Abstract
We developed and optimized a new modified amplified fragment length polymorphism (AFLP) typing method to obtain a multibanding fingerprint that can be separated by agarose gel electrophoresis. Both to maximize the discriminatory power and to facilitate the computer-assisted analysis, bacterial DNA was digested with four different restriction enzymes. After ligation of adaptors to the DNA fragments, PCR testing of various single primers was performed. Two single primers that gave optimal results with regard to band resolution and discriminatory power were selected and combined. The computer-assisted analysis of fingerprint patterns was performed with Pearson's product-moment correlation values of densitometric curves, without assigning bands to peaks. Thus, the analysis is not subject to human interpretation errors. With this method, we investigated two outbreaks of multiresistant Klebsiella pneumoniae in an intensive care unit and various sporadic isolates of K. pneumoniae and Klebsiella oxytoca. Cluster analysis of isolates analyzed in different experiments and on different gels showed that fingerprint patterns clustered correctly according to subspecies or to the outbreaks. Multienzyme multiplex PCR AFLP revealed that the first outbreak was caused by two different types of strains. Outbreak two was caused by yet another strain of K. pneumoniae. In conclusion, the typing method used here is easy to perform and highly reproducible, and due to generation of complex banding patterns, it has a higher discriminatory power. Furthermore, the multienzyme multiplex PCR fingerprints are easy to analyze, and a reliable database can be stored in the computer to facilitate comparison of future isolates of Klebsiella spp. The method can be performed in every clinical microbiology laboratory.
An adequate epidemiological typing system is essential for identification and control of outbreaks of bacterial pathogens. Since conventional typing methods such as biotyping, serotyping, phage typing, and bacteriocin typing allow sufficient differentiation only when used in combination, they are not very suitable for epidemiological studies. Molecular typing methods can present accurate tools in epidemiological investigations. Several molecular typing methods have been used in the investigation of bacterial outbreaks (9, 13, 19, 20). Pulsed-field gel electrophoresis is most often used but is technically demanding and time-consuming. PCR-based typing methods with enterobacterial repetitive intergenic consensus (ERIC) or repetitive element sequences have limited discriminatory power. Randomly amplified polymorphic DNA has been shown to have limited reproducibility (2, 10).
In contrast, amplified fragment length polymorphism (AFLP)-based typing systems have been shown to be highly reproducible (8). Since the AFLP method was first published (18), several restriction enzyme combinations have been used for typing of bacteria. In addition, two methods of analysis of AFLP-generated banding patterns have been used, analysis by polyacrylamide gel electrophoresis and by agarose gel electrophoresis. On polyacrylamide gels, band sizes range from 50 to 500 bp, and at most two enzymes are used for AFLP typing procedures (3, 8, 16). Most of the AFLP-generated fingerprints are analyzed with agarose gel electrophoresis, with band sizes ranging from 100 to 4,000 bp, and are based on one enzyme and a single adaptor (1, 14).
The AFLP typing systems with electrophoresis on polyacrylamide gels and fluorescent labels to be analyzed on automated sequencers are not widely accessible for the hospital microbiology laboratory, where an accurate epidemiological typing system is most warranted. In addition to the high costs, it requires skilled specialists. Agarose gel-based AFLP typing can be performed in every clinical microbiology laboratory.
Analysis of fingerprint patterns can be performed with GelComparII/Bionumerics software. With fingerprint patterns of low complexity the band positions are usually assigned to peaks and the cluster analysis is performed on the basis of band presence or absence. The resulting interpretation of banding patterns can be complicated by variable parameters such as gel electrophoresis quality, different technicians and subjective judgment of bands.
As a consequence, the database storage of the fingerprint patterns obtained over time and resulting from different experiments and run on different gels can still present difficulties in epidemiological typing of bacterial pathogens. This drawback also hampers the interlaboratory exchange of typing patterns.
Our objective was to develop an AFLP-based typing system with four different restriction enzymes and multiple primers to generate a complex banding pattern that is separated by agarose gel electrophoresis. The generation of a more complex banding pattern was intended to serve two purposes: first, to obtain optimal discriminatory power compared to the single- enzyme single-primer approach, and second, to facilitate the computer-assisted analysis with Bionumerics software in order to avoid subjective and variable interpretation of bands.
We developed and optimized the multienzyme multiplex PCR AFLP method with isolates of Klebsiella spp. Klebsiella pneumoniae strains are frequently isolated from patient materials and are the causative pathogen in 3 to 7% of nosocomial infections (7). In the hospital environment, K. pneumoniae strains expressing multiple resistance, e.g., resistance to aminoglycosides and cephalosporins, are isolated with increasing frequency. Several hospital outbreaks with these multiresistant strains have been documented (5, 20).
An easy-to-perform and reliable genotypic typing method could be a helpful tool in recognizing and controlling these hospital outbreaks. We evaluated the multienzyme AFLP method by investigation of two Klebsiella outbreaks in an intensive care unit.
MATERIALS AND METHODS
Bacterial isolates.
A total of 79 Klebsiella isolates, comprising 63 K. pneumoniae isolates and 16 K. oxytoca isolates, were used in this study. All isolates were cultured from patient specimens presented for culture at our clinical microbiology laboratory. Isolates were identified by conventional NCCLS-recommended biochemical methods and completed with Api 20E (bioMerieux SA, Lyon, France) test kit. Multiple isolates from one patient were cultured at least 1 week apart. Both epidemiologically unrelated and related isolates were analyzed. One isolate each of Serratia marcescens and Xanthomonas maltophilia were included to present the outgroup in the computer-assisted analysis.
DNA extraction.
Genomic DNA was extracted with the QiAmp DNA minikit (Qiagen, Hilden, Germany). After addition of 5 μl of RNase (1 U; Sigma), the concentration of DNA was determined with a Genequant spectrophotometer (260 nm; Genequant, Pharmacia, United Kingdom).
Restriction-ligation reaction.
Enzymes were chosen to recognize relatively GC-rich sequences (PstI and NheI) and relatively AT-rich sequences (EcoRI and XbaI). Double-stranded oligonucleotide adaptors were chosen to block the restriction recognition sites after ligation to the target DNA, allowing single-tube restriction and ligation. Primers were complementary to the ligated adaptors including the restriction site (Table 1, e.g., px and pn) or to the adaptor sequence only (pxn). In the case of a frequent-cutting enzyme, 3′ selective bases were added (Table 1, pst primers).
TABLE 1.
Restriction enzymes, adaptors, and primers
| Restriction enzyme | Recognition site | Adaptor oligonucleotidesa | Primer | Primer name |
|---|---|---|---|---|
| XbaI | 5′T↓CTAGA | 5′CTAGTACTGGCAGACTCT | 5′AGAGTCTGCCAGTACTAGA | px |
| AGATC↓T | 3′ATGACCG | 5′AGAGTCTGCCAGTACTAG | pxn | |
| NheI | 5′G↓CTAGC | 5′AGAGTCTGCCAGTACTAGC | pn | |
| CGATC↓G | 5′AGAGTCTGCCAGTACTAGCG | pn-g | ||
| PstI | 5′CTGCA↓G | 5′CTCGTAGACTGCGTACATGCA | 5′GACTGCGTACATGCAGG | pst-g |
| G↓ACGTC | 3′CATCTGACGCATGT | 5′GACTGCGTACATGCAGAG | pst-ag | |
| 5′GACTGCGTACATGCAGAT | pst-at | |||
| EcoRI | 5′G↓AATTC | 5′AATTGGTACGCAGTCTAC | 5′GTAGACTGCGTACCAATTC | peco |
| CTTAA↓G | 3′CCATGCGTCAGATGCTC |
One of the oligonucleotides of each adaptor was phophorylated at its appropriate 5′ site. Adaptors were chosen from references 4, 8, and 15.
The restriction-ligation reaction was performed with 500 ng of DNA in a final volume of 30 μl. It consisted of restriction enzymes EcoRI, PstI, XbaI, and NheI (20 U each; Roche Molecular Biochemicals), 3 μl of T4 ligase buffer, 1 U of T4 ligase (Roche Molecular Biochemicals), and 20 pmol of each adaptor oligonucleotide (see Table 1). The restriction-ligation mixture was incubated at 37°C for 3 h. Subsequently, the DNA was precipitated with 2.5 M ammonium acetate in 100 μl of chilled absolute ethanol. After the DNA was washed with 70% ethanol, it was resuspended in 100 μl of TE (Tris-EDTA) buffer and stored at 4°C.
PCR.
PCRs were carried out with Ready-To-Go beads (Amersham Pharmacia, Little Chalfont, United Kingdom), supplemented with 1 μl of MgCl2 (25 mM). Then 10 pmol of each primer and 100 ng of chromosomal template DNA in a final reaction volume of 25 μl were used. The reaction mixtures were preheated for 4 min at 94°C in a DNA thermocycler (Perkin Elmer GeneAmp PCR System 2400). Thirty-three amplification cycles of 1 min at 94°C, 1 min at 60°C, and 2.5 min at 72°C were performed.
Analysis of banding patterns.
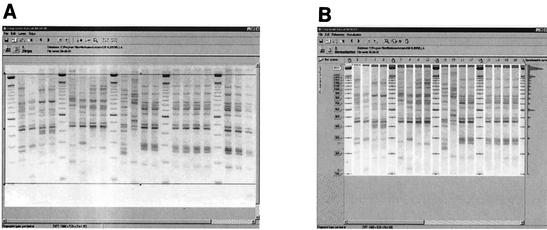
Analysis of 10 μl of each PCR product was performed by agarose gel electrophoresis on a 1.5% agarose gel (MP agarose, Roche Molecular Biochemicals). Marker DNA (100-bp ladder; Gibco-BRL, Life Technologies, Paisley, Scotland) was loaded after every four samples. Analysis of banding patterns on gel images was performed with the software program Gelcompar II included in Bionumerics (Applied Maths, Kortrijk, Belgium). The gel images were imported in the gel analysis software Bionumerics. The images were fitted in the framework where the borders are touching the upper 2,000-bp marker band and below the 100-bp marker band (Fig. 1A). During the next step, marker lanes were indicated as references, and the positions of references were indicated. After normalization of the banding pattern, the image was stored (Fig. 1B). Cluster analysis of the fingerprints was performed with the unweighted pair group method using arithmetic averages, and similarities between AFLP patterns were calculated with the Pearson product-moment correlation coefficient (17).
FIG. 1.
Computer-assisted analysis of multienzyme multiplex PCR AFLP fingerprint patterns with GelComparII/Bionumerics software. (A) The window of analysis is marked by the upper and lower marker bands of 2,000 and 100 bp. (B) Band sizing and normalization are performed with the bands in marker lanes. No bands are assigned to the fingerprints obtained from Klebsiella isolates.
ERIC-typing.
For comparison, 57 Klebsiella isolates were also typed with primers ERIC1 (5′-ATGTAAGCTCCTGGGGATTCAC-3′) and ERIC2 (5′-AAGTAAGTGACTGGGGTGAGCG-3′). The PCR with ERIC primers was carried out with 100 ng of chromosomal template DNA and 35 amplification cycles of 30 s at 94°C, 30 s at 25°C, and 1.5 min at 72°C.
RESULTS AND DISCUSSION
An AFLP-based typing method with three restriction enzymes was recently described (15). In that case, the additional enzymes were intended to reduce the number of bands in the typing pattern. In contrast, the use of four restriction enzymes in this study was aimed at increasing the number of bands.
To obtain a fingerprint pattern that represents the complete bacterial genome, DNA fragments to be amplified should range from 100 to 2,000 bp when analyzed with agarose gel electrophoresis. The theoretical chance that a 6-bp-recognizing restriction enzyme cuts is 1 in 46. Consequently, four enzymes would statistically cut the bacterial genome into approximately 1,000-bp fragments.
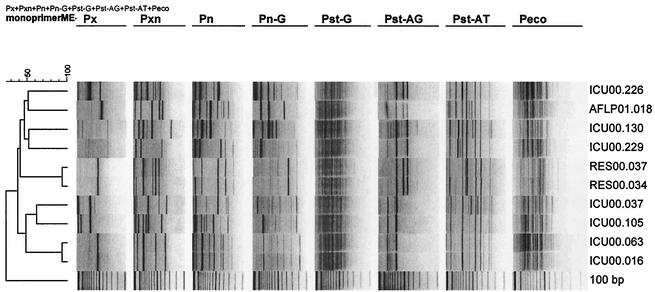
To investigate the discriminatory power of each of the single primers in typing of different strains, a selection of 10 Klebsiella isolates was made. Chromosomal DNA of these isolates was digested with all four enzymes, and all adaptors were ligated in a single-tube restriction-ligation reaction. The restriction-ligation mixture was subjected to PCR with each one of the primers mentioned in Table 1. The patterns of the isolates were analyzed, and the number of types, the resolution of the banding patterns on the gel, and the number of bands were compared (Fig. 2). Discriminatory power and band resolution were found to vary between each of the single primers. For example, a discrimination between strains RES00.037 and RES00.034 was observed with primers pxn and pn, while typing with other primers resulted in identical patterns between these strains. Identical patterns between strains ICU.063 and ICU.016 were generated with each primer (Fig. 2).
FIG. 2.
Fingerprint types obtained with single primers. The primers used are indicated above the images. Strain designations are shown to the right.
In order to increase the complexity of fingerprints, two primers were combined. In addition to a-a and b-b fragments obtained from two single primers, additional fragments are amplified between restriction sites a and b. Primer combination pxn and pst-at gave the most satisfactory typing results with regard to discriminatory power and band resolution compared to other primer combinations.
For comparison, 57 Klebsiella isolates were typed with ERIC primers (not shown), a single primer (pxn), and the primer combination pxn/pst-at. The numbers of types generated with these three methods were 34, 33, and 38 different fingerprint types, respectively. Thus, multienzyme AFLP shows a considerable improvement in discriminatory power compared to the other methods.
The multienzyme AFLP method is very flexible for optimizing discriminatory power. It is expected that a suitable set of primers can be selected for typing of any bacterial pathogen.
Reproducibility of the method was 100%. Repeated analysis of one strain or strains isolated from one patient always showed the same fingerprint type. Nevertheless, it is recommended that a positive control with known typing pattern be included to ensure digestion of DNA with all four restriction enzymes and ligation of adaptors. Also, one should be aware that previously amplified fragments might contaminate typing patterns of newly analyzed strains. Therefore, a negative control should also be included.
Analysis and interpretation of fingerprint patterns obtained in different runs or different laboratories often result in discordant results. For example, the single-enzyme single-primer AFLP typing used by the European Working Group on Legionella Infections failed to obtain consistency of typing results between various laboratories (1). The main cause of the observed discordance may be due to the arbitrary assignment of bands to peaks. Similarity of patterns calculated on the basis of band presence versus band absence is used with patterns of low complexity. With complex banding patterns, it is more accurate to calculate the product-moment correlation coefficient of each pair of densitometric curves (8, 12, 17).
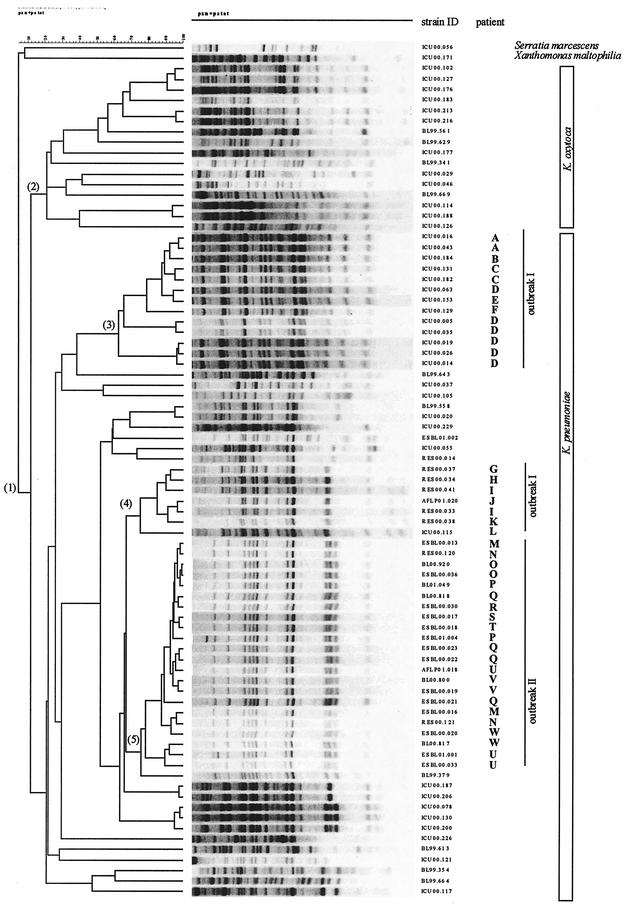
Different experiments and different gels added to the cluster analysis of all strains included in this study and resulted in the dendrogram shown in Fig. 3. The first branching-off in the dendrogram separates Klebsiella species that are grouped in cluster 1 from the outgroup presented by one isolate each of Serratia marcescens and Xanthomonas maltophilia. The second branching-off separates K. oxytoca strains grouped in cluster 2 from K. pneumoniae strains. Linkage level of species is <10% and that of subspecies is <20%. The clusters 3 and 4 comprise strains associated with outbreak I, while cluster 5 comprises strains that were involved in outbreak II.
FIG. 3.
Dendrogram of fingerprint patterns of Klebsiella isolates (group 1). Clusters comprise K. oxytoca isolates (group 2) and K. pneumoniae isolates of outbreak I (groups 3 and 4) and outbreak II (group 5) amid sporadic K. pneumoniae isolates. Patients involved in the outbreaks are designated A to W.
As the whole densitometric curve of the gel track is measured without the assignment of bands, the method is relatively insensitive to different concentrations and gel qualities. This study shows that although DNA concentrations and gel qualities vary, and hence the number of bands visible to the naked eye differ (e.g., Fig. 3, patient D), these apparent differences are absorbed by the computerized analysis.
In evaluating the multienzyme multiple-primer approach with outbreak-related and sporadic isolates of Klebsiella spp., we found that fingerprint patterns obtained in different experiments and different gels clustered correctly according to subspecies or to the outbreaks. A surprising outcome was the finding that outbreak I was caused by two different types of Klebsiella strains, while the antibiograms were similar and did not suggest a difference.
Thus, reliable computerized analysis will greatly promote database storage of typing patterns obtained over time. Interlaboratory exchange of typing data is expected to lead to higher concordance of results as interpretation errors are omitted.
Agarose gel electrophoresis-based AFLP typing is robust, reproducible, reliable, and simple. It can be performed routinely in 1.5 days in every microbiology laboratory. The multienzyme multiple-primer approach gives an excellent coverage of the bacterial genome and may contribute to the identification of epidemic clones or facilitate phylogenetic studies. The method is broadly applicable for typing of bacterial pathogens. We are currently trying this method on other pathogenic bacterial species to investigate its usefulness in phylogenetic studies and its feasibility in interlaboratory exchange of typing data.
Acknowledgments
We gratefully acknowledge the assistance of Kim van der Zwaluw (National Institute for Public Health and the Environment [RIVM], Bilthoven, The Netherlands) in analysis with Gelcompar/Bionumerics software.
REFERENCES
- 1.Fry, N. K., J. M. Bangsborg, S. Bernander, J. Etienne, B. Forsblom, V. Gaia, P. Hasenberger, D. Lindsay, A. Papoutsi, C. Pelaz, M. Struelens, S. A. Uldum, P. Visca, and T. G. Harrison. 2000. Assessment of intercentre reproducibility and epidemiological concordance of Legionella pneumophila serogroup 1 genotyping by amplified fragment length polymorphism analysis. Eur. J. Clin. Microbiol. Infect. Dis. 19:773-780. [DOI] [PubMed] [Google Scholar]
- 2.Gori, A., F. Espinasse, A. Deplano, C. Nonhoff, M. H. Nicolas, and M. Struelens. 1996. Comparison of pulsed-field gel electrophoresis and randomly amplified DNA polymorphism analysis for typing extended-spectrum β-lactamase-producing Klebsiella pneumoniae. J. Clin. Microbiol. 34:2448-2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huys, G., L. Rigouts, K. Chemlal, F. Portaels, and J. Swings. 2000. Evaluation of amplified fragment length polymorphism analysis for inter- and intraspecific differentiation of Mycobacterium bovis, M. tuberculosis, and M. ulcerans. J. Clin. Microbiol. 38:3675-3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mazurek, G. H., V. Reddy, B. J. Marston, W. H. Haas, and J. T. Crawford. 1996. DNA fingerprinting by infrequent-restriction-site amplification. J. Clin. Microbiol. 34:2386-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pagani, L., M. Perilli, R. Migliavacca, F. Luzzaro, and G. Amicosante. 2000. Extended-spectrum TEM-and SHV-type β-lactamase-producing Klebsiella pneumoniae strains causing outbreaks in intensive care units in Italy. Eur. J. Clin. Microbiol. Infect. Dis. 19:765-772. [DOI] [PubMed] [Google Scholar]
- 6.Pena, C., M. Pujol, C. Ardanuy, A. Ricrt, R. Pallres, J. Linares, J. Ariza, and F. Gudiol. 2001. An outbreak of hospital-acquired Klebsiella pneumoniae bacteraemia, including strains producing extended-spectrum beta-lactamase. J. Hosp. Infect. 47:53-59. [DOI] [PubMed] [Google Scholar]
- 7.Podschun, R., and U. Ullmann. 1998. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin. Microbiol. Rev. 11:589-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Savelkoul, P. H. M., H. J. M. Aarts, J. De Haas, L. Dijkshoorn, B. Duim, M. Otsen, J. L. Rademaker, L. Schouls, and J. A. Lenstra. 1999. Amplified-fragment length polymorphism: the state of an art. J. Clin. Microbiol. 37:3083-3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sechi, L. A., T. Spanu, M. Sanguinetti, I. Dupre, L. Masucci, A. Siddu, G. Tortorolo, G. Vento, L. Maggio, A. Cambieri, S. Zanetti, and G. Fadda. 2001. Molecular analysis of Klebsiella pneumoniae strains isolated in paediatric wards by ribotyping, pulsed field gel electrophoresis and antimicrobial susceptibilities. Microbiologica 24:35-45. [PubMed] [Google Scholar]
- 10.Shannon, K., K. Fung, P. Stapleton, R. Anthony, E. Power, and G. French. 1998. A hospital outbreak of extended-spectrum β-lactamase-producing Klebsiella pneumoniae investigated by RAPD typing and analysis of the genetics and mechanisms of resistance. J. Hosp. Infect. 39:291-300. [DOI] [PubMed] [Google Scholar]
- 11.Silva, J., R. Gatica, C. Aguilar, Z. Becerra, U. Garza-Ramos, M. Velazquez, G. Miranda, B. Leanos, F. Solorzano, and G. Echaniz. 2001. Outbreak of infection with extended-spectrum beta-lactamase-producing Klebsiella pneumoniae in a Mexican hospital. J. Clin. Microbiol. 39:3193-3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Speijer, H., P. H. M. Savelkoul, M. J. Bonten, E. E. Stobberingh, and H. T. Tjhie. 1999. Application of different genotyping methods for Pseudomonas aeruginosa in an endemic setting in an intensive care unit. J. Clin. Microbiol. 37:3654-3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su, L. H., H. S. Leu, Y. P. Chiu, J. H. Chia, A. J. Kuo, C. F. Sun, T. Y. Lin, T. L. Wu, and the Infection Control Group. 2000. Molecular investigation of two clusters of hospital-aquired bacteraemia caused by multiresistant Klebsiella pneumoniae with pulsed-field gel electrophoresis and infrequent restriction site PCR. J. Hosp. Infect. 46:110-117. [DOI] [PubMed] [Google Scholar]
- 14.Valsangiacomo, C., F. Baggi, V. Gaia, T. Balmelli, R. Peduzzi, and J. C. Piffaretti. 1995. Use of amplified fragment polymorphism in molecular typing of Legionella pneumophila and application to epidemiological studies. J. Clin. Microbiol. 33:1716-1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Wurff, A. W., Y. L. Chan, N. M. van Straalen, and J. Schouten. 2000. TE-AFLP: combining rapidity and robustness in DNA fingerprinting. Nucleic Acids Res. 28:E105.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der Zwet, W. C., G. A. Parlevliet, P. H. Savelkoul, J. Stoof, A. M. Kaiser, J. G. Koeleman, and C. M. Vandenbroucke-Grauls. Nosocomial outbreak of gentamicin-resistant Klebsiella pneumoniae in a neonatal intensive care unit controlled by a change in antibiotic policy. J. Hosp. Infect. 42:295-302. [DOI] [PubMed]
- 17.Vauterin, L., and P. Vauterin. 1992. Computer-aided objective comparison of electrophoresis patterns for grouping and identification of microorganisms. Eur. Microbiol. 1:37-42. [Google Scholar]
- 18.Vos, P., R. Hogers, M. Bleeker, M. Reijans, T. Van de Lee, M. Hornes, A. Frijters, J. Pot, J. Peleman, M. Kuiper, and M. Zabeua. 1995. AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res. 23:4407-4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Youssef, M. T., H. I. Malkawi, A. A. Shurman, and A. O. Andremont. 1999. Molecular typing of multiresistant Klebsiella pneumoniae isolated from children from northern Jordan. J. Trop. Pediatr. 45:271-277. [DOI] [PubMed] [Google Scholar]
- 20.Yuan, M., H. Aucken, L. M. C. Hall, T. L. Pitt, and D. M. Livermore. 1998. Epidemiological typing of Klebsiellae with extended-spectrum β-lactamases from European intensive care units. J. Antimicrob. Chemother. 41:527-539. [DOI] [PubMed] [Google Scholar]