Abstract
OBJECTIVE
We compared the reproducibility and accuracy of conventional clinical examination of the diabetic foot to monofilament examination. We also sought to simplify the monofilament examination by reducing it to fewer touch points.
METHODS
In a cross-sectional study at 10 centers in the United States, Canada, and Switzerland, general internists and residents performed a structured history and physical examination for neuropathy on the feet of diabetic patients. Independent examination by two observers included monofilament sensation, pinprick, vibration, position sense, and ankle reflexes.
MAIN RESULTS
A total of 304 patients were examined by at least one practitioner, and 200 received duplicate examinations. Monofilament examination and ankle reflexes had the best reproducibility, with moderate agreement (κ=0.59); pinprick, position, and vibration sense had fair agreement (κ=0.28–0.36). No component of the history or physical examination, singly or in aggregate, was both sensitive and specific for identifying a patient with an abnormal monofilament examination. A simplified monofilament examination using only 4 sites per foot (total 8 sites) detected 90% of patients with an abnormal 16-site monofilament evaluation.
CONCLUSIONS
Conventional clinical examination had low reproducibility and correlated poorly with monofilament examination for the identification of the at-risk patient. The Semmes-Weinstein monofilament examination, a reproducible, valid, and generalizable test of foot sensation, is recommended as the screening procedure of choice for examining diabetic feet.
Keywords: diabetes, foot ulcer, diabetic neuropathy, clinical examination, screening
Ascreening test for the identification of diabetic patients at risk of neuropathic foot ulcers and amputation is highly desirable. More than 50,000 amputations occur yearly in the United States in diabetic patients, and peripheral neuropathy plays a role in up to 80% of these.1–4 Approximately 25% of all diabetic patients eventually develop foot complications, and 1% per year undergo amputation.5 Intensive foot care including foot education, appropriate shoes, and foot clinics, along with optimal treatment of ulcers, infection, and peripheral vascular disease, may prevent up to 90% of these amputations.1,6,7 In the absence of the resources to apply all of these preventive measures to every diabetic patient, simple screening methods that identify patients at risk of ulceration and subsequent amputation would direct appropriate preventive procedures to those most likely to benefit.
The optimal screening test is simple and quick to perform, yields the same results when carried out by different observers (high interobserver reproducibility), accurately measures or predicts a clinically important condition (high validity against an independent and clinically meaningful criterion reference standard), and can be used by various examiners when assessing a wide range of patients (high generalizabililty).8,9 Furthermore, screening should lead to improved clinical outcomes.
Although no test has yet been demonstrated to fulfill all of these criteria, examination of light touch perception with the Semmes-Weinstein 5.07 monofilament is the most promising. Examination with the monofilament is inexpensive and simple to perform. It was superior at distinguishing patients with and without foot ulceration when compared with other clinical examination maneuvers, such as temperature perception, vibration perception by biothesiometer, or ankle reflexes (cross-sectional validity).10–15 It was also a powerful predictor of ulceration and amputation (predictive validity).16,17 In a prospective community-based study, abnormal monofilament sensation at any of 8 plantar sites on either foot was associated with a relative risk (RR) of 15 for the development of foot ulcers.16,17 A stronger but less common predictor was a history of previous ulceration or amputation (RR of 72), and the combination of monofilament examination and history of foot complications yielded a sensitivity of 85% and specificity of 82%. Moreover, a randomized trial of diabetic foot screening (monofilament status, vibration sensation by biothesiometer, and palpation of pedal pulses), followed by intervention in identified high-risk patients, reduced subsequent ulceration and amputation.18
On the basis of these data, many organizations, including the United States Public Health Service and the American Diabetes Association, recommend an annual monofilament examination as a screen of diabetic patients for neuropathic feet at risk of ulcers and amputation.7,19,20
The monofilament examination may be reproducible, but studies of precision are limited by small sample size, constrained spectrum of disease (e.g., exclusively young patients, reexamining only those subjects with abnormal findings), or having the assessments completed by a limited number of trained examiners.5,21–24 We therefore designed this study to determine the interrater reproducibility of the monofilament foot examination in diabetic patients at risk of foot complications, and to determine the reproducibility and validity of history and standardized neurologic assessment of the feet. We also sought to identify those sites of the 8-point-per-foot monofilament examination that were the best predictors of abnormal monofilament sensation status.
METHODS
Participating Centers
Ten centers from across the United States, Canada, and Switzerland participated in the study. Investigators were all members of the Clinical Examination Interest Group of the Society of General Internal Medicine. The majority of examiners were university-affiliated general internists, although the second examiner for each patient was often an internal medicine resident or physician's assistant.
Study Population
Diabetic patients attending outpatient clinics or general internal medicine inpatients who were aged 18 years or older were asked to participate in the study. Signed consent was obtained. Exclusion criteria were any cognitive impairment of the patient by history or during explanation of consent procedure; previous history of stroke affecting the legs; or history or physical examination revealing an active foot ulcer, foot infection, amputation, foot ischemia (gangrene or pregangrene), or peripheral edema.
Clinical Examination
Patients sat on an examining table or reclined in a hospital bed. Two clinicians examined and interviewed each patient separately, with only one examiner in the room at a time to ensure blinding. Both examinations were performed on the same day.
Pinprick sensation was tested with a sterile or unused safety pin over the plantar aspect of the distal first, third, and fifth toe of each foot with the stimulus applied once per site. Patients were asked to identify when they felt a sensation, and whether it was sharp or dull. Findings were scored as sharp, dull, or absent for each site.
Position sense was assessed at the interphalangeal joint of each great toe for a 10° change. The toe was held at both sides with one hand while using the other hand to move the distal phalanx up or down. The fingers were positioned at the medial and lateral aspects of the toe. After demonstrating “this is up, this is down” while moving the toe to that position, three trials for each foot were performed. The patient was asked to choose “up or down?” and the three responses per foot were scored as correct or incorrect.
Vibration sensation was tested with a 128 or 256 Hz tuning fork over the distal big toe and medial malleolus. Examiners were instructed to strike the fork gently against the palm so that vibration could be felt for 10 to 15 seconds. Examiners demonstrated the sensation of vibration on the patient's sternum or patella prior to examining the feet. Patients were asked initially, and after 5 seconds, whether they perceived vibration. Patients who felt vibration both initially and after 5 seconds were scored as normal. Vibration not perceived at all was scored as absent, and vibration perceived initially but not at 5 seconds was scored as abnormal.
Ankle reflexes were obtained at both ankles. With the patient sitting or lying, the examiner was instructed to gently dorsiflex the foot and strike the Achilles tendon briskly with the reflex hammer. If no reflex was obtained, the attempt was repeated with reinforcement, by having patients pull their fingers apart just before the reflex was elicited, to verify the absence of reflex. The reflex was scored as 0 (absent with reinforcement), 1 (present, decreased), 2 (normal), 3 (increased), or 4 (greatly increased, with clonus).
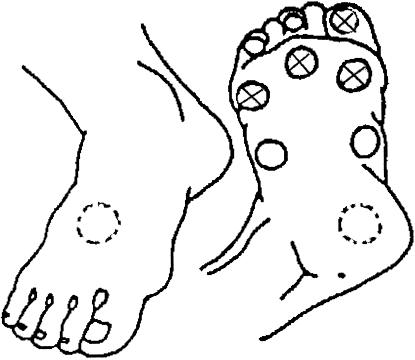
Monofilament sensation was tested with the Semmes-Weinstein 5.07 monofilament at 10 touch sites per foot, as previously described.19 Nine plantar sites (distal great toe, third toe, and fifth toe; first, third, and fifth metatarsal heads; medial foot, lateral foot, and heel) and one dorsal site were tested (Fig. 1). The monofilament was applied until it buckled, and held for 1 second. Examiners demonstrated the monofilament sensation on one of the patient's hands. A two-choice forced algorithm was used: each site was touched once during one of two time periods, while counting “one, two.” Patients were asked to state the time interval (1 or 2) during which the stimulus was felt, or to state that they could not tell. The examiner recorded the individual test site results (correct or incorrect) on a diagram of the two feet, and recorded the total correct score for each foot. For each foot, if a single site was incorrect, then that site was tested two more times. If both additional tests were correct, then the site was recorded as correct. Otherwise, the site was recorded as incorrect.
FIGURE 1.
Ten touch points (9 plantar and 1 dorsal), and the model 1 “a priori” abridged 4-point monofilament examination.
Abnormal monofilament examination was defined as incorrect stimulus identification at any of 8 plantar sites on either foot, with the heel and dorsum of the foot excluded. This definition was chosen because it is thought that calluses on the heel lead to a large number of false- positive examinations for neuropathy, and this was the definition used in the prospective validation study.17
Each examiner recorded the history after the physical examination. Items recorded for all patients included the patient's age, type and duration of diabetes, use of insulin, history of foot infection, ulcer, hospitalization for foot complications, and history in the last week of numbness, burning, pain, falling asleep sensation, or pins and needles in the feet.
Statistical Analysis
Reproducibility of physical examination components was measured by the κ statistic, or “agreement beyond chance,” with 95% confidence intervals (CIs). The κ statistic was interpreted as previously described, with κ of 0.20 to 0.39 representing fair agreement, and 0.40 to 0.59 representing moderate agreement.25 All test results were dichotomized as completely normal versus presence of any abnormality. For analyses comparing the traditional neurologic examination to the monofilament, the monofilament was considered the reference standard because of its superior ability to predict future foot ulcers, as described above. For these analyses, only the first examiner's data were used. Sensitivity, specificity, and likelihood ratio (LR) were calculated for components of the history and physical examination.
The sensitivity of a 4-site-per-foot monofilament examination, chosen a priori, was compared with the full 8-site-per-foot examination. The 4 chosen sites (great toe and first, third, and fifth metatarsal heads) are common sites of foot ulceration, and were predictive of monofilament status in a pilot study. Study physicians were unaware of the hypothesis being tested.
In addition, we used logistic regression techniques to find the most sensitive shortened monofilament examination. The outcome variable was a positive 16-point monofilament examination (8 points on each foot), defined as insensation at any of the sites. Sites were added in matched pairs to the model (i.e., the left and right great toe were added simultaneously) in a stepwise fashion. The model was complete when addition of any further pair of sites did not make a statistically significant improvement in the variance explained by the model (and therefore the sensitivity of the examination). The model was created using the first 175 patients and validated using the subsequent 129 patients. To evaluate the effect of clustering of examination findings within providers or sites or both, generalized estimating equation analysis was performed using examiner and the site as a covariate.
RESULTS
Ten university-affiliated centers in the United States, Canada, and Switzerland recruited a total of 304 patients. Duplicate examinations were performed in 200 patients. Patients had a median age of 63 years (range, 18–89 years), and a median duration of diabetes of 8 years (85% type 2;Table 1). Twelve per cent of patients had a prior foot infection, 6% had prior foot ulcer, and 3% had been hospitalized for foot infection. More than half (57%), or 172 of the 304 patients were found to be insensate at one or more sites of the 16-site monofilament examination (8 sites per foot). Patients without a duplicate examination were similar to those with a duplicate examination in all demographic characteristics.
Table 1.
Demographic and Clinical Characteristics of the Patients Enrolled in the Study
| Characteristic | |
|---|---|
| Demographics | |
| Median age (range), years | 63 (18–89) |
| Sex, % | |
| Male | 71 |
| Female | 29 |
| Race, % | |
| White | 80 |
| Hispanic | 8 |
| African American | 7 |
| Other | 5 |
| Diabetes characteristics | |
| Median duration (range), years | 8(2 weeks–63 years) |
| Medication, % | |
| Taking insulin | 49 |
| Oral agents only or no medication | 51 |
| Foot disease characteristics, % | |
| History of foot infection | 12 |
| History of foot ulcer | 6 |
Reproducibility between examiners ranged from κ statistics of 0.28 to 0.59 for the various examination techniques (Table 2). The reproducibility of the monofilament examination (normal vs any insensate site) was moderate with κ of 0.59 (95% CI 0.48, 0.71). Ankle reflexes also had moderate agreement (κ 0.59; 95% CI 0.47, 0.71), whereas pinprick, position sense, and vibration all had fair interobserver agreement (κ 0.28–0.36).
Table 2.
Interobserver Reproducibility (κ) of Physical Examination Components (Agreement Beyond Chance, κ) and 95% Confidence Intervals for 200 Patients Examined Independently by Two Observers
| Finding | Reproducibility | 95% Confidence Interval |
|---|---|---|
| Monofilament | 0.59 | 0.48, 0.71 |
| Ankle reflex | 0.59 | 0.47, 0.71 |
| Pinprick | 0.36 | 0.21, 0.51 |
| Position | 0.28 | 0.09, 0.48 |
| Vibration | 0.31 | 0.18, 0.45 |
Agreement beyond chance was measured at individual touch sites for the monofilament examination (Table 3). The 6 distal plantar sites (first, third, and fifth toes and their metatarsal heads) had moderate reproducibility (κ 0.38–0.54), whereas the arches, heel, and dorsum had fair reproducibility (κ 0.22–0.38).
Table 3.
Interobserver Reproducibility (Agreement Beyond Chance, κ) of Individual Touch Sites for Semmes-Weinstein Monofilament Examination of the Feet
| Site | Right Foot | Left Foot |
|---|---|---|
| Big toe | 0.54 | 0.50 |
| Third toe | 0.44 | 0.42 |
| Fifth toe | 0.40 | 0.38 |
| First metatarsal | 0.48 | 0.44 |
| Third metatarsal | 0.46 | 0.52 |
| Fifth metatarsal | 0.49 | 0.44 |
| Medial arch | 0.25 | 0.34 |
| Lateral arch | 0.31 | 0.26 |
| Heel | 0.22 | 0.35 |
| Dorsum | 0.29 | 0.38 |
The patient's subjective sensations lacked sensitivity for detecting abnormal monofilament status (Table 4). Individually, burning, numbness, pain, pins and needles, or the sensation of the foot falling asleep had low sensitivity (26%–49%) and moderate specificity (79%–85%). An aggregate of all five questions increased sensitivity to only 65%, with a specificity of 62%.
Table 4.
Diagnostic Test Properties of Clinical Findings for the Presence of Abnormal Monofilament Examination Status*
| Finding | TP | FP | FN | TN | Accuracy | Sensitivity | Specificity | LRPositive | LRNegative |
|---|---|---|---|---|---|---|---|---|---|
| Subjective sensations | |||||||||
| Burning | 37 | 17 | 131 | 113 | 0.50 | 0.22 | 0.87 | 1.68 | 0.90 |
| Numbness | 83 | 27 | 85 | 103 | 0.62 | 0.49 | 0.79 | 2.38 | 0.64 |
| Pain | 43 | 20 | 125 | 109 | 0.51 | 0.26 | 0.84 | 1.65 | 0.88 |
| Pins and needles | 54 | 20 | 114 | 110 | 0.55 | 0.32 | 0.85 | 2.09 | 0.80 |
| Foot asleep | 43 | 19 | 125 | 111 | 0.52 | 0.26 | 0.85 | 1.75 | 0.87 |
| Any of above 5 | 111 | 49 | 61 | 81 | 0.64 | 0.65 | 0.62 | 1.71 | 0.57 |
| Age >65 years, diabetes mellitus >10 years, numbness, or male | 145 | 98 | 27 | 32 | 0.59 | 0.84 | 0.25 | 1.12 | 0.64 |
| Neurologic examination | |||||||||
| Ankle reflex | 96 | 36 | 76 | 95 | 0.63 | 0.56 | 0.73 | 2.03 | 0.61 |
| Pinprick | 142 | 77 | 29 | 52 | 0.65 | 0.83 | 0.40 | 1.39 | 0.42 |
| Position | 34 | 2 | 135 | 126 | 0.54 | 0.20 | 0.98 | 12.9 | 0.81 |
| Vibration | 87 | 32 | 85 | 99 | 0.61 | 0.51 | 0.76 | 2.07 | 0.65 |
| Any of above 4 | 160 | 92 | 12 | 38 | 0.66 | 0.93 | 0.29 | 1.31 | 0.24 |
Abnormal monofilament status was defined as incorrect stimulus identification at any of 8 plantar sites on either foot. TP indicates true positive; FP, false positive; FN, false negative; TN, true negative; LR, likelihood ratios for positive and negative test results.
The patient's subjective sensations along with other components of the history were studied in multivariate analyses. The independent predictors of abnormal monofilament status were age (dichotomized at 65 years), duration of diabetes (dichotomized at 10 years), subjective numbness, and male gender. A model based on the presence of any of these four characteristics increased sensitivity for detection of abnormal monofilament examination to 84%, but decreased specificity to 25%.
The diagnostic test properties of physical examination components were studied individually and in aggregate (see Table 4). Abnormal position sense was highly specific for abnormal monofilament status (98%, LR positive = 12.9), but a normal finding had low sensitivity (20%, LR negative = 0.81). A combination of all four tests (ankle reflex, pinprick, position, and vibration sense) was associated with high sensitivity (93%) but low specificity (29%) when normality was defined as normal results on all four items.
To develop a shortened monofilament examination, an a priori model (model 1) and a logistic regression model (model 2) were examined. Model 1 (consisting of the great toe and base of the first, third, and fifth metatarsals, and chosen on the basis of the literature and the sites of high frequency of ulceration) detected 154 (90%) of 172 patients with an abnormal 16-site monofilament examination, with an overall accuracy of 94%. Model 2 was developed by stepwise logistic regression in the first 175 patients (89 of whom were insensate at one site or more) and validated in the next 129 patients (83 of whom had abnormal monofilament examination). In model 2, only the 8 touch sites used by Rith-Najarian in his prospective validation study were used.17 Four points per foot, consisting of the third and fifth toes and the first and third metatarsal heads, identified 83 (93%) of 89 patients with an abnormal monofilament examination in the derivation set and 79 (95%) of 83 patients in the validation set. No further touches added statistically significant information. When examiner or site or both were added to the model, only the first and third metatarsal heads offered statistically significant improvement about global insensation.
The conventional neurologic examination, including pinprick, vibration, position sense, and ankle reflexes, took an average of 233 seconds, whereas the 10-point-per-foot monofilament examination took 165 seconds (p < .001). The 4-point-per-foot monofilament examination took 39 seconds.
DISCUSSION
The Semmes-Weinstein 5.07 monofilament has been recommended as the diagnostic test of choice for the detection of diabetic patients with feet at risk of ulcers and amputation.7,20 We have shown that the monofilament examination is reproducible, generalizable across a spectrum of patients and examiners, and practical. More than 30 general internists, medical residents, and physicians' assistants from 10 centers in three countries participated. In this study and a previous study,26 most examiners had little or no previous experience with the monofilament examination, yet achieved good reproducibility. This suggests that primary care providers need only limited written instruction to use the monofilament. Patients encompassed a wide spectrum of ages and duration and severity of diabetes, and had neither current ulcers nor a previous amputation. We thus identified those patients in whom we would want to apply a screening test for assessing risk of foot complications.
An effective screening test requires high sensitivity and acceptable specificity. A negative test should rule out disease. A better summary measure may be the LR, which incorporates both the sensitivity and specificity. The LR indicates the degree by which a particular test result increases or decreases the probability of having a target disorder. For a screening test, the LR for a negative result should approach 0, such that the target disorder is ruled out. The clinical history was not sufficiently sensitive to rule out abnormal monofilament examination status, nor was any individual component of the physical examination. A predictive rule consisting of any abnormality in the four components of the physical examination (ankle reflexes, pinprick, vibration, and position sense) had good sensitivity (93%), and relatively low LR for a negative test result (0.24), for identifying abnormal monofilament examination in patients. A similar multicomponent screening examination was detailed in guidelines from the Centers for Disease Control and Prevention.27 However, with a specificity of only 29%, and a LR positive of 1.31, a large number of patients will be incorrectly identified as high risk.
The optimal screening test should also be practical. The typical monofilament examination took over 2 minutes, which is an improvement over the multicomponent conventional examination, but still may be too long for the average general internist encounter. We investigated whether an abridged examination would provide similar information. Our a priori model consisting of 4 sites per foot (great toe and first, third, and fifth metatarsal heads) identified 90% of patients with an abnormal monofilament examination and performed as well as the 94% sensitivity of the model derived by logistic regression. Both the a priori and the logistic regression models used 4 sites per foot, and no further sites were independent predictors of abnormal monofilament status. The a priori sites may be more clinically relevant, as they were chosen to represent areas where ulceration is frequent. Further investigation after the analysis including examiner and site revealed that all examinations which had four touches per foot, all on the toes and metatarsal heads, including the first and third metatarsal heads, had 90% to 93% sensitivity in both the derivation and validation sets of patients. We therefore recommend that the busy primary care provider use an abbreviated examination with four touches on each foot, including the first and third metatarsal heads and two other toes or metatarsal heads. The 4-site-per-foot examination required less than 1 minute to complete. Foot care providers with the time to perform a more complete examination may reasonably choose to do so.
We acknowledge limitations to our study. First, although the examiners were blinded to one another's results, the monofilament assessment was performed after the clinical examination, and thus was not blinded to results of neurologic testing. However, any expectation bias introduced by this order of examination would have been minimized by the monofilament testing procedure. The monofilament test relies on the patient's response to a fixed force that induces buckling of the monofilament device. This makes it distinctly different from vibration, pinprick, and reflex pinprick testing, in which the observer applies a stimulus that is not consistent. The observer could introduce bias in the decision about where to place the monofilament, but this is also standardized, and bias is minimized through application at multiple sites. If expectation bias were present, it would be likely to increase the correlation of monofilament testing with neurologic examination, but high correlation was not found.
Second, our study was limited to the clinical examination for neuropathic signs and symptoms. We did not assess foot deformity or peripheral vascular disease, which may predict foot complications independently of monofilament status. Foot deformity assessment, however, is not highly reproducible,27 and the additional prognostic information may be limited.17 The predictive value of clinical examination for peripheral vascular disease requires study. In a study of risk stratification and intervention, in which high-risk individuals were identified by a screening test of foot sensation (monofilament examination and biothesiometer) and palpation of pedal pulses, reduction in foot ulcers and amputations was demonstrated.18
Though we realize the Semmes-Weinstein 5.07 monofilament is not yet in wide use as a tool for assessing the risk of foot complications, it is emerging as the best tool available for that purpose. Primary care providers performed the test well with simple written instructions; thus, emphasis should be on increasing awareness and availability of the monofilament. This consciousness can be raised by standard approaches to guidelines enforcement, such as sending content experts into the field. A current barrier is the lack of a recognized diagnostic or procedure code for monofilament examination (or, indeed, any kind of foot evaluation in patients with diabetes). Adoption of such a code would allow documentation of and conceivably even billing for foot screening, which would promote its performance.28
We recommend screening of all diabetic patients by monofilament testing of the feet and eliciting a history of foot ulcers and amputation. We suggest that no other neurologic tests be done for the purposes of screening. All patients with diabetes should be offered basic foot care education; regular monofilament examinations may facilitate identification of those patients who should receive more intense education and closer follow-up, or who might benefit from referral to specialized foot care clinics.
REFLECTIONS
What to Call Me
Call me doc and I'm an old codger
at a country store, a well-chewed cigar
in the corner of my mouth,
ready to declare the wonder of Epsom salt.
Call me doctor and I will itemize
your benefits, risks, and alternatives,
distant and calm
as reading a timetable.
Call me Doctor Berlin and I will stretch
across the fissure of detachment
and approach you like a father
reaching for his grown child.
Call me Richard and the mirrors
in my eyes will vanish
fast as alcohol from skin,
but you may not know the man you see.
Or sing childhood names
I forgot when I swore the oath.
For there are days I can't remember
what to call myself, in whose name I heal.
Richard M. Berlin, MD
Richmond, Mass.
FINALIST, 1999 Creative Medical Writing Contest
Acknowledgments
Dr. Edelman is supported by a VA Health Services Research Career Development Award.
The International Cooperative Group for Clinical Examination Research included the following site coordinators and participating centers: Durham Veterans Affairs Center, Durham, NC (David Edelman, David Simel); Madigan Army Medical Center, Tacoma, Wash (Jeff Jackson); Mayo Clinic, Jacksonville, Fla (Mark Parkulo); McMaster University, Hamilton, Ont, Canada (Marek Smieja, Dereck Hunt, Jim Nishikawa, Herzel Gerstein, Rose Hatala); Minneapolis Veterans Affairs Hospital, Minneapolis, Minn (Craig Roth); San Antonio Veterans Affairs Hospital, San Antonio, Tex (John Williams); Universite de Lausanne, Switzerland (Jacques Cornuz, Maria Gueorguiev); University of Kentucky, Lexington, Ky (Don Holleman); University of Texas, San Antonio, Tex (Pat Wathen, Bob Badgett); University of Texas Southwestern, Dallas, Tex (Jim Wagner); University of Toronto, Toronto, Ont, Canada (Ed Etchells); and University of Wisconsin, Madison, Wis (Mae Hla).
REFERENCES
- 1.Bild DE, Selby JV, Sinnock P, Browner WS, Braveman P, Showstack JA. Lower-extremity amputation in people with diabetes: epidemiology and prevention. Diabetes Care. 1989;12:24–31. doi: 10.2337/diacare.12.1.24. [DOI] [PubMed] [Google Scholar]
- 2.Boulton AJM. The diabetic foot: neuropathic in aetiology? Diabetic Med. 1990;7:852–8. doi: 10.1111/j.1464-5491.1990.tb01318.x. [DOI] [PubMed] [Google Scholar]
- 3.Harati Y. Diabetes and the nervous system. Endocrinology and Metab Clin of North Am. 1996;25:325–59. doi: 10.1016/s0889-8529(05)70327-3. [DOI] [PubMed] [Google Scholar]
- 4.Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation: basis for prevention. Diabetes Care. 1990;131:513–21. doi: 10.2337/diacare.13.5.513. [DOI] [PubMed] [Google Scholar]
- 5.Nelson RG, Gohdes DM, Everhart JE, Hartner JA, et al. Lower-extremity amputations in NIDDM: 12-yr follow-up study in Pima Indians. Diabetes Care. 1988;11:8–16. doi: 10.2337/diacare.11.1.8. [DOI] [PubMed] [Google Scholar]
- 6.Borssen B, Bergenheim T, Lithner F. Preventive treatment of foot deformities in type I diabetic patients aged 15–50 years—an epidemiological and prospective study. J Intern Med. 1996;240:219–25. doi: 10.1046/j.1365-2796.1996.37866000.x. [DOI] [PubMed] [Google Scholar]
- 7.Caputo GM, Cavanagh PR, Ulbrecht JS, Gibbons GW, Karchmer AW. Assessment and management of foot disease in patients with diabetes. N Engl J Med. 1994;331:854–60. doi: 10.1056/NEJM199409293311307. [DOI] [PubMed] [Google Scholar]
- 8.Jaeschke R, Guyatt G Sackett DL for the Evidence-Based Medicine Working Group. Users' guides to the medical literature, III: how to use an article about a diagnostic test, A: are the results of the study valid? JAMA. 1994;271:389–91. doi: 10.1001/jama.271.5.389. [DOI] [PubMed] [Google Scholar]
- 9.Jaeschke R, Guyatt G Sackett DL for the Evidence-Based Medicine Working Group. Users' guides to the medical literature, III: how to use an article about a diagnostic test, B: what are the results and will they help me in caring for my patients? JAMA. 1994;271:703–7. doi: 10.1001/jama.271.9.703. [DOI] [PubMed] [Google Scholar]
- 10.Birke JA, Sims DS. Plantar sensory threshold in the ulcerative foot. Lepr Rev. 1986;57:261–7. doi: 10.5935/0305-7518.19860028. [DOI] [PubMed] [Google Scholar]
- 11. Boyko EJ, Smith DG, Ahroni JH. A prospective study of risk factors for diabetic foot ulcer. Rehab R&D Prog Report. 1994;318–9. [DOI] [PubMed]
- 12.Holewski JJ, Stess RM, Graf PM, Grunfeld C. Aesthesiometry: quantification of cutaneous pressure sensation in diabetic peripheral neuropathy. J Rehab Res Dev. 1988;25:1–10. [PubMed] [Google Scholar]
- 13.McNeely MJ, Boyko EJ, Ahroni JH, et al. The independent contributions of diabetic neuropathy and vasculopathy in foot ulceration: how great are the risks? Diabetes Care. 1995;18:216–9. doi: 10.2337/diacare.18.2.216. [DOI] [PubMed] [Google Scholar]
- 14.Olmos PR, Cataland S, O'dorisio TM, Casey CA, Smead WL, Simon SR. The Semmes-Weinstein monofilament as a potential predictor of foot ulceration in patients with noninsulin-dependent diabetes. Am J Med Sci. 1995;309:76–82. doi: 10.1097/00000441-199502000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Sosenko JM, Kato M, Soto R, Bild DE. Comparison of quantitative sensory-threshold measures for their association with foot ulceration in diabetic patients. Diabetes Care. 1990;13:1057–61. doi: 10.2337/diacare.13.10.1057. [DOI] [PubMed] [Google Scholar]
- 16.Gohdes D, Rith-Najarian S. Foot disease in diabetes. N Engl J Med. 1995;332:269–70. Letter. [PubMed] [Google Scholar]
- 17.Rith-Najarian SJ, Stolusky T, Gohdes DM. Identifying diabetic patients at high risk for lower-extremity amputation in a primary health care setting: a prospective evaluation of simple screening criteria. Diabetes Care. 1992;15:1386–9. doi: 10.2337/diacare.15.10.1386. [DOI] [PubMed] [Google Scholar]
- 18.McCabe CJ, Stevenson RC, Dolan AM. Evaluation of a diabetic foot screening and protection programme. Diabetic Med. 1998;15:80–4. doi: 10.1002/(SICI)1096-9136(199801)15:1<80::AID-DIA517>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 19.Duffy JC, Patout CA. Management of the insensitive foot in diabetes: lessons learned from Hansen's disease. Military Med. 1990;155:575–9. [PubMed] [Google Scholar]
- 20.Mueller MJ. Identifying patients with diabetes mellitus who are at risk for lower-extremity complications: use of Semmes-Weinstein monofilaments. Phys Ther. 1996;76:68–71. doi: 10.1093/ptj/76.1.68. [DOI] [PubMed] [Google Scholar]
- 21.Diamond JE, Mueller MJ, Delitto A, Sinacore DR. Reliability of a diabetic foot evaluation. Phys Ther. 1989;69:797–802. doi: 10.1093/ptj/69.10.797. [DOI] [PubMed] [Google Scholar]
- 22.Klenerman L, McCabe C, Cogley D, Crerand S, Laing P, White M. Screening for patients at risk of diabetic foot ulceration in a general diabetic outpatient clinic. Diabetic Med. 1996;13:561–3. doi: 10.1002/(SICI)1096-9136(199606)13:6<561::AID-DIA112>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 23.Valk GD, de Sonnaville JJJ, vanHoutum WH, et al. The assessment of diabetic polyneuropathy in daily clinical practice: reproducibility and validity of Semmes Weinstein monofilaments examination and clinical neurological examination. Muscle & Nerve. 1997;20:116–8. doi: 10.1002/(sici)1097-4598(199701)20:1<116::aid-mus19>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 24.Adler AI, Boyko EJ, Ahroni JH, Stensel V, Forsberg RC, Smith DG. Risk factors for diabetic peripheral sensory neuropathy: results of the Seattle Prospective Diabetic Foot Study. Diabetes Care. 1997;20:1162–7. doi: 10.2337/diacare.20.7.1162. [DOI] [PubMed] [Google Scholar]
- 25.Sackett DL. A primer on the precision and accuracy of the clinical examination. JAMA. 1992;267:2638–44. [PubMed] [Google Scholar]
- 26.Edelman D, Sanders L, Pogach LM. Reproducibility and accuracy among primary care providers of a screening examination for foot ulcer risk among diabetic patients. Preventive Med. 1998;27(1):274–8. doi: 10.1006/pmed.1998.0263. [DOI] [PubMed] [Google Scholar]
- 27. Centers for Disease Control and Prevention. The prevention and treatment of complications of diabetes mellitus: a guide for primary care practitioners. January 1991.
- 28.Mayfield JA, Strand T, Toya AR. A call for specific codes for diabetes foot and eye care. Diabetes Care. 1995;18(3):418–21. doi: 10.2337/diacare.18.3.418. [DOI] [PubMed] [Google Scholar]