Abstract
OBJECTIVE
To examine the relation between selected nonoccupational risk factors and surgery for carpal tunnel syndrome.
DESIGN
Case-control study using an administrative database.
PARTICIPANTS
Enrollees of New Jersey Medicare or Medicaid programs during 1989 to 1991.
MEASUREMENTS
The outcome of interest was open or endoscopic carpal tunnel release. We examined the relation between carpal tunnel release and diabetes mellitus, thyroid disease, inflammatory arthritis, hemodialysis, pregnancy, use of corticosteroids, and hormone replacement therapy.
MAIN RESULTS
In multivariate models, inflammatory arthritis was strongly associated with carpal tunnel release (odds ratio [OR] 2.9; 95% confidence interval [CI] 2.2, 3.8). However, corticosteroid use also appeared to be associated with a greater likelihood of undergoing carpal tunnel release, even in the absence of inflammatory arthritis (OR 1.6; 95% CI 1.2, 2.1). Diabetes had a weak but significant association with carpal tunnel release (OR 1.4; 95% CI 1.2, 1.8), as did hypothyroidism (OR 1.7; 95% CI 1.1, 2.8), although patients with hyperthyroidism did not have any change in risk. Women who underwent carpal tunnel release were almost twice as likely to be users of estrogen replacement therapy as controls (OR 1.8; 95% CI 1.0, 3.2).
CONCLUSIONS
Although inflammatory arthritis is the most important nonoccupational risk factor for carpal tunnel release, these data substantiate the increase in risk associated with diabetes and untreated hypothyroidism. Further investigation in detailed clinical studies will be necessary to confirm whether changes in corticosteroid use and hormone replacement therapy offer additional means of risk reduction for this common condition.
Keywords: carpal tunnel syndrome, risk factors, thyroid disease, diabetes mellitus, corticosteroids
Hand and wrist complaints are common in primary care, affecting 7% to 10% of the U.S. population at any point in time, and prompt many physician office visits.1 Carpal tunnel syndrome (CTS) is a frequent diagnosis considered in such patients and is the most frequent entrapment neuropathy. Approximately 1.6% of adults describe symptoms consistent with CTS,2 characterized by paresthesias and pain in the distribution of the median nerve, and occasional weakness leading to upper extremity functional impairment.3 The syndrome can often be treated effectively with splinting and avoidance of repetitive motions and awkward wrist positions; however, carpal tunnel release is ultimately performed in 25% to 50% of these patients.4 Despite the prevalence and morbidity of CTS, its nonoccupational risk factors are poorly defined.
Two large studies have attempted to define the risk factors for CTS. De Krom and colleagues conducted a case-control analysis in the Netherlands and found repetitive motion, past hysterectomy without oophorectomy, recent menopause, and higher body-mass index to be positively associated with CTS.5 A 20-year uncontrolled population-based survey in Rochester, Minn, suggested that trauma, rheumatoid arthritis, use of hormonal agents, oophorectomy, diabetes mellitus, and pregnancy were commonly associated with CTS.6 Other conditions reported in the literature in association with CTS are hemodialysis,7 pseudogout,8 polymyalgia rheumatica,9 hypothyroidism,10 hyperthyroidism,11 acromegaly,12 carpal stenosis,13 and a patent median artery.14 However, these associations are quantified poorly or not at all. Many past studies of risk factors for CTS are difficult to interpret because of small sample size and, frequently, lack of a control population.
The relative paucity and poor quality of research on risk factors for CTS has implications for clinical management. When evaluating patients with newly diagnosed CTS, clinicians are not sure which, if any, diagnostic tests should be performed to exclude potentially related conditions such as thyroid disease, diabetes mellitus, and inflammatory arthritis. Further, the limited evidence regarding etiologic associations hampers efforts at prevention and nonsurgical treatment.
If used judiciously, health care encounter data from health insurance programs can provide data on medical conditions, medication use, and surgical procedures, facilitating epidemiologic studies of select conditions in very large populations. We used such a claims database to characterize the magnitude of CTS risk associated with commonly described conditions such as inflammatory arthritis, diabetes mellitus, and hypothyroidism. We also performed analyses exploring the risk of carpal tunnel release associated with estrogen replacement therapy.
METHODS
Subjects
Study subjects were enrolled in the New Jersey Medicaid, Medicare, and Pharmacy Assistance for the Aged and Disabled (PAAD) programs during the period 1989 to 1991. Services for which data are available include all hospitalizations, physician visits, nursing home care (through Medicaid), and medications (through Medicaid and PAAD). During the study period, essentially all drugs were covered without formulary restrictions. Deductibles and copayments were absent or minimal, permitting comprehensive capture of medication use for enrolled subjects. All traceable personal identifiers were removed from the claims data prior to analysis.
We began by identifying all subjects who underwent an open or endoscopic carpal tunnel release procedure (International Classification of Diseases, Ninth Clinical Modification[ICD-9-CM] procedure code 04.43, or Physicians’ Current Procedural Terminology (CPT) procedure codes 64721 or 29848) during the study period. Subjects were eligible to become case subjects only once, and the date of carpal tunnel surgery was considered the index date. For every case, six eligible subjects who had not already become case subjects as of the index date were selected as control subjects. Control subjects were frequency-matched by age and gender with case subjects. We did not match on race or insurance status. Case subjects and control subjects were required to be active system users, defined as having filled at least one prescription for any drug during the periods 1 to 180 days and 180 to 365 days prior to the index date.
Variables
Initially, we explored the associations of carpal tunnel release with thyroid disease, diabetes mellitus, inflammatory arthritis, pregnancy, and hemodialysis. The variables of interest were defined by diagnostic codes, disease-specific medications, or procedure codes. Patients were considered to carry a diagnosis, use specific medications, or undergo a procedure if these were recorded in the year prior to the index date. The presence of diabetes was defined by ICD-9-CM diagnosis codes 250.00–250.99 or the use of insulin or oral hypoglycemic agents. Hypothyroidism was studied in two ways; subjects were identified as having diagnosed hypothyroidism if they had physician visits for ICD-9-CM diagnosis codes 243.00 or 244.00–244.99, or were users of thyroid replacement preparations, primarily thyroxine, as based on filled prescription data. Because many people carried the diagnosis of hypothyroidism but were not taking replacement drugs, we studied each group separately. In a similar fashion, hyperthyroidism was defined by diagnosis codes and thyroid ablation procedure codes (ICD-9-CM diagnosis codes 242.00–242.99, 245.00–245.99, and 648.1) and by use of medications to ablate or suppress thyroid function.
For inflammatory arthritis, patients either carried one of several diagnoses or were taking disease-modifiying antirheumatic drugs (DMARDs). The inflammatory arthritis category included rheumatoid arthritis (ICD-9-CM diagnosis codes 714.0–714.9), diffuse diseases of connective tissue such as systemic lupus erythematosus, systemic sclerosis, inflammatory muscle disease (ICD-9-CM diagnosis codes 710.00–710.99), crystalline arthritis (ICD-9-CM diagnosis codes 712.0–712.9), and seronegative inflammatory arthritides such as Reiter’s disease (ICD-9-CM diagnosis codes 711.1 and 711.3), psoriatic arthritis (ICD-9-CM diagnosis code 696.0), arthritis related to inflammatory bowel disease (ICD-9-CM diagnosis code 713.1), and ankylosing spondylitis (ICD-9-CM diagnosis codes 720.0–720.9). Disease-modifying agents were oral methotrexate, penicillamine, azathioprine, and gold. Sulfasalazine was not included because most people using this medication have diagnoses other than inflammatory arthritis. Oral corticosteroids, although important medications in the treatment of inflammatory arthritis, have many nonrheumatic uses. Hence, we analyzed corticosteroid use separately. Secondary analyses were performed to explore whether patients who underwent carpal tunnel release may have been prescribed corticosteroids as treatment for their carpal tunnel symptoms (confounding by indication). We therefore categorized corticosteroid use according to proximity to the index date, defining four groups of corticosteroid users: those who filled prescriptions only in the 30 days prior to the index date; those who filled prescriptions only in the 60 days prior to the index date; those who filled prescriptions only in the 90 days prior to the index date; and those who filled prescriptions at other times. The first group was most likely to have the corticosteroids prescribed to reduce CTS symptoms and the last group was least likely.
Pregnancy was defined as the 9-month period before a diagnosis code for a spontaneous or surgical delivery (ICD-9-CM diagnosis and procedure codes V27.0–V27.9, 72.0–72.9, 73.0–73.9, 74.0–74.9, or CPT procedure codes 59400, 59410, 59412, 59414, 59510, 59515, and 59525) or the 5-month period before a therapeutic or spontaneous abortion (ICD-9-CM diagnosis and procedure codes 630.00–633.9, and 75.0, or CPT procedure codes 59100, 59120, 59121, 59130, 59135, 59136, 59140, 59150, 59812, 59820, 59821, 59830, 59840, 59841, 59850, 59851, 59852, and 59870). Chronic hemodialysis was identified by the appropriate diagnosis or procedure codes (ICD-9-CM diagnosis code 39.95, or CPT procedure codes 90935 and 90937), and did not include patients with only the diagnosis of chronic renal failure.
We also assessed the risk of carpal tunnel surgery associated with the use of estrogen replacement therapy. Subjects were stratified into three age groups: 18–45, 46–65, and 66 and over. The youngest group was considered the referent group. Race was dichotomized into white and all others.
Analysis
We initially measured the association between carpal tunnel release and various risk factors with contingency tables. Strength of association was represented with the odds ratio (OR) and 95% confidence intervals (CIs), and statistical significance was assessed with the χ2test. We entered each variable separately into the final multivariate logistic regression model. Because corticosteroids were independently related to carpal tunnel release in adjusted models, we further investigated this relation in a model that excluded all subjects who had a diagnosis of inflammatory arthritis or were using disease-modifying antirheumatic drugs. All analyses were carried out using SAS (SAS Institute, Cary, NC).
RESULTS
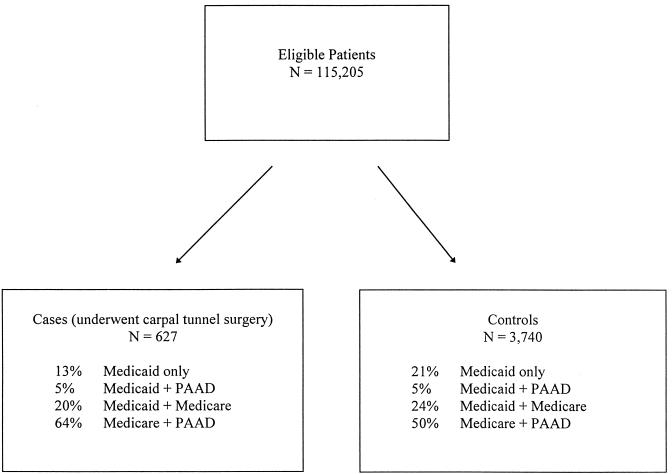
Case and control subjects were drawn from Medicare, Medicaid, and PAAD in similar proportions (Fig. 1). The population was predominantly over 64 years old (73%), female (74%), and white (72%) (Table 1). Several risk factors emerged as significant predictors of carpal tunnel release; on univariate analysis, case subjects were more likely to have inflammatory arthritis, diabetes, or hypothyroidism, require hemodialysis, or use corticosteroids or estrogen replacement therapy. No statistically significant differences were found between case and control subjects for rates of hyperthyroidism (based on diagnosis or therapy), pregnancy, or the use of thyroid replacement therapy (Table 1).
FIGURE 1.
Source of study population.
Table 1.
Population Studied
| Patient Attributes | CaseSubjects, %(n = 626) | ControlSubjects, %(n = 3,618) |
|---|---|---|
| Age>64 years | 71 | 73 |
| Female* | 81 | 73 |
| White | 75 | 71 |
| Inflammatory arthritis* | 14 | 5 |
| Diabetes* | 26 | 19 |
| Hypothyroid* | 4 | 2 |
| Hemodialysis* | 3 | 0 |
| Pregnancy | 0 | 1 |
| Corticosteroid use* | 14 | 8 |
| Estrogen replacement use* | 5 | 3 |
p <.01.
We then developed a multivariate model of factors associated with carpal tunnel release in which each variable was adjusted for the others. These results are displayed in Table 2. Several variables remained significant risk factors after adjustment, including female gender (OR 1.6; 95% CI 1.3, 2.0), inflammatory arthritis (OR 3.1; 95% CI 2.2, 4.2), diabetes (OR 1.4; 95% CI 1.2, 1.8), hypothyroidism (OR 1.7; 95% CI 1.1, 2.8), corticosteroid use (OR 1.6; 95% CI 1.2, 2.1), hemodialysis (OR 9.0; 95% CI 4.2, 19.6), and hormone replacement therapy (OR 1.8; 95% CI 1.0, 3.2). The overall model had moderate explanatory power (c = 0.64); age remained a nonsignificant predictor. Inserting hyperthyroidism and thyroid replacement use into the model as variables added nothing to its predictive value. Models also were run with inflammatory arthritis defined only with diagnostic codes or by DMARD use. Both definitions of inflammatory arthritis were significantly associated with CTS surgery in unadjusted and multivariate analyses, and other variable point estimates were not changed.
Table 2.
Association Between Carpal Tunnel Releaseand Potential Risk Factors
| Unadjusted | Adjusted* | |||
|---|---|---|---|---|
| Risk Factor | OR | 95% CI | OR | 95% CI |
| Age, years | ||||
| 45–64 | 1.2 | 1.0, 1.5 | 1.1 | 0.8, 1.6 |
| 65+ | 0.9 | 0.8, 1.1 | 1.0 | 0.7, 1.3 |
| Female† | 1.7 | 1.3, 2.1 | 1.6 | 1.3, 2.0 |
| Inflammatory arthritis† | 3.1 | 2.4, 4.1 | 3.1 | 2.2, 4.2 |
| Diabetes mellitus† | 1.4 | 1.2, 1.8 | 1.4 | 1.2, 1.8 |
| Hypothyroidism | 1.9 | 1.2, 3.1 | 1.7 | 1.1, 2.8 |
| Hemodialysis† | 8.4 | 4.0, 17.7 | 9.0 | 4.2, 19.6 |
| Corticosteroid use† | 1.9 | 1.4, 2.4 | 1.6 | 1.2, 2.1 |
| Estrogen replacement use | 2.0 | 1.2, 3.6 | 1.8 | 1.0, 3.2 |
Adjustments made for race and all other variables in the table.
p <.01.
We then analyzed a stratified sample to further define whether corticosteroid use was an independent predictor of carpal tunnel release. In a model that excluded all subjects who had inflammatory arthritis or used disease-modifiying antirheumatic drugs, individual point estimates for each predictor were stable, and corticosteroid use continued to have a significant association with carpal tunnel surgery (OR 1.5; 95% CI 1.1, 2.1). To examine the possibility that this relation was an artifact of preoperative corticosteroid use, we analyzed the risk associated with corticosteroid use in the 30 days, 60 days, and 90 days prior to the index date. Odds ratios for each of these groups did not differ from 1.0, but those who were prescribed corticosteroids prior to 90 days had an OR of 2.2, indicating that the association of corticosteroids with CTS surgery was not likely an artifact of confounding by indication. In addition, when we excluded patients who began using a corticosteroid in the 30 days prior to carpal tunnel surgery, the model estimates did not change and the point estimate for corticosteroid use remained stable (OR 1.6; 95% CI 1.2, 2.1).
DISCUSSION
Although CTS is common and causes considerable disability, many of its risk factors are poorly defined. We assembled the largest population to date of patients undergoing carpal tunnel release to quantify the risk of surgery for patients with CTS associated with important common conditions and medications. As had been suggested by prior literature, inflammatory arthritis conferred a nearly threefold increase in risk of carpal tunnel surgery (OR 2.9; 95% CI 2.2, 3.8). Other significant risk factors were diabetes (40% increase in risk), the diagnosis of hypothyroidism (70% increase), and hemodialysis, which was associated with a ninefold increase in risk of carpal tunnel surgery. Patients taking thryoid replacement medication did not appear to have an increased risk of CTS surgery, suggesting a potentially useful area for further research. Use of hormone replacement therapy appeared to be associated with a higher rate of requiring carpal tunnel release (80% increase). In addition, we found an unexpected 60% increase in risk of carpal tunnel release in patients taking corticosteroids, even in the absence of evidence for concomitant inflammatory arthritis.
Several aspects of our study design limit the strength of the conclusions. First, we studied predictors of a procedure, carpal tunnel release, and not the clinical condition, CTS. Although both are important epidemiologic end points, it is possible that patients who undergo carpal tunnel release represent the most severe cases or that they receive more intensive medical care. This could lead to more extensive laboratory testing and thus make it more likely that they would also be diagnosed with other conditions. Second, the clinical conditions and medications we studied as potential risk factors were defined by diagnosis codes or medication prescriptions, not chart review, and thus may have potential inaccuracies. This issue pervades studies of administrative data and requires that our findings be confirmed in detailed clinical studies.15,16 Specifically, the association with steroid use could be confounded if diagnoses of inflammatory arthritis were not recorded or if physicians were prescribing corticosteroids before subjecting patients to carpal tunnel release. We explored this possibility by separately analyzing patients who only used corticosteroids within 90 days of their carpal tunnel release. No effect of corticosteroid use was seen in this group, but the effect was strengthened in longer-term users, suggesting that the increased risk observed is not due to confounding by indication. Our population contained few subjects who became pregnant, limiting our ability to quantify the risk associated with this factor. Finally, we could not adjust for clinical characteristics not contained in the data set such as obesity, a putative risk factor for CTS,17 and repetitive forceful motion. The latter problem is attenuated by studying a largely unemployed population.
Although only observational in nature and based on limited clinical detail, these results have credible pathophysiologic explanations. Pathologic studies document that the inflammatory tenosynovitis associated with crystal arthritis can cause compression of the median nerve in the carpal tunnel.18 This is presumably true for other types of inflammatory arthritis as well. Uncontrolled hypothyroidism is associated with myxedematous deposition and thickened synovial fluid, which are thought to entrap the median nerve in the carpal tunnel.9 Diabetes causes a peripheral neuropathy through abnormal glycosylation of protein end products; however, it is doubtful that diabetic neuropathy is the primary cause of CTS in these patients. Diabetic vascular changes and tendinopathies may also predispose toward CTS.19 Hemodialysis is known to stimulate β2-microglobulin production, which may deposit as amyloid in soft tissues.20 Presumably, similar β2-microglobulin amyloid deposition in the carpal tunnel causes median nerve entrapment. There have been no pathologic studies of oral corticosteroids use and CTS, but three hypotheses related to other known causative factors can be considered. First, oral corticosteroids have a mineralocorticoid effect and thus cause expansion of fluid volume and generalized edema, which could compress the median nerve in the fixed space of the carpal tunnel. Second, corticosteroid-associated CTS may be related to undiagnosed hyperglycemia causing glycosylation of tissue end products as in diabetes. Third, corticosteroid use is known to cause lipomatosis,21 which may entrap the median nerve in the carpal tunnel. If this finding is confirmed in other studies, it could have important clinical implications.
Acknowledgments
The authors thank Drs. Neil Solomon and Paula Birnbaum for providing encouragement and valuable feedback. In addition, Dr. Barry Simmons provided thoughtful comments on an earlier version of this manuscript.
This work was funded in part by National Institutes of Health Multipurpose Arthritis Center Grant AR36308. Dr. Solomon is a recipient of a Physician Scientist Development Award from the Arthritis Foundation and American College of Rheumatology, and Dr. Katz is a recipient of an Investigator Award from the Arthritis Foundation.
REFERENCES
- 1.Cunningham LS, Kelsey JL. Epidemiology of musculoskeletal impairments and associated disability. Am J Public Health. 1984;74:574–9. doi: 10.2105/ajph.74.6.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanaka S, Wild DK, Seligman PJ, et al. The US prevalence of self-reported carpal tunnel syndrome: 1988 National Health Interview Survey Data. Am J Public Health. 1994;84:1846–8. doi: 10.2105/ajph.84.11.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dawson DM. Entrapment neuropathies of the upper extremity. N Engl J Med. 1993;329:2013–8. doi: 10.1056/NEJM199312303292707. [DOI] [PubMed] [Google Scholar]
- 4.Yamaguchi DM, Lipscomb PR, Soule EH. Carpal tunnel syndrome. Minn Med. 1965;48:22–33. [PubMed] [Google Scholar]
- 5.De Krom MC, Knipschild PG, Kester AD, et al. Carpal tunnel syndrome: prevalence in the general population. J Clin Epidemiol. 1992;45:373–6. doi: 10.1016/0895-4356(92)90038-o. [DOI] [PubMed] [Google Scholar]
- 6.Stevens JC, Beard CM, O’Fallon WM, Kurland LT. Conditions associated with carpal tunnel syndrome. Mayo Clin Proc. 1992;67:541–8. doi: 10.1016/s0025-6196(12)60461-3. [DOI] [PubMed] [Google Scholar]
- 7.Bicknell JM, Lim AC, Raroque HG, Tzamaloukas AH. Carpal tunnel syndrome, subclinical median mononeuropathy, and peripheral polyneuropathy: common early complications of chronic peritoneal and hemodialysis. Arch Phys Med Rehabil. 1991;72:378–81. [PubMed] [Google Scholar]
- 8.Pattrick MG, Watt I, Dieppe PA, Doherty M. Peripheral nerve entrapment at the wrist in pyrophosphate arthropathy. J Rheumatol. 1988;15:1254–7. [PubMed] [Google Scholar]
- 9.Ahmed T, Braun AI. Carpal tunnel syndrome with polymyalgia rheumatica. Arthritis Rheum. 1978;21:221–3. doi: 10.1002/art.1780210207. [DOI] [PubMed] [Google Scholar]
- 10.Dekel S, Papaioannou T, Rushworth G, Coates R. Idiopathic carpal tunnel syndrome caused by carpal stenosis. BMJ. 1980;280:1297–9. doi: 10.1136/bmj.280.6227.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lavey EB, Pearl RM. Patent median artery as a cause of carpal tunnel syndrome. Ann Plast Surg. 1981;7:236–8. [PubMed] [Google Scholar]
- 12.O’Duffy JD, Randall RV, MacCarty CS. Median neuropathy in acromegaly: a sign of endocrine overactivity. Ann Intern Med. 1973;78:379–83. doi: 10.7326/0003-4819-78-3-379. [DOI] [PubMed] [Google Scholar]
- 13.Dorwart BB, Schumacker HR. Joint effusions, chondrocalcinosis and other rheumatic manifestations in hypothyroidism: a clinicopathologic study. Am J Med. 1975;59:780–90. doi: 10.1016/0002-9343(75)90463-5. [DOI] [PubMed] [Google Scholar]
- 14.Roquer J, Cano JF. Carpal tunnel syndrome and hyperthyroidism: a prospective study. Acta Neurol Scand. 1993;88:149–52. doi: 10.1111/j.1600-0404.1993.tb04207.x. [DOI] [PubMed] [Google Scholar]
- 15.Ray WA, Griffin MR. Use of Medicaid data for pharmacoepidemiology. Am J Epidemiol. 1989;129:837–49. doi: 10.1093/oxfordjournals.aje.a115198. [DOI] [PubMed] [Google Scholar]
- 16.Bright RA, Avorn J, Everitt DE. Medicaid data as a resource for epidemiologic studies: strengths and limitations. J Clin Epidemiol. 1989;42:937–45. doi: 10.1016/0895-4356(89)90158-3. [DOI] [PubMed] [Google Scholar]
- 17.Nathan PA, Keniston RC, Myers LD, Meadows KD. Obesity as a risk factor for slowing of sensory conduction of the median nerve in industry: a cross-sectional and longitudinal study involving 429 workers. J Occup Med. 1992;34:379–83. [PubMed] [Google Scholar]
- 18.Lagier R, Boivin G, Gerster JC. Carpal tunnel syndrome associated with mixed calcium pyrophosphate dihydrate and apatite crystal deposition in tendon synovial sheath. Arthritis Rheum. 1984;27:1190–5. doi: 10.1002/art.1780271018. [DOI] [PubMed] [Google Scholar]
- 19.Schulte L, Roberts MS, Zimmerman C, Ketler J, Simon LS. A quantitative assessment of limited joint mobility in patients with diabetes: goniometric analysis of upper extremity passive range of motion. Arthritis Rheum. 1993;36:1429–43. doi: 10.1002/art.1780361016. [DOI] [PubMed] [Google Scholar]
- 20.Gejyo F, Odani S, Yamada T, et al. β2-microglobulin: a new form of amyloid protein associated with chronic hemodialysis. Kidney Int. 1986;30:385–90. doi: 10.1038/ki.1986.196. [DOI] [PubMed] [Google Scholar]
- 21.Rawlings CE, Bullard DE, Caldwell DS. Peripheral nerve entrapment due to steroid-induced lipomatosis of the popliteal fossa: case report. J Neurosurg. 1986;64:666–8. doi: 10.3171/jns.1986.64.4.0666. [DOI] [PubMed] [Google Scholar]