvirus 2 DNA in its available brain tissue (4.84 copies per 25 mg), but the sample possibly did not represent the area with maximal viral load.
The amount of viral DNA measured in the different porcine tissues varied by orders of magnitude. The highest values were detected in the pharynx and in the skin of pig 3. However, ovine herpesvirus 2 DNA was also detected in the nasal mucosa and the lungs of other animals, which gave rise to the question of whether ovine herpesvirus 2 may be excreted by affected animals.
In the present report, the previous, unfortunately ill-recognized literature concerning the occurrence of porcine malignant catarrhal fever was confirmed (3, 10, 22, 23), and our knowledge of porcine malignant catarrhal fever is extended in this article. The evidence is based on a combination of identification and quantitation of ovine herpesvirus 2 DNA in a variety of tissues from diseased pigs and on the exclusion of primary porcine gammaherpesviruses.
Acknowledgments
We thank E. Bürgi, P. Jaros, B. Gerzner, and K. Süss as well as C. Casura and A. Brändli for helping with sample collection. Enlightening discussions with and technical assistance of the following persons are also gratefully acknowledged: R. Fatzer, T. Franz, E. Loepfe, and A. Vögtlin.
This work was supported by the Swiss Federal Veterinary Office and the University of Zurich.
REFERENCES
- 1.Anonymous. 1989. Reglement über die Durchführung des Beratungs- und Gesundheitsdienstes in der Schweinehaltung (SGD-Reglement). Eidgenössisches Volkswirtschaftsdepartement, Bern, Switzerland.
- 2.Baxter, S. I., I. Pow, A. Bridgen, and H. W. Reid. 1993. PCR detection of the sheep-associated agent of malignant catarrhal fever. Arch. Virol. 132:145-159. [DOI] [PubMed] [Google Scholar]
- 3.Bratberg, B. 1980. Acute vasculitis in pigs: a porcine counterpart to malignant catarrhal fever, p. 353. 10th Congress of the International Pig Veterinary Society. Reproset, Copenhagen, Denmark.
- 4.Bridgen, A., and H. W. Reid. 1991. Derivation of a DNA clone corresponding to the viral agent of sheep-associated malignant catarrhal fever. Res. Vet. Sci. 50:38-44. [DOI] [PubMed] [Google Scholar]
- 5.Brown, C. C., and L. L. Bloss. 1992. An epizootic of malignant catarrhal fever in a large captive herd of white-tailed deer (Odocoileus virginianus). J. Wildl. Dis. 28:301-305. [DOI] [PubMed] [Google Scholar]
- 6.Chmielewicz, B., M. Goltz, and B. Ehlers. 2001. Detection and multigenic characterization of a novel gammaherpesvirus in goats. Virus Res. 75:87-94. [DOI] [PubMed] [Google Scholar]
- 7.Ehlers, B., K. Borchers, C. Grund, K. Frölich, H. Ludwig, and H. J. Buhk. 1999. Detection of new DNA polymerase genes of known and potentially novel herpesviruses by PCR with degenerate and deoxyinosine-substituted primers. Virus Genes 18:211-220. [DOI] [PubMed] [Google Scholar]
- 8.Ehlers, B., S. Ulrich, and M. Goltz. 1999. Detection of two novel porcine herpesviruses with high similarity to gammaherpesviruses. J. Gen. Virol. 80:971-978. [DOI] [PubMed] [Google Scholar]
- 9.Goltz, M., F. Widen, M. Banks, S. Belak, and B. Ehlers. 2000. Characterization of the DNA polymerase loci of porcine cytomegaloviruses from diverse geographic origins. Virus Genes 21:249-255. [DOI] [PubMed] [Google Scholar]
- 10.Holmgren, N., N.-E. Björklund, and B. Persson. 1983. Fall av akut vaskulit hos svin påvisade i Sverige. Svensk Vet. 35:103-106. [Google Scholar]
- 11.Hüssy, D., N. Stäuber, C. M. Leutenegger, S. Rieder, and M. Ackermann. 2001. Quantitative fluorogenic PCR assay for measuring ovine herpesvirus 2 replication in sheep. Clin. Diagn. Lab. Immunol. 8:123-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11a.Hüssy, D., F. Janett, S. Albini, N. Stäuber, R. Thun, and M. Ackermann. 2002. Analysis of the pathogenic basis for shedding and transmission of ovine gamma herpesvirus 2. J. Clin. Microbiol. 40:4700-4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurtze, H. 1950. Uebertragung des “Bösartigen Katarrhalfiebers des Rindes” auf ein Schwein. Dtsch. Tieraerztl Wochenschr. 57:261. [Google Scholar]
- 13.Leutenegger, C. M., D. Klein, R. Hofmann-Lehmann, C. Mislin, U. Hummel, J. Boni, F. Boretti, W. H. Guenzburg, and H. Lutz. 1999. Rapid feline immunodeficiency virus provirus quantitation by polymerase chain reaction using the TaqMan fluorogenic real-time detection system. J. Virol. Methods 78:105-116. [DOI] [PubMed] [Google Scholar]
- 14.Li, H., T. C. McGuire, U. U. Müller-Doblies, and T. B. Crawford. 2001. A simpler, more sensitive competitive inhibition enzyme-linked immunosorbent assay for detection of antibody to malignant catarrhal fever viruses. J. Vet. Diagn. Investig. 13:361-364. [DOI] [PubMed] [Google Scholar]
- 15.Li, H., D. T. Shen, D. P. Knowles, J. R. Gorham, and T. B. Crawford. 1994. Competitive inhibition enzyme-linked immunosorbent assay for antibody in sheep and other ruminants to a conserved epitope of malignant catarrhal fever virus. J. Clin. Microbiol. 32:1674-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Løken, T., M. Aleksandersen, H. Reid, and I. Pow. 1998. Malignant catarrhal fever caused by ovine herpesvirus-2 in pigs in Norway. Vet. Rec. 143:464-467. [DOI] [PubMed] [Google Scholar]
- 17.Metzler, A. E. 1991. The malignant catarrhal fever complex. Comp. Immunol. Microbiol. Infect. Dis. 14:107-124. [DOI] [PubMed] [Google Scholar]
- 18.Michie, C. 2001. Xenotransplantation, endogenous pig retroviruses and the precautionary principle. Trends Mol. Med. 7:62-63. [DOI] [PubMed] [Google Scholar]
- 19.Morselli, R. 1901. La febbre catarrhale maligna dei bovini è contagiosa? G. R. Soc. Vet. 1901:813-815.
- 20.Müller-Doblies, U. U., J. Egli, H. Li, U. Braun, and M. Ackermann. 2001. Bösartiges Katarrhalfieber in der Schweiz 1.Teil: Epidemiologie. Schweiz. Arch. Tierheilkd. 143:173-183. [PubMed] [Google Scholar]
- 21.Müller-Doblies, U. U., H. Li, B. Hauser, H. Adler, and M. Ackermann. 1998. Field validation of laboratory tests for clinical diagnosis of sheep-associatedmalignant catarrhal fever. J. Clin. Microbiol. 36:2970-2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okkenhaug, H., and O. Kjelvik. 1995. Ondartet katarrfeber hos gris. Norsk Vet. 107:199-203. [Google Scholar]
- 23.Pohlenz, J., H.-U. Bertschinger, and W. Koch. 1974. A malignant catarrhal fever-like syndrome in sows, p. V15-1-V15-3. 3rd Congress of the International Pig Veterinary Society. Presses de l’Inprimerie ESPIC, Toulouse, France.
- 24.Reid, H. W., D. Buxton, W. A. McKelvey, J. A. Milne, and W. T. Appleyard. 1987. Malignant catarrhal fever in Père David's deer. Vet. Rec. 121:276-277. [DOI] [PubMed] [Google Scholar]
- 25.Schultheiss, P. C., J. K. Collins, T. R. Spraker, and J. C. DeMartini. 2000. Epizootic malignant catarrhal fever in three bison herds: differences from cattle and association with ovine herpesvirus-2. J. Vet. Diagn. Investig. 12:497-502. [DOI] [PubMed] [Google Scholar]
- 26.Ulrich, S., M. Goltz, and B. Ehlers. 1999. Characterization of the DNA polymerase loci of the novel porcine lymphotropic herpesviruses 1 and 2 in domestic and feral pigs. J. Gen. Virol. 80:3199-3205. [DOI] [PubMed] [Google Scholar]