Abstract
OBJECTIVE
To determine the clinical factors associated with delayed protease inhibitor initiation.
DESIGN
Chart review and telephone survey.
SETTING
General medicine practice at an academic medical center in Boston, Mass.
PATIENTS
One hundred ninety patients living with HIV and a viral load of more than 10,000 copies/ml.
MEASUREMENTS AND MAIN RESULTS
The main outcome measurement was time to first protease inhibitor prescription after first elevated HIV viral load (>10,000 copies/ml). In this cohort, 190 patients had an elevated viral load (median age 39; 87% male; 12% history of injection drug use; 63% AIDS; 53% with depression; 17% history of pneumocystis pneumonia; 54% CD4 <200). In Cox proportional hazards modeling, significant univariate correlates for delayed protease inhibitor initiation were higher CD4 cell count (hazard ratio [HR] 2.38 for CD4 200–500 compared with <200, 95% confidence interval [CI] 1.59, 3.57; and HR 8.33 for CD4> 500; 95% CI 2.63, 25.0), higher viral load (HR 0.43 for each 10-fold increase; 95% CI 0.31, 0.59), injection drug use (HR 2.08; 95% CI 1.05, 4.17), AIDS (HR 0.24; 95% CI 0.15, 0.36), and history of pneumocystis pneumonia (HR 0.32; 95% CI 0.21, 0.49). In multivariate models adjusted for secular trends in protease inhibitor use, factors significantly associated with delay of protease inhibitor initiation (p < .05) were higher CD4 cell count (for CD4 200–500, HR 2.63; 95% CI 1.61, 4.17; for CD4> 500, HR 11.11; 95% CI 3.57, 33.33), higher viral load (HR 0.66 for each 10-fold increase; 95% CI 0.45, 0.98), history of pneumocystis pneumonia (HR 0.57; 95% CI 0.37, 0.90), history of depression (HR 1.49; 95% CI 1.03, 2.13), and history of injection drug use (HR 2.70; 95% CI 1.35, 5.56).
CONCLUSIONS
HIV-infected patients with higher CD4 cell counts or a history of depression or history of injection drug use have significant and lengthy delays of protease inhibitor therapy. Although some delays may be clinically appropriate, enhancement of provider and patient education might prove beneficial. Further research should examine reasons for delays in protease inhibitor initiation and their appropriateness.
Keywords: HIV, protease inhibitors, depression, injection drug use
In clinical studies, suppression of HIV viral load reduces progression to AIDS.1,2 Combination therapy with protease inhibitors can halt viral replication within days, leading rapidly to unmeasurable serum viral load3 and decreased lymphoid viral replication.4 Such findings support early and aggressive treatment of HIV infection. In mid-1996, an international panel of experts recommended beginning combination antiviral therapy for all patients with HIV viral loads greater than 5,000 to 10,000 copies/ml, regardless of CD4 cell count. The panel also recommended therapy for patients with symptomatic HIV disease or CD4 cell counts below 500.5,6 Consistent adherence to therapy prevents the development of resistant virus.7,8
Barriers to rapid use of protease inhibitors in office practice are largely unknown. We examined factors associated with delays in protease inhibitor use for patients with elevated viral loads.
METHODS
Study Population
We conducted detailed chart reviews of all patients with HIV treated at a general internal medicine practice at Beth Israel Deaconess Medical Center. The approximately 30 faculty, 100 house officers, and 10 nurse practitioners at the practice provide more than 50,000 patient visits annually to general medicine patients. Medical records are completely computerized, including all notes, laboratory reports, and medication and problem lists.9 Approximately 30% of patients have Medicaid or receive free care from the hospital when they meet income criteria. Infectious disease specialists consult within the practice.10
In December 1996, we identified all patients with known HIV infection. We found 289 patients whom we considered “active” (defined as having visited the practice within 18 months prior to December 1996 and having no evidence of transfer of care out of the practice). We excluded 14 hospital employees to protect their confidentiality and performed detailed, structured chart reviews on records for the remaining 275 patients. Of these, 190 patients were found to have at least one viral load above 10,000 copies/ml and are the focus of this report. Of the 190 patients with elevated viral load, 129 (68%) completed a separate telephone survey that provided additional demographic data.11 The Beth Israel Deaconess Medical Center Committee on Clinical Investigations approved the consent process and study design.
Data Collection
Chart Review
Using a structured instrument, we abstracted clinical data from computerized medical records for all 275 active patients for a 2-year period (July 1, 1995, through June 30, 1997). One of the investigators (KMF) performed 175 of the chart reviews. The remaining 100 charts were reviewed by two trained physician chart reviewers, and 10% of these charts were also reviewed by a study investigator (KMF). In addition, all dates of protease inhibitor initiation and viral loads were confirmed for all patients by a study investigator (KMF). An error rate of less than 2% was observed. The reviews included demographic information (age, sex, HIV risk factors, and health insurance), CD4 cell counts, viral load measurements with dates, HIV-related complications, hospital admissions, primary care visits and visits to infectious disease clinic with dates, and names of all prescription medications. We noted start and stop dates for protease inhibitors, as well as patient refusals of protease inhibitors. We also collected history of anxiety, depression, and injection drug use. History of depression or anxiety was recorded as being present if it appeared in either the problem list or progress notes during the study period. Injection drug use was recorded when reported in either the problem list or progress notes at any time in the past. Active injection drug use was identified when either relapse or discussion of active use was present in the problem list or progress notes during the 2-year study period.
Information about clinicians included level of training (faculty vs resident) and number of HIV patients at the start of the study period. We viewed physicians as low-volume HIV providers if they had fewer than four patients with HIV in their practice during the 2-year study period. We chose this cutoff because the distribution of patient volume for our providers during this period was such that providers generally cared for few (e.g., one to three) or many more patients with HIV.
Telephone Survey
We surveyed all patients who agreed to telephone interviews between April 22 and June 30, 1997. Prior to beginning, patients gave verbal informed consent to be interviewed. The mean interview length was 22 minutes. The interview queried patients about demographic characteristics (education, self-described race, current employment status, and income) and details about use of complementary or alternative therapies.11
Statistical Analyses
We used descriptive statistics (SAS statistical software, SAS Institute, Cary, NC) to characterize our study population. Our outcome of interest in the remaining analyses was time (in days) to protease inhibitor initiation following first detection of an elevated viral load (>10,000 copies/ml). Patients who did not receive protease inhibitors during the study period contributed person-time until they were censored at the end of follow-up. The CD4 cell counts used were those measured most recently before detecting the first elevated viral load. Categorical terms for CD4 cell counts best fit the data based on strength of the parameter estimates and because of the nonlinear relation between the logarithm of the hazard and CD4 cell count. Because of a concern that protease inhibitor therapy might be delayed pending consultation with infectious disease specialists, we included a term for this in our models. Infectious disease consultation was modeled as a time-varying covariate, allowing its value to change during calendar time of the study. A variable representing history of injection drug use at any time was used in modeling (active use was rare and not modeled). We used a base-10 logarithmic transformation of viral load based on the distribution of this variable and to improve interpretability.
Using univariate Cox proportional hazards modeling, we identified significant predictors of time to protease inhibitor initiation. We selected the best multivariate model by fitting a Cox model using forward stepwise selection, eliminating previously selected variables with the smallest nonsignificant partial F statistic.12 Variables that were initially eliminated were examined again as potential confounders using a change in the parameter estimate of 10% as criterion for confounding.13 We searched for, but did not find, interactions between depression, anxiety, or injection drug use and either sex or AIDS. Similarly, we did not find interactions between any of the statistically significant variables from the univariate analyses. Obviously collinear terms were not included together in models, including any combination of CD4 cell count less than 200, history of pneumocystis pneumonia, and AIDS diagnosis. We did not find evidence for collinearity between other terms.
The final model included all significant variables and a term for calendar date of first elevated viral load to adjust for secular trends in protease inhibitor use. We report the inverse of the hazard ratios (HRs) such that increasing hazard is associated with delayed protease inhibitor therapy, with 95% confidence intervals (CIs) and p values. To check for proportionality of hazards, we examined time-varying covariates for those variables in our final model. No covariates had statistically significant terms for nonproportionality. We estimated unadjusted median days to protease inhibitor initiation using the Kaplan-Meier product limit for each covariate in the final Cox model.
To check for effects of availability (commercial or clinical trials) of protease inhibitors over the study period and the dissemination of practice guidelines, we ran the above analyses two more times. First, we included only cases with a first elevated viral load after January 1, 1996 (when the FDA approved the first protease inhibitor). In another set of models, we included only cases with a first elevated viral load after July 1, 1996 (first guidelines for protease inhibitors released).5 To determine whether patients with a history of depression or injection drug use were more likely to refuse protease inhibitors, we used χ2tests. To determine whether patients with higher CD4 cell count, depression, or injection drug use histories may have been receiving nonnucleoside reverse transcriptase inhibitors instead of protease inhibitors, we modeled use of these drugs using logistic regression. We carried out both univariate models and models adjusted for CD4 cell count (in the case of depression or injection drug use), pneumocystis history, viral load, and time period.
Patients who completed the telephone survey were included in a subgroup analysis. Respondents and nonrespondents were compared using χ2tests. We examined effects of self-described race, income, education, and employment status using the same univariate technique as above. We then entered each term individually (using the same final model form and covariates as from the full analysis) to check for multivariate statistical significance or evidence of confounding. We also examined the impact of other covariates in multivariate models for this subgroup but ultimately kept the same final model.
RESULTS
As stated, 190 of 275 clinically active, HIV-seropositive patients had at least one elevated viral load. Of the 85 patients without an elevated viral load, 12 never had viral load levels checked during the study period. Of these, six had not been seen in the previous year; of the other six, three had CD4 cell counts less than 500.
The median age of the 190 patients was 39 years; 87% were male (Table 1). Unprotected male homosexual contact was the most common HIV risk factor (78%). Although 12% had a history of injection drug use, only three patients were active users during the study period. Fifty-four percent had a CD4 cell count less than 200 before their first elevated viral load, and 53% had a diagnosis of depression.
Table 1.
Patient Characteristics (n = 190)
| Characteristic | n(%) |
|---|---|
| Age (median), years | 39 |
| Female | 25 (13) |
| HIV risk factor | |
| Unprotected homosexual male contact | 147 (77) |
| Injection drug use | 22 (12) |
| Heterosexual contact or transfusion | 21 (11) |
| CD4 cell count before first elevated viral load | |
| <200 | 103 (54) |
| 200–500 | 66 (35) |
| >500 | 21 (11) |
| AIDS diagnosis | 120 (63) |
| History of pneumocystis pneumonia | 32 (17) |
| History of depression | 101 (53) |
| Care by low-volume physician | 57 (30) |
| Care by faculty-level physician | 133 (70) |
Of the 190 patients, 130 were prescribed protease inhibitors during the study period, and 60 contributed person-time in the analysis until the end of follow-up. Table 2 shows unadjusted HRs for time to protease inhibitor initiation from the univariate analyses using the entire 2 years of follow-up. Higher CD4 cell counts and history of injection drug use were associated with delays in initiating protease inhibitor therapy. More rapid initiation was associated with history of an AIDS-defining diagnosis, history of pneumocystis pneumonia, higher viral loads, and more frequent viral load checks and clinic visits. Other specific AIDS-defining diagnoses, other HIV risk factors, and history of anxiety were not significant in univariate analyses. Sixteen patients refused protease inhibitor therapy. These refusals were not significantly associated with history of injection drug use or depression. Neither low physician volume of patients with HIV nor faculty status was associated significantly with delay in protease inhibitor use.
Table 2.
Unadjusted Analysis of Time to ProteaseInhibitor Initiation
| Factor | HazardRatio | 95% CI |
|---|---|---|
| CD4 cell count before elevated viral load | ||
| <200* | 1.00 | — |
| 200–500 | 2.38 | 1.59, 3.57 |
| >500 | 8.33 | 2.63, 25.0 |
| History of AIDS-defining diagnosis | 0.24 | 0.15, 0.36 |
| History of pneumocystis pneumonia | 0.32 | 0.21, 0.49 |
| Tenfold increase in initial elevated viral load | 0.43 | 0.31, 0.59 |
| Known to be HIV positive <6 years | 1.05 | 0.75, 1.49 |
| Female | 1.30 | 0.76, 2.22 |
| HIV risk factor | ||
| Unprotected homosexual male contact | 0.77 | 0.50, 1.19 |
| Injection drug use | 2.08 | 1.05, 4.17 |
| Heterosexual contact or transfusion | 1.41 | 0.85, 2.33 |
| History of anxiety | 1.06 | 0.67, 1.61 |
| History of depression | 1.28 | 0.91, 1.79 |
| Private insurance | 1.31 | 0.93, 1.85 |
| Number of viral load checks (total)† | 0.86 | 0.80, 0.93 |
| Number of infectious disease consultant visits†‡ | 1.15 | 0.60, 21.7 |
| Number of RN and MD visits in past year† | 0.94 | 0.91, 0.97 |
| Low-volume physician (<4 patients with HIV) | 1.15 | 0.78, 1.69 |
| Faculty physician | 0.88 | 0.59, 1.30 |
| Involvement in clinical trial in past year | 0.70 | 0.48, 1.02 |
Referent group.
Per one unit increase.
Time-varying covariate.
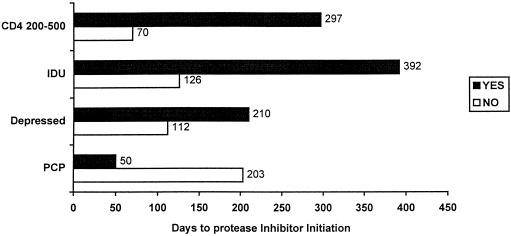
Table 3 shows the final multivariate Cox proportional hazards model for time to protease inhibitor initiation. We adjusted for date of patient entry into the study to account for secular trends in protease inhibitor use during the study period. Higher CD4 cell counts were associated with delays in protease inhibitor initiation. Patients with CD4 cell counts of 200 to 500 had delay of protease inhibitor initiation compared with those with CD4 cell counts below 200 (HR 2.63 vs 1.0), while those with CD4 cell counts above 500 had greater delays (HR 11.11). Magnitude of the first elevated viral load was also associated with speed of protease inhibitor use, with an HR of 0.66 associated with each 10-fold increase in viral load. Patients with a history of pneumocystis pneumonia had an HR of 0.57 compared with those without this history. Patients with a history of depression or injection drug use had significant delays in use of protease inhibitors. Patients with depression had an HR of 1.49 for protease inhibitor initiation compared with those without a depression history, while those with an injection drug use history had an HR of 2.70 compared with those without such a history. Figure 1 shows the unadjusted median days from detection of elevated viral load to protease inhibitor initiation for each categorical variable remaining in the final model.
Table 3.
Multivariate Cox Model for Time to Protease Inhibitor Initiation*
| Factor | HazardRatio | 95% CI | p Value |
|---|---|---|---|
| CD4 cell count | |||
| <200 | 1.00 | — | — |
| 200–500 | 2.63 | 1.61, 4.17 | <.001 |
| >500 | 11.11 | 3.57, 33.33 | <.001 |
| Tenfold increase in initial elevated viral load | 0.66 | 0.45, 0.98 | .038 |
| History of pneumocystis | 0.57 | 0.37, 0.90 | .016 |
| History of depression | 1.49 | 1.03, 2.13 | .032 |
| History of injection drug use | 2.70 | 1.35, 5.56 | .005 |
Model is adjusted for calendar date of first elevated viral load.
FIGURE 1.
Median days to protease inhibitor initiation for each categorical factor in the final model (unadjusted). Patients with CD4 cell counts of 200 to 500 had large absolute delays in protease inhibitor initiation when compared with patients with CD4 cell counts less than 200. Although we are unable to report an exact median number of days to protease initiation for patients with CD4 cell counts greater than 500 (owing to a large number of censored subjects), the median is at least 560 days. Similarly, patients with a history of injection drug use (IDU) and depression had large delays in protease inhibitor initiation when compared with patients without those factors. Those patients with a history of pneumocystis carinii pneumonia (PCP) were started on protease inhibitors more rapidly.
Physician factors appeared unrelated to speed of protease inhibitor initiation. Variables indicating low volume of patients with HIV (<4 patients per physician) and faculty status (vs housestaff ) were added individually to the final model and yielded HR of 0.95 (95% CI 0.62, 1.47) and HR of 1.08 (95% CI 0.70, 1.64), respectively.
In two more analyses using only the last 18 months or 12 months of follow-up, our results were similar to those reported in Table 3(which included the full 2 years of follow-up). In analyses using logistic regression to predict any use of nonnucleoside reverse transcriptase inhibitors, we found that patients with depression (odds ratio [OR] 0.87; 95% CI 0.32, 2.39) or injection drug use (OR 0.91; 95% CI 0.22, 3.76) were no more likely to be on these medications during the study period. Patients with CD4 cell counts of 200 to 500 were no more likely to be on nonnucleoside reverse transcriptase inhibitors (OR 1.05; 95% CI 0.26, 4.25) when compared with those with CD4 below 200. Patients with CD4 cell counts above 500 were much less likely to be on any nonnucleoside reverse transcriptase inhibitors (OR 0.19; 95% CI 0.05, 0.76).
The subgroup of patients (n = 129) for whom more data were available from telephone surveys were similar to the nonrespondents according to age, sex, socioeconomic factors, baseline CD4 cell count, and viral load. The subgroup analysis showed no evidence of delays in protease inhibitor use by race, educational attainment, work status, or income. For nonwhites, the HR was 1.06 (95% CI 0.62, 1.82) and for those with at least some college education (vs none), the HR was 0.83 (95% CI 0.55, 1.28). For patients working full time, the HR was 1.12 (95% CI 0.71, 1.79), while for patients on disability, the HR was 0.88 (95% CI 0.53, 1.45). For patients with yearly incomes above $30,000, the HR was 1.28 (95% CI 0.76, 2.13). None of these findings was statistically significant.
DISCUSSION
We report significant and lengthy delays in protease inhibitor initiation for patients with higher CD4 cell counts or a history of depression or injection drug use. To our knowledge, we are the first to report delays due to these factors. As expected, higher viral load or history of pneumocystis pneumonia was associated with more rapid protease inhibitor initiation. Socioeconomic factors including race, educational attainment, work status, and income were not significantly related to delay of protease inhibitor initiation, although we had limited power to assess these factors.
Our findings have important implications. Although protease inhibitors were started promptly after the first viral load for most patients with AIDS-defining diagnoses, we found delays among patients with higher CD4 cell counts. Published guidelines recommend treatment for viral loads greater than 5,000 to 10,000 copies/ml regardless of CD4 cell count.5,6,14 Delay in patients with higher CD4 cell counts may represent a knowledge deficit or reluctance to treat on the part of physicians or patients. However, the decision to initiate antiretroviral treatment when viral load is mildly elevated is a matter of clinical judgment in some situations.14 Initiating protease inhibitors is an important clinical decision, particularly because treatment is lifelong, dosing regimens are difficult, adverse drug effects are common, and the therapies are not curative. For these reasons, some flexibility on the part of the clinician may be necessary and some delays may be clinically appropriate.
In our study population, the prevalence of depression was greater than 50% during the 2-year study period. Although we had fewer patients with injection drug use histories, this is a common HIV risk factor in the United States.15 Thus, delays in prescribing protease inhibitors for patients with depression or prior injection drug use would be substantial if extrapolated to all persons with HIV and would permit longer periods of uninhibited viral replication. Recent studies demonstrate decreased morbidity and mortality associated with combination therapy with protease inhibitors for HIV infection.16–18 No published data assess the effects of delays in starting protease inhibitors. Nonetheless, such delays could attenuate the observed survival benefit.
We are unaware of other studies of the relation between higher CD4 counts or depression and delays in protease inhibitor use. Palella et al. reported that injection drug users were less likely than others to receive protease inhibitor prescriptions, but they did not report effect estimates. They also found that patients with private health insurance were more likely to receive protease inhibitors.16 Insurance type was not significant in our study, perhaps because Massachusetts provides access to drugs for the treatment of HIV infection for all patients without adequate insurance. Celentano et al.19 and Strathdee et al.20 found low rates of protease inhibitor therapy among injection drug users with HIV in Baltimore and British Columbia.
We did not find any significant association between physician experience with HIV care or level of training and delay of protease inhibitor use. Strathdee et al. reported lower rates of antiviral use in injection drug users by physicians with less HIV experience in a free HIV treatment program in British Columbia.20 In our setting, the ready availability of infectious disease consultations and periodic teaching conferences about HIV care10 may reduce practice differences related to an individual physician's volume of HIV patients.
Our study has several limitations. Its findings may not be generalizable beyond academic practices. We have limited information about patient preferences for antiviral therapy, which might affect therapeutic decisions. However, we found little evidence of antiviral therapy refusal, and no association between refusal and factors associated with delays. In addition, we did not find evidence that patients with higher CD4 cell count, depression, or injection drug use history were differentially prescribed nonnucleoside reverse transcriptase inhibitors in lieu of protease inhibitors. We have limited power to detect delays in protease inhibitor initiation related to level of physician training or HIV patient volume, but effect estimates from adjusted analyses were close to 1.0 for both these factors.
We used medical record reviews to determine history of depression or injection drug use, including active or past drug use, which may have underestimated the actual prevalence in our population. However, for our purposes (i.e., examining delays in protease inhibitor use), physicians' perceptions of whether the patient is depressed or has used injection drugs may be the pertinent consideration. We found a similar prevalence of depression to that reported by Mayne et al.,21 who determined that 58% of their HIV study population had significantly depressed affect at least once during their study. Although the 12% rate of injection drug use in our population is lower than the 1996 –1997 U.S. rates of 23% for seropositive men and 33% for seropositive women,15 we found HIV risk factors clearly documented in most initial visit notes. Therefore, our low injection drug use prevalence is most likely accurate.
Our final models were based on data from July 1995 through June 1997. At the beginning of this interval, protease inhibitors were available through expanded access programs and through protocols. Saquinavir was approved by the Food and Drug Administration in December 1995. Despite general consensus that protease inhibitor use was appropriate for patients with elevated viral loads, the first recommendations for use were not published until June 1996.5 Delays in starting protease inhibitors during the first part of the study period may have been due to differences in access to drugs or protocols by physicians. However, this study took place in a single primary care practice in which HIV experts within the practice kept all physicians informed about drug availability by announcements. Also, we adjusted our models for secular trends. We conducted analyses including only the last year or 18 months of follow-up and found that the results did not differ materially.
Whether the observed delays for patients with depression or history of injection drug use are clinically appropriate remains unclear. Singh et al. found statistically significant lower adherence in patients with a history of depression, but not in patients with history of injection drug use.22 They conducted their study before the availability of protease inhibitors, and availability of more effective therapeutic regimens may alter the observed associations. Clinicians might expect patients with depression or active injection drug use to have poor adherence to follow-up or complex pharmacologic therapy. Clinicians may also be concerned about polypharmacy and drug interactions for depressed patients. We could not determine the timing of discussions between patients and doctors about protease inhibitors in relation to depressive symptoms, treatment of depression, or injection drug use. For persons with severe depression or active injection drug use, delaying protease inhibitor use may be appropriate while these conditions are addressed and may result in improved outcomes.
In conclusion, we found that higher CD4 cell count, depression, and injection drug use history are associated with substantial delays in protease inhibitor therapy. The reasons for these delays are not clear, and the clinical appropriateness of delays for patients with depression or injection drug use is unknown. As clinical guidelines recommend therapy for patients with elevated viral loads regardless of CD4 count,5,6,14 educational initiatives may be needed to improve physician and patient acceptance of protease inhibitors when clinically appropriate. More data are needed about the impact of depression and history of injection drug use on adherence to antiviral therapy and treatment decisions for patients with HIV. Future studies should explore physician and patient attitudes and beliefs about protease inhibitor use in the setting of higher CD4 cell count, depression, and injection drug use.
REFLECTIONS
Pre-Op
Ants,
that is what they are—
scurrying soldiers
draped in blue.
I rest, watching
from my little life.
Their faces dangle.
Words are muffled,
breathed away.
I am quiescent and deaf.
My name signs itself
to a contract and
I'm rolled like a baby
into the nursery,
placed on a steel crib.
Softly, the man injects sleep
into my veins,
a lucid sleep,
pure as teardrops.
A cacophony of voices
crowds my forehead,
shoving and pushing
at my eyes.
I'm told to count;
One hundred, ninety-nine,
ninety-eight …
then they're gone.
Vanessa Lops
San Diego, Calif
FINALIST, 1999 Creative Medical Writing Contest
Acknowledgments
Dr. Fairfield was supported by National Research Service Award PE11001-09. Partial funding for this project was provided by the Center for Alternative Medicine Research (National Institutes of Health Grant U24 AR3441), The John E. Fetzer Institute, the Friends of Beth Israel Deaconess Medical Center, the Kenneth J. Germeshausen Foundation, and the J.E. and Z.B. Butler Foundation.
REFERENCES
- 1.O'brien TR, Blattner WA, Waters D, et al. Serum HIV-1 RNA levels and time to development of AIDS in the Multicenter Hemophilia Cohort Study. JAMA. 1996;276:105–10. [PubMed] [Google Scholar]
- 2.Mellors JW, Kingsley LA, Rinaldo CR, et al. Quantitation of HIV-1 RNA in plasma predicts outcome after seroconversion. Ann Intern Med. 1995;122:573–9. doi: 10.7326/0003-4819-122-8-199504150-00003. [DOI] [PubMed] [Google Scholar]
- 3.Perelson AS, Neumann AU, Markowitz M, Leonard JM, Ho DD. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science. 1996;271:1582–6. doi: 10.1126/science.271.5255.1582. [DOI] [PubMed] [Google Scholar]
- 4.Cohen OJ, Pantaleo G, Holodniy M, et al. Decreased human immunodeficiency virus type 1 plasma viremia during antiretroviral therapy reflects downregulation of viral replication in lymphoid tissue. Proc Natl Acad Sci USA. 1995;92:6017–21. doi: 10.1073/pnas.92.13.6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carpenter CCJ, Fischl MA, Hammer SM, et al. for the International AIDS Society. Antiretroviral Therapy for HIV infection in 1996: recommendations of an international panel. JAMA. 1996;276:146–54. [PubMed] [Google Scholar]
- 6.Carpenter CCJ, Fischl MA, Hammer SM, et al. for the International AIDS Society. Antiretroviral Therapy for HIV infection in 1997: updated recommendations of the International AIDS Society—USA Panel. JAMA. 1997;277:1962–9. [PubMed] [Google Scholar]
- 7.Deeks SG, Smith M, Holodniy M, Kahn JO. HIV-1 protease inhibitors: a review for clinicians. JAMA. 1997;277:145–53. [PubMed] [Google Scholar]
- 8.Williams A, Friedland G. Adherence, compliance, and HAART. AIDS Clinical Care. 1997;9:51–4,58. [PubMed] [Google Scholar]
- 9.Safran C, Rury C, Rind DM, Taylor WC. A computer-based outpatient medical record for a teaching hospital. MD Comput. 1991;8:291–9. [PubMed] [Google Scholar]
- 10.Makadon HJ, Delbanco SF, Delbanco TL. Caring for people with AIDS and HIV infection in hospital-based primary care practice. J Gen Intern Med. 1990;5:446–50. doi: 10.1007/BF02599436. [DOI] [PubMed] [Google Scholar]
- 11.Fairfield KM, Eisenberg DM, Davis RB, Libman H, Phillips RS. Patterns of use, expenditures, and perceived efficacy of complementary and alternative therapies in HIV-infected patients. Arch Intern Med. 1998;158:2257–64. doi: 10.1001/archinte.158.20.2257. [DOI] [PubMed] [Google Scholar]
- 12.Kleinbaum DG, Kupper LL, Muller KE. Applied Regression Analysis and Other Multivariable Methods. Belmont, Calif: Duxbury Press; 1988. [Google Scholar]
- 13.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129:125–37. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 14.US Department of Health and Human Services. Guidelines for the Use of Antiretroviral Agents in HIV-Infected Adults and Adolescents. Washington, DC: US Department of Health and Human Services. 1998;47(RR–5):43–82. [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. HIV/AIDS Surveillance Report. Atlanta, Ga: Centers for Disease Control and Prevention; 1997. :9 (No. 1). [Google Scholar]
- 16.Palella FJ, Delaney KM, Moorman AC, Loveless MO, Fuhrer J. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 17. Detels R, Munoz A, McFarlane G, et al. Increased survival time to AIDS and death associated with use of combined therapy in the multicenter AIDS cohort study. Presented at the 5th Conference on Retroviral Opportunistic Infection, Feb 1–5, 1998. Abstract 115.
- 18. McNaghten AD, Hanson DL, Jones JL, Ward JW, Dworkin MS. The effects of antiretroviral therapy and opportunistic illness primary chemoprophylaxis on survival after AIDS. Presented at the 5th Conference on Retroviral Opportunistic Infection, Feb 1–5, 1998. Abstract 81.
- 19.Celentano DD, Vlahov D, Cohn S, Shadle VM, Obasanjo O, Moore RD. Self-reported antiretroviral therapy in injection drug users. JAMA. 1998;280:544–6. doi: 10.1001/jama.280.6.544. [DOI] [PubMed] [Google Scholar]
- 20.Strathdee SA, Palepu A, Cornelisse PG, et al. Barriers to use of free antiretroviral therapy in injection drug users. JAMA. 1998;280:547–9. doi: 10.1001/jama.280.6.547. [DOI] [PubMed] [Google Scholar]
- 21.Mayne TJ, Vittinghoff E, Chesney MA, Barrett DC, Coates TJ. Depressive affect and survival among gay and bisexual men infected with HIV. Arch Intern Med. 1996;156:2233–8. [PubMed] [Google Scholar]
- 22.Singh N, Squier C, Sivek C, Wagener M, Nguyen MH, Yu VL. Determinants of compliance with antiretroviral therapy in patients with human immunodeficiency virus: prospective assessment with implications for enhancing compliance. AIDS Care. 1996;8:261–9. doi: 10.1080/09540129650125696. [DOI] [PubMed] [Google Scholar]