Abstract
Mycobacterium ulcerans causes Buruli ulcer disease (BUD), an ulcerative skin disease emerging mainly in West Africa. Laboratory confirmation of BUD is complicated as no “gold standard” for diagnosis exists. A nested primer PCR based on IS2404 has shown promise as a diagnostic assay. We evaluated the IS2404-based PCR to detect M. ulcerans DNA in tissue specimens from 143 BUD patients diagnosed according to the World Health Organization BUD clinical case definition in Ghana. Comparisons were made with culture and histopathology results. Variables influencing detection rate tested in this PCR protocol included the amount of tissue used and the stage of disease. The nested PCR was repeated on DNA extracted from a different part of the same biopsy specimen of 21 culture-positive samples. Of all 143 specimens, 107 (74.8%; 95% confidence interval, 68 to 82%) showed the presence of M. ulcerans DNA by PCR. Of the 78 histology-confirmed BUD patient samples, 64 (83%) were PCR positive. Detection rates were influenced neither by the amount of tissue processed for PCR nor by the stage of disease (preulcerative or ulcerative). Taken together, the two nested PCR tests on the subset of 21 culture-positive samples were able to detect M. ulcerans DNA in all 21 culture-confirmed patients. For future studies, small tissue samples, e.g., punch biopsy samples, might be sufficient for case confirmation.
Mycobacterium ulcerans causes Buruli ulcer disease (BUD), which is a devastating disease characterized by necrotizing, ulcerative lesions of subcutaneous fat and the overlying skin. Ulceration, with typically undermined edges, is preceded by a preulcerative stage. The preulcerative stage is characterized by a firm nontender nodule, edema, or plaque, with large areas of indurated skin (18). In recent years, the condition has emerged in several West African countries (1, 18). The diagnosis of BUD is usually made on the basis of clinical presentation alone. Laboratory confirmation is difficult. Swabs of lesions often do not show acid-fast bacilli (AFB) by microscopic examination, and histopathology, although a robust confirmatory test, has a low detection rate for AFB. Culture of M. ulcerans is difficult, since it is a notoriously slow-growing mycobacterium and culture media are frequently contaminated by fungi and bacteria due to the extra time required for incubation to observe mycobacterial colonies. A more sensitive and specific diagnostic assay is urgently needed.
PCR methods that have been developed are based on the 16S rRNA gene (11), the hsp65 gene (12), or the insertion sequence IS2404 (14). In 1999, Guimaraes-Peres et al. (8) evaluated two nested PCRs: the nested IS2404-based PCR and the nested 16S rRNA gene-based PCR. IS2404-based PCR was positive only with M. ulcerans isolates and the closely related M. shinshuense. The 16S rRNA gene-based PCR was positive not only for these two strains but also for M. marinum. These authors studied 65 clinical specimens from 26 patients with histologically confirmed BUD by using 1 g of tissue. The two PCR methods showed similar detection rates, with 22 out of 26 patients confirmed for M. ulcerans. However, the use of IS2404-based PCR as a detection method for M. ulcerans showed better specificity, required less time, and was less costly than the 16S rRNA gene-based PCR (8).
In the autumn of 2000, a case-control study of BUD in Ghana was carried out by the Centers for Disease Control and Prevention and the Emory University School of Medicine, Atlanta, Ga.; the Ministries of Health, Accra, Ghana; and the University Hospital Groningen, Groningen, The Netherlands. Specimens obtained from clinically suspected BUD patients were studied by PCR as described by Guimaraes-Peres et al. (8); other case confirmation methods included culture, AFB in smears of lesions, and histopathology. This is the first time that this PCR method has been used in a large study population with 0.1 g of tissue. In the study by Guimaraes-Peres et al., 1 g of tissue fragments was used (8). We describe the use of the nested IS2404-based PCR as a diagnostic tool in a set of 143 clinically diagnosed BUD patients and evaluated the effect of repeating the nested PCR on the detection rate in a subset of tissues from patients who had culture-confirmed BUD. The influence of the amount of tissue and the stage of BUD (preulcerative versus ulcerative) on the detection rate was also studied.
MATERIALS AND METHODS
Study population.
From September to November 2000, a case-control study was performed in three areas in Ghana where BUD is endemic. The aims of this study were surveillance, serodiagnosis, and identification of modifiable risk factors and host factors for BUD. These results will be reported separately. We enrolled 158 patients who met the World Health Organization clinical criteria for BUD (4). Tissue specimens were collected from patients in order to confirm diagnosis by culture of M. ulcerans, histopathology, and PCR for M. ulcerans DNA. Culture and histopathology were performed by standard techniques already published (9). The results of the histopathologic features present in the specimens from this study will be described in detail separately.
Of 158 BUD patients enrolled, 143 patients had tissue samples available for PCR and culture, and 122 patients had adequate tissue for histopathology. By culture, 56 patients were confirmed for BUD. Of these 56 positive cultures, 21 were performed at Emory University School of Medicine and were confirmed by high-pressure liquid chromatography (HPLC) by the Centers for Disease Control and Prevention at the time of PCR analysis. In this subset of 21 patients, we studied the value of a second PCR on a separate piece of tissue from the original biopsy sample.
The study was approved by the medical ethics committees of participating institutions.
Tissue collection and transport.
Elliptical biopsy samples were obtained from the margin of the active ulcer during routine surgical treatment of the patients. In the case of nodules, elliptically shaped samples were cut out of the excised nodule. Tissue specimens, ranging from 0.1 to 2 g, were immediately placed into 2-ml test tubes containing P5 transport medium, which is a modified Dubos medium (Fisher Scientific, Suwanee, Ga.) supplemented with oleic acid-albumin-dextrose-catalase (Remel, Lenexa, Kans.), PANTA (Becton Dickinson and Co., Franklin Lakes, N.J.) (15), and 0.5% agar (Fisher Scientific) (3). Tissue was transported at 4°C and stored at −20°C until the start of the isolation procedure.
DNA isolation from tissue.
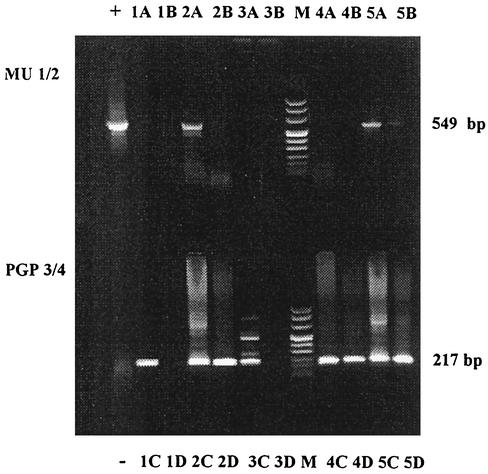
The isolation procedure and the PCR method are similar to the procedures used by Guimaraes-Peres et al. (8). Briefly, from each tissue specimen, 0.1 g was sterilely separated. We used subcutaneous tissue initially if this was available. The tissue was homogenized with a sterile piston in 500 μl of digestion buffer (30 mM Tris-HCl, 30 mM EDTA, 5% Tween 20, 0.5% Triton X-100, 800 mM guanidine hydrochloride), followed by the addition of 20 μl of proteinase K (20-mg/ml stock solution). The solution was vortexed and incubated with rotation for 30 min at 60°C. After incubation, each sample was sonicated for 8 min in a water bath sonicator at room temperature. The tissue homogenate was pelleted by centrifugation, supernatant was transferred to a sterile tube, and 40 μl of diatomaceous earth (Bio-Rad, Hercules, Calif.) was added. Each sample was incubated in a water bath for 1 h at 37°C. Following incubation, the pellet was washed twice with 70% ethanol and once with acetone. The pellet was dried at 60°C for 5 min and resuspended in 100 μl of sterile TE buffer (10 mM Tris, 1 mM EDTA, pH 8.0). Finally each solution was incubated for 20 min at 65°C and centrifuged at 2,000 × g, and the supernatant was transferred to a sterile tube and used for PCR. Positive and negative controls were included in each run. For each patient, a sample spiked with 5 ng of M. ulcerans DNA was included (Fig. 1).
FIG. 1.
Typical nested PCR amplification findings. Following the first (top) and second (bottom) rounds of PCR amplification with primer pairs MU1-MU2 and PGP3-PGP4, respectively, 15 μl of sample was applied to a 1.6% agarose gel for electrophoretic separation. +, positive control; −, negative control after MU1-MU2 and PGP3-PGP4. Lanes 1A and 1C, spiked samples of sample 1; 1B, sample 1 after MU1-MU2 stage; 1D, sample 1 after PGP3-PGP4 stage. Samples 2 to 5 are categorized in the same way. Lane M, DNA molecular size markers. After PCR, sample 1 was negative, sample 2 was positive, 3 was negative, and samples 4 and 5 were positive.
The selection of the part of tissue collected could have influenced PCR results, since M. ulcerans affects the subcutaneous fat. In this study, we chose to test the subcutaneous tissue, where we would expect the majority of AFB to be present, in the first nested PCR. A second nested PCR was run on another part of 0.1 g of the same tissue sample for the 21 culture-confirmed BUD patients. In the second PCR we targeted the dermal tissue for inclusion. The dermal tissue from 13 specimens was used; however, only subcutaneous fat tissue was available in six cases for the second nested PCR, and in two others only liquid remnant was available for further testing (Table 1).
TABLE 1.
Nested IS2404-based PCR results of the first and second PCRs for 21 culture- and HPLC-confirmed cases of BUD
| Patient | Lesion | First PCR
|
Second PCR
|
||
|---|---|---|---|---|---|
| Part of tissue | Result | Part of tissue | Result | ||
| 1 | Nodule/ulcera | Epi-/hypodermalb | + | (Epi)dermalc | + |
| 2 | Nodulea/ulcer | Hypodermal | − | Hypodermal | + |
| 3 | Edema/ulcera | Hypodermal | + | (Epi)dermal | + |
| 4 | Ulcer | (Epi)dermal | + | (Epi)dermal | + |
| 5 | Plaque | Hypodermal | − | (Epi)dermal | + |
| 6 | Nodulea/ulcer | Hypodermal | + | (Epi)dermal | + |
| 7 | Nodule | Hypodermal | + | (Epi)dermal | + |
| 8 | Plaque | Hypodermal + necrotic | − | (Epi)dermal | + |
| 9 | Ulcer | Hypodermal | + | Hypodermal | + |
| 10 | Nodulea/edema/ulcer | Hypodermal + necrotic | + | Hypodermal | + |
| 11 | Ulcer | Hypodermal | + | (Epi)dermal | + |
| 12 | Ulcer | Hypodermal | + | (Epi)dermal | + |
| 13 | Nodules | Hypodermal | + | Hypodermal | + |
| 14 | Ulcer | Epi-/hypodermal | + | (Epi)dermal | − |
| 15 | Nodule | Hypodermal | + | Hypodermal | + |
| 16 | Ulcera/scar | Hypodermal | + | (Epi)dermal | − |
| 17 | Ulcer | Epi-/hypodermal | + | (Epi)dermal | + |
| 18 | Ulcer | Hypodermal | − | (Epi)dermal | + |
| 19 | Nodule/ulcera | Hypodermal | + | Fluid in tube | + |
| 20 | Nodule | Hypodermal | + | Fluid in tube | + |
| 21 | Nodule | Hypodermal | + | Hypodermal | + |
Tissue used for PCR.
Epidermal or hypodermal.
Epidermal or dermal.
DNA amplification.
Ten microliters of the sample acquired after the DNA isolation procedure was amplified in a 50-μl reaction mixture containing 20 pmol of each primer (MU1 and MU2), 1 U of Faststart Taq DNA polymerase (Roche, Indianapolis, Ind.), 200 μM (each) deoxyribonucleotide triphosphate, 1.5 mM MgCl2, and PCR buffer.
The primers used in this amplification were MU1 (5′-GGCAGGCTGCAGATGGCAT-3′) and MU2 (5′-GGCAGTTACTTCACTGCACA-3′) (14), directed at the IS2404 sequence and producing a 549-bp fragment in the presence of M. ulcerans genomic DNA.
The thermocycling profile was as follows: denaturation at 94°C for 5 min; 40 cycles of 94°C for 1 min, 66°C for 1 min, and 72°C for 1 min; and a final extension at 72°C for 7 min. Throughout the cycling time, the heated lid function was used at 108°C. Agarose gel electrophoresis was done to confirm the status of negative and positive controls. If the controls for the first PCR were correct, the procedure continued with the following step of the nested PCR. One microliter of product from the first PCR was used in a 25-μl reaction mixture with primers PGP3 (5′-GGCGCAGATCAACTTCGCGGT-3′) and PGP4 (5′-CTGCGTGGTGCTTTACGCGC-3′) (8). These primers produce a 217-bp product. The buffers and other reaction components were added at concentrations identical to those in the first PCR step. The same thermocycling profile was used, except for a change of the annealing temperature, from 66 to 64°C.
Agarose gel electrophoresis.
Agarose gels (1.6%) in Tris-borate-EDTA buffer were prepared with ethidium bromide. Fifteen microliters of each PCR mixture was mixed with loading dye and added to the wells. The products from the first PCR, with primers MU1 and MU2, were loaded in the top row of wells while the products from the second PCR, with primers PGP3 and PGP4, were loaded in the bottom row of wells. One lane of each row on the agarose gel included molecular weight standards (MWmarker VIII; Roche). Gels were run at 90 V for 30 to 45 min. The DNA fragments were visualized with UV light and were documented on film (Polaroid). The protocol was designed for a nested PCR, and results were based on the presence of a 217-bp fragment resulting from the second PCR round with the PGP3 and PGP4 primers.
Statistical analysis.
The χ2 test was used to compare detection rates for preulcerative and ulcerative lesions. McNemar's test was used to compare outcomes of culture-confirmed cases in the first and second PCRs with a statistical significance of α = 0.05.
RESULTS
Tissue was collected and evaluated by PCR for 143 biopsy specimens of 143 clinically diagnosed BUD patients. By the nested IS2404-based PCR protocol developed by Guimaraes-Peres et al. (8), 107 tissues (74.8%, 95% confidence interval, 68 to 82%) showed the presence of M. ulcerans DNA. Of the 107 PCR-positive specimens, 6 were from patients with a histopathological diagnosis other than BUD: three filarial nodules, one keratin cyst, one deep fungus (subcutaneous zygomycosis), and one squamous cell carcinoma. Of the 78 histology-confirmed BUD patients, 64 (83%) were PCR positive. Only three patient specimens showed a negative result in the sample spiked with M. ulcerans DNA. When repeated, these spiked samples were positive. An example of a gel from the results is shown in Fig. 1.
A second PCR was run on another part of 0.1 g of the same tissue sample for 21 culture-confirmed BUD patients. Table 1 shows the PCR results with the original sample (first nested PCR) and additional material (second nested PCR). In summary, M. ulcerans DNA was present in 17 (81%) of the 21 specimens in the first nested PCR. The second nested PCR showed positive results in 19 (90%) of the 21 specimens, with a positive nested PCR for the four cases that had been negative in the first nested PCR. Two samples with a positive nested PCR in the first PCR tested negative in the second. Detection rates in the first PCR were not different from detection rates in the second PCR (P = 0.69, McNemar's test). The change of tissue type (hypodermal-dermal or epidermal) in the second nested PCR did not have an influence on the detection rate, with 12 (86%) of the 14 dermal or epidermal specimens and 22 (85%) out of 26 hypodermal specimens testing positive. When the first and the second nested PCR results are taken together, 100% of the culture-confirmed patients studied were positive.
There was no difference in rates of PCR positivity between preulcerative (nodule, plaque, or edema) and ulcerative lesions (P = 0.459, χ2 test). Of the 30 nodules tested, 24 (80%) tested positive by PCR. Only one of the six tissue samples (17%) from plaques or edemas tested positive by nested PCR. Three of the tissues from plaques or edemas that tested negative by PCR were confirmed for BUD by both culture and histopathology. Of the 103 ulcers, 78 (76%) tested positive by PCR. For four tissue samples from patients with multiple lesions, it was unclear from which lesion the tissue was obtained.
DISCUSSION
In a study performed in the autumn of 2000, tissues from 143 clinically diagnosed BUD patients were tested by the IS2404-based protocol. Of these tissues, 74.8% tested positive by PCR. High specificity and sensitivity with low detection limits are known benefits of the IS2404-based PCR (8, 13, 14, 17). The main differences between the study of Guimaraes-Peres et al. (8) and the present study are the high number of study participants, the inclusion of different disease stages, and the quantity of tissue used. Only 0.1 g of tissue was acquired from tissue excised during surgery, with a diagnostic yield similar to the specimen size of 1.0 g. We therefore speculate that similar small amounts of tissue may suffice in future studies. Such tissue samples might be obtained by simple punch biopsy. Punch biopsy samples have proven to be sufficient for diagnosis of other mycobacterial infections. For the diagnosis of M. leprae infections, PCR can be performed on sections of 5 μm of frozen or paraffin-embedded punch skin biopsy samples (6). For the identification of mycobacterial DNA in cutaneous lesions of sarcoidosis, 15 to 20 sections of 5 μm of punch biopsy samples have been used (10). Punch biopsies can be used for diagnosis of cutaneous tuberculosis with PCR dot blotting since both culture and histopathological examination are difficult due to the paucity of organisms (2).
The study by Guimaraes-Peres et al. (8) demonstrated PCR positivity in 55 of 65 (85%) specimens of 26 histologically confirmed patients. We found a similar detection rate in our study, with 64 of the 78 (82%) patients who were confirmed by histopathology also testing positive by PCR. As shown, the reduction in quantity of tissue processed for PCR did not influence the detection rates. In this study, 21 (15%) of 143 patients did not have subcutaneous tissue, and thus the specimens were considered inadequate for histopathologic evaluation. However, there was sufficient tissue for PCR evaluation of the 143 specimens received. M. ulcerans DNA was detected at similar rates in preulcerative and ulcerative tissues.
Of the PCR-positive specimens, six were obtained from patients with other diseases according to the histopathology, one of which was squamous cell carcinoma. Such tumors can develop in a long-standing BUD ulcer (7); either the PCR test result for this patient might be considered a false positive or possibly M. ulcerans DNA persisted in the lesion. Likewise, it cannot be ruled out that M. ulcerans DNA was truly present in the five other patients diagnosed with conditions other than BUD.
For a subset of 21 tissues of culture-positive patients, a second nested PCR was performed on a separate piece of tissue from the original biopsy sample. The results of the PCR were independent of the tissue selected. For these 21 culture- and HPLC-confirmed samples, PCR results were expected to be positive. In both the first and the second nested PCR, several samples tested negative. A false-negative PCR may be caused by the time between collection and testing of the specimen, the absence of bacilli in the specimen tested, or technical limitations (5, 6, 10, 19). Taken together, the first and second nested PCRs detected 100% of samples from the 21 culture-confirmed patients. A second nested PCR on a separate piece of tissue from the original biopsy sample was found to increase the diagnostic capability. Therefore, tissue samples of 0.1 g seem to be sufficient for PCR evaluation. Future studies are needed to explore the influence of the sampling site on the detection rate as well as the utility of PCR in identifying optimal resection margins in the surgical management of patients suffering from Buruli ulcer.
Acknowledgments
This research was supported in part by a grant from the Centers for Disease Control and Prevention (HHS U50/CCU416560), a grant from the NWO (Dutch Organization for Scientific Research), from the foundation “De Drie Lichten” in The Netherlands, and from the Jan Kornelis Cock foundation.
We thank C. K. Ishikawa, S. L. Kihlstrom, and K. M. Dobos at Emory University for their assistance. We gratefully acknowledge Ablordey and D. Ofori-Adjei at Noguchi Memorial Institute, Accra, Ghana; S. Etuaful at St. Martin's Catholic Hospital, Agroyesum, Ghana; E. Klutse at Dunkwa Government Hospital, Upper Denkyira District, Ghana; E. Quarshie at Presbyterian Hospital, Agogo, Ghana; and G. Amofah at the Ministry of Health, Republic of Ghana, Accra, Ghana, for their assistance with data and specimen collection.
REFERENCES
- 1.Amofah, G., F. Bonsu, C. Tetteh, J. Okrah, K. Asamoa, K. Asiedu, and J. Addy. 2002. Buruli ulcer in Ghana: results of a national case search. Emerg. Infect. Dis. 8:167-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arora, S. K., B. Kumar, and S. Sehgal. 2000. Development of a polymerase chain reaction dot-blotting system for detecting cutaneous tuberculosis. Br. J. Dermatol. 142:72-76. [DOI] [PubMed] [Google Scholar]
- 3.Asiedu, K., M. Raviglione, and R. Sherpbier (ed.). 1998. Report: International Conference on Buruli Ulcer Control and Research, Yamoussoukro, Cote d'Ivoire, 6 to 8 July 1998. Document WHO/TB/98.252. World Health Organization, Geneva, Switzerland. [Online.]
- 4.Asiedu, K., R. W. Scherpbier, and M. Raviglione. 2000. Buruli ulcer—Mycobacterium ulcerans infection. World Health Organization, Geneva, Switzerland.
- 5.Burkardt, H. J. 2000. Standardization and quality control of PCR analyses. Clin. Chem. Lab. Med. 38:87-91. [DOI] [PubMed] [Google Scholar]
- 6.de Wit, M. Y., W. R. Faber, S. R. Krieg, J. T. Douglas, S. B. Lucas, N. Montreewasuwat, S. R. Pattyn, R. Hussain, J. M. Ponnighaus, and R. A. Hartskeerl. 1991. Application of a polymerase chain reaction for the detection of Mycobacterium leprae in skin tissues. J. Clin. Microbiol. 29:906-910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans, M. R., S. N. Etuaful, G. Amofah, O. Adjei, S. Lucas, and M. H. Wansbrough-Jones. 1999. Squamous cell carcinoma secondary to Buruli ulcer. Trans. R. Soc. Trop. Med. Hyg. 93:63-64. [DOI] [PubMed] [Google Scholar]
- 8.Guimaraes-Peres, A., F. Portaels, P. de Rijk, K. Fissette, S. R. Pattyn, J. van Vooren, and P. Fonteyne. 1999. Comparison of two PCRs for detection of Mycobacterium ulcerans. J. Clin. Microbiol. 37:206-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.King, C. H., D. A. Ashford, K. M. Dobos, E. A. Spotts Whitney, P. L. Raghunathan, J. Guarner, and J. W. Tappero. 2001. Mycobacterium ulcerans infection and Buruli ulcer disease: emergence of a public health dilemma, p. 137-152. In W. M. Scheld, W. A. Craig, and J. M. Hughes (ed.), Emerging infections 5. ASM Press, Washington, D.C.
- 10.Li, N., A. Bajoghli, A. Kubba, and J. Bhawan. 1999. Identification of mycobacterial DNA in cutaneous lesions of sarcoidosis. J. Cutan. Pathol. 26:271-278. [DOI] [PubMed] [Google Scholar]
- 11.Portaels, F., J. Aguiar, K. Fissette, P. A. Fonteyne, H. De Beenhouwer, P. de Rijk, A. Guedenon, R. Lemans, C. Steunou, C. Zinsou, J. M. Dumonceau, and W. M. Meyers. 1997. Direct detection and identification of Mycobacterium ulcerans in clinical specimens by PCR and oligonucleotide-specific capture plate hybridization. J. Clin. Microbiol. 35:1097-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts, B., and R. Hirst. 1997. Immunomagnetic separation and PCR for detection of Mycobacterium ulcerans. J. Clin. Microbiol. 35:2709-2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ross, B. C., P. D. Johnson, F. Oppedisano, L. Marino, A. Sievers, T. Stinear, J. A. Hayman, M. G. Veitch, and R. M. Robins-Browne. 1997. Detection of Mycobacterium ulcerans in environmental samples during an outbreak of ulcerative disease. Appl. Environ. Microbiol. 63:4135-4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ross, B. C., L. Marino, F. Oppedisano, R. Edwards, R. M. Robins-Browne, and P. D. Johnson. 1997. Development of a PCR assay for rapid diagnosis of Mycobacterium ulcerans infection. J. Clin. Microbiol. 35:1696-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saxegaard, F. 1985. Isolation of Mycobacterium paratuberculosis from intestinal mucosa and mesenteric lymph nodes of goats by use of selective Dubos medium. J. Clin. Microbiol. 22:312-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stienstra, Y., W. T. van der Graaf, G. J. te Meerman, T. H. The, L. F. de Leij, and T. S. Van Der Werf. 2001. Susceptibility to development of Mycobacterium ulcerans disease: review of possible risk factors. Trop. Med. Int. Health 6:554-562. [DOI] [PubMed] [Google Scholar]
- 17.Stinear, T., J. K. Davies, G. A. Jenkin, J. A. Hayman, F. Oppedisano, and P. D. Johnson. 2000. Identification of Mycobacterium ulcerans in the environment from regions in Southeast Australia in which it is endemic with sequence capture-PCR. Appl. Environ. Microbiol. 66:3206-3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Der Werf, T. S., W. T. van der Graaf, J. W. Tappero, and K. Asiedu. 1999. Mycobacterium ulcerans infection. Lancet 354:1013-1018. [DOI] [PubMed] [Google Scholar]
- 19.Vaneechoutte, M., and J. Van Eldere. 1997. The possibilities and limitations of nucleic acid amplification technology in diagnostic microbiology. J. Med. Microbiol. 46:188-194. [DOI] [PubMed] [Google Scholar]