Abstract
In 1998, 21 inhabitants of a German nursing home fell ill with acute gastroenteritis after consumption of minced beef heart (P. Graf and L. Bader, Epidemiol. Bull. 41:327-329, 2000). Two residents died during hospital treatment. Seventeen Clostridium perfringens strains were collected from two different dishes and from patients' stool samples and autopsy materials. A majority of these isolates was not typeable by restriction fragment length polymorphism-pulsed-field gel electrophoresis (PFGE). Subsequent ribotyping of C. perfringens distinguished four different groups. The same ribopattern was detected in a minced beef heart dish, in autopsy material from the two deceased patients, and additionally in stool samples from six further residents who had fallen ill with diarrhea. Three further ribopatterns from food and autopsy materials could be differentiated. As chromosomal macrorestriction with subsequent PFGE is generally regarded more useful than ribotyping for molecular strain analysis, four selected isolates were lysed in parallel with a standard protocol and two nucleases inhibiting modifications. Neither of these methods could differentiate all of the isolates. These results suggest that PFGE with the current standard protocols is not able to characterize all C. perfringens isolates from food-borne disease investigations and that ribotyping is still a helpful method for molecular identification of clonal relationships.
Clostridium perfringens is a gram-positive, spore-forming, anaerobic rod. This bacterium can spoil food, and some strains produce an enterotoxin (C. perfringens enterotoxin [CPE]) that is released upon lysis of the vegetative cell during sporulation in the small intestine. CPE causes food-borne disease in humans and some animals (3, 14, 17). As C. perfringens is also part of the intestinal flora, molecular typing is important for investigating clonal relationships in outbreaks and for studying the molecular epidemiology of this microorganism. Several molecular methods have been used successfully for C. perfringens strain differentiation; among them are serotyping (12), plasmid isolation (4, 11), sodium dodecyl sulfate-polyacrylamide gel electrophoresis (9a), pulsed-field gel electrophoresis (PFGE) (16, 19), and ribotyping (1). We describe the examination of 17 food-borne disease-related C. perfringens isolates by ribotyping (7) and PFGE with three different cell lysis methods. PFGE is presumed to offer greater discriminatory power, typeability, and reproducibility than many other DNA-based typing methods, including ribotyping, and has been applied successfully for genotyping of various C. perfringens isolates (16, 19, 20). Nevertheless, DNA degradation problems with certain strains due to endogenous bacterial nucleases, which are rather common among clostridial isolates, have been reported (5, 7, 19, 20). In our study, preliminary PFGE of isolates involved in a food-borne disease outbreak (6) failed. Therefore, ribotyping was performed subsequently and PFGE was repeated, including two protocols with nuclease-inhibiting supplements.
Strains
Table 1 shows the origins of the 17 C. perfringens isolates investigated. Isolates were cultured, purified, identified, and kept on a Microbank (Mast Diagnostica, Reinfeld, Germany) at −18°C. Before PFGE and ribotyping were performed, the purity of the isolates was ensured by culturing them twice on Columbia sheep blood agar (Unipath, Ltd., Basingstoke, Hampshire, United Kingdom).
TABLE 1.
PFGE and ribotyping results of 17 C. perfringens isolates involved in a food-borne disease outbreak
| Isolate(s) (n = 17) | Origin | PFGEa | Ribotype | CPE result |
|---|---|---|---|---|
| 728, 729, 730, 732, 733, 734 | 6 patients' stool samples | NT | 1 | Positive |
| 866 | Autopsy, patient B, stomach content | NT | 1 | Positive |
| 867 | Autopsy, patient B, small intestine | NT | 1 | Positive |
| 869 | Autopsy, patient S, stomach content | NT | 1 | Positive |
| 11227a | Minced beef heart | NT | 1 | Positive |
| 650 | Autopsy patient B, small intestine | —b | 2 | Negative |
| 11227 b | Minced beef heart | NT | 2 | Negative |
| 18006 | Meat dish Wilderertöpfchen | —b | Not tested | |
| 648 | Autopsy, patient B, duodenum | NT | 4 | Negative |
| 652 | Autopsy, patient S, duodenum | NT | 4 | Negative |
| 653 | Autopsy, patient S, jejunum | NTc | 4 | Negative |
| 654 | Autopsy, patient S, colon | NT | 4 | Negative |
NT, strain(s) not typeable in two laboratories using the standard PFGE protocol. Isolates in bold were not typeable with either the standard protocol, PIV plus 37% formaldehyde, or PIV plus 7 M urea in parallel (third laboratory).
—, isolate not analyzed by PFGE.
Isolate 653 was not typeable via PFGE in two laboratories but yielded weak bands in the third laboratory in the parallel run of PIV, PIV plus 37% formaldehyde, and PIV plus 7 M urea.
Ribotyping was carried out as described by Grimont and Grimont (7). Briefly, clostridia were grown anaerobically overnight in brain heart infusion broth (Unipath, Ltd.). DNA was isolated by the guanidium thiocyanate method of Pitcher et al. (15), including the modifications described by Björkroth and Korkeala (2). Five micrograms of DNA was cleaved with EcoRI (Qbiogene, Heidelberg, Germany) in accordance with the manufacturer's instructions. DNA concentrations were determined with a UV spectrophotometer (UV/VIS spectrometer Lambda 2; Perkin-Elmer, Norwalk, Conn.). DNA fragments were separated in 0.8% agarose gels (24 h, 25 V). Digoxigenin-labeled phage lambda DNA (Roche Diagnostics, Mannheim, Germany) was used as a molecular size marker. DNA fragments were Southern blotted (18) to a nylon membrane (Roche Diagnostics). DNA was fixed at 180°C for 0.5 h, prehybridized for 2 to 4 h in a 58°C water bath, and hybridized overnight at 58°C; the rest of the procedure was done in accordance with the instructions supplied with the Roche Diagnostics digoxigenin DNA labeling and detection kit. The DNA probe was prepared from Escherichia coli 16S and 23S rRNA (Roche Diagnostics) in accordance with the instructions supplied with the kit. The pattern was read visually.
PFGE was first carried out as described recently (8, 13, 19) and repeated after ribotyping with the standard buffer plus two modifications. After being cultured overnight in brain heart infusion broth, cells were harvested by centrifugation (1,000 × g, 4°C, 15 min) and resuspended in parallel in 600 μl of pure PIV buffer (10 mM Tris, 1 M NaCl, pH 7.6), PIV buffer with formaldehyde (10 mM Tris, 1 M NaCl, 3.7% formaldehyde) as previously reported (10), and PIV buffer with urea (10 mM Tris, 1 M NaCl, 7 M urea). Restriction of chromosomal DNA with SmaI (New England Biolabs, Beverly, Mass.) followed. Electrophoresis was performed in a 1% pulsed-field agarose gel (Bio-Rad, Hercules, Calif.; dissolved in 0.5× Tris-borate-EDTA) in the Gene NavigatorÔ System (Pharmacia Biotech, Uppsala, Sweden) at 200 V. The electrophoresis time was 21 h with initial and final switching times of 1 and 30 s. The gel was stained in ethidium bromide solution (0.5 μg ml−1 in 0.5× Tris-borate-EDTA) and analyzed visually with the Gel Doc 1000 imaging system (Bio-Rad).
The CPE-producing capability of the isolates was tested with a commercially available CPE reverse passive latex agglutination test kit (PET-RPLA; Oxoid, Basingstoke, United Kingdom) in accordance with the manufacturer's recommendations.
PFGE was carried out with all 17 strains in two different laboratories with the standard PIV buffer (19) without interpretable results. After ribotyping of all 17 strains, PFGE was repeated in the third laboratory with four isolates (653, 730, 11227a, and 11227b) representing three different ribotypes by using in parallel the standard PIV buffer procedure (19) and two further nuclease-inhibiting modifications with 3.7% formaldehyde and 7 M urea, respectively (Table 1).
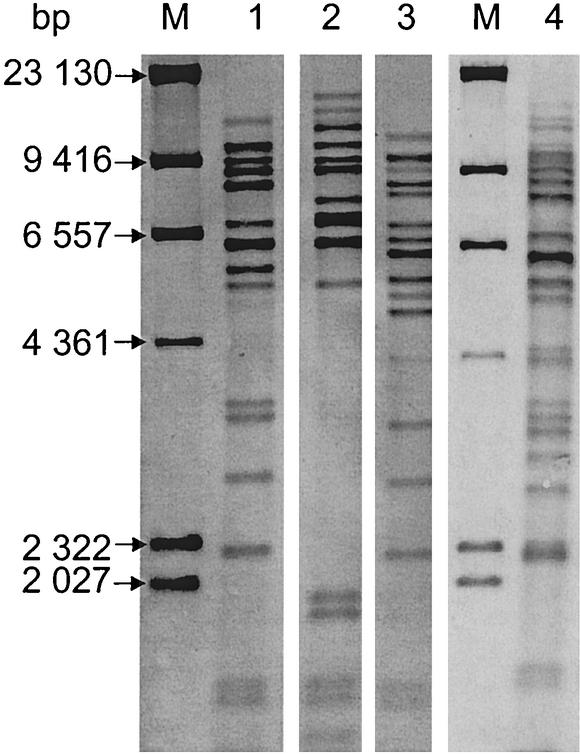
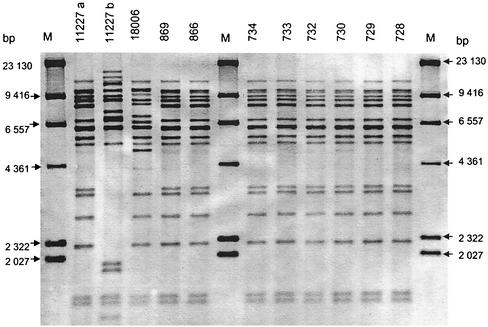
The results of ribotyping and PFGE done with the three different DNA preparation methods are listed in Table 1 together with the results of CPE detection. As shown in Fig. 1, four distinct ribotype patterns were detected among the 17 C. perfringens isolates. The results of one membrane with the ribopatterns of 11 C. perfringens isolates possibly involved in the outbreak are shown in Fig. 2.
FIG. 1.
Four ribopatterns (lanes 1 to 4) found among 17 C. perfringens isolates possibly involved in a food-borne disease outbreak. The isolates' numbers and origins are given in Table 1. Lanes M contained molecular size markers.
FIG. 2.
Ribopatterns, on one membrane (lanes 1 to 3), of 11 of 17 C. perfringens isolates possibly involved in a food-borne disease outbreak. The isolates' numbers and origins are given in Table 1. Lanes M contained molecular size markers.
Table 1 shows that six C. perfringens isolates (no. 728, 729, 730, 732, 733, and 734) from patients' stool samples, three isolates from the two deceased patients' autopsy material (no. 866, 867, and 869), and one food isolate from minced beef heart (no. 11227a) belonged to ribopattern 1. Ribopattern 2 was detected twice, in minced beef heart (no. 11227 b) and in patient B's autopsy material (no. 650). Ribopattern 3 was detected only once, in a meat dish called Wilderertöpfchen (no. 18006). Four C. perfringens isolates derived from autopsy materials from patients B and S (no. 648, 652, 653, and 654) belonged to ribopattern 4.
PFGE of all 17 strains in two laboratories produced no interpretable results. After ribotyping of all 17 strains, PFGE was repeated in the third laboratory with four isolates (no. 653, 730, 11227a, and 11227b) representing three different ribotypes and using in parallel the standard PIV buffer procedure (19) and two further nuclease-inhibiting modifications. Only one C. perfringens isolate (no. 653), representing ribotype 4, gave a weakly visible pattern with all three protocols. The other three isolates showed degraded DNA on all lanes far beyond the 15-kb band of the marker.
The ribotyping results obtained suggest that all 17 of the C. perfringens isolates tested could, theoretically, have been involved in the outbreak. Three C. perfringens isolates with three different ribopatterns were detected in foods. Ribopattern 1 (Table 1 and Fig. 1) was detected in isolates that most probably represent the outbreak, as it was found in minced beef heart, six stool samples from different patients, and various autopsy materials of the two deceased patients. These findings, together with the positive CPE results, give a strong hint that the food isolate with pattern 1 was responsible for the outbreak. Ribopattern 2 was also detected in the minced beef heart isolate but only in one autopsy isolate from one patient. Furthermore, these two isolates were CPE negative. Therefore, these C. perfringens isolates can be classified as not involved in the outbreak.
Ribopattern 3 was exclusively detected in the meat dish Wilderertöpfchen. It is unlikely to be involved in the outbreak, because it was not found in any isolate originating from the patients. Ribopattern 4 was detected in four isolates of autopsy material from the two deceased patients. It is remarkable that these two individuals carried isolates with this same ribopattern. Nevertheless, no obvious connection with the outbreak can be stated and the CPE result was also negative.
As PFGE gave no interpretable results, the use of another DNA-based typing technique would be helpful in evaluating the present results of these isolates.
When ribotyping and PFGE are compared in this context, it is remarkable that 1 single C. perfringens isolate (no. 653) of 17 yielded results after repeated PFGE in three different laboratories. This shows that the use of PFGE, which is regarded as superior to ribotyping, can imply difficulties in the investigation of the genetic relationships of certain isolates involved in epidemic situations. It can be concluded that the current protocols for restriction fragment length polymorphism-PFGE seem to be not necessarily applicable to all strains of C. perfringens.
Besides the standard PFGE protocol, two special nuclease-inhibiting procedures were used in our study. Neither of them could differentiate all of the isolates beyond the results obtained with the standard protocol. This shows, in accordance with many researchers who have reported problems with Clostridium sp. DNA degradation (1, 8, 9, 10, 16, 19), that modified PFGE protocols with improved protection against endonucleases activity need to be developed. However, few results concerning the PFGE typeability of C. perfringens strains involved in food-borne diseases have been published (16; Klein et al., Proc. 37th Arbeitstagung Arbeitsgebietes Lebensmittelhyg.). The results of this study show that PFGE with the current standard protocols is insufficient for reliable differentiation of C. perfringens isolates from epidemiological investigations, e.g., in food-borne disease outbreaks, and that ribotyping is still a helpful tool for the characterization of certain isolates.
Acknowledgments
We thank P. Graf, Department of Health and Environment, Municipal Authority, Munich, Germany, and H. Beck, Department of Health Service, South Bavaria, Oberschleissheim, Germany, for providing data concerning the outbreak and isolates.
REFERENCES
- 1.Bidet, P., V. Lalande, B. Salauze, B. Burghoffer, V. Avesami, M. Delmee, A. Rossier, F. Barbut, and J. C. Petit. 2000. Comparison of PCR-ribotyping, arbitrarily primed PCR, and pulsed-field gel electrophoresis for typing Clostridium difficile. J. Clin. Microbiol. 38:2484-2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Björkroth, J., and H. Korkeala. 1996. rRNA gene restriction patterns as a characterization tool for Lactobacillus sake strains producing ropy slime. Int. J. Food Microbiol. 30:293-302. [DOI] [PubMed] [Google Scholar]
- 3.Duncan, C. L., D. H. Strong, and M. Sebald. 1972. Sporulation and enterotoxin production by mutants of Clostridium perfringens. J. Bacteriol. 110:378-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eisgruber, H., M. Wiedmann, and A. Stolle. 1995. Use of plasmid profiling as a typing method for epidemiologically related Clostridium perfringens isolates from food poisoning cases and outbreaks. Lett. Appl. Microbiol. 20:290-294. [DOI] [PubMed] [Google Scholar]
- 5.Forsblom, B., A. Palmu, P. Hirvonen, and H. Jousimiessomer. 1995. Ribotyping of Clostridum perfringens. Clin. Infect. Dis. 20(Suppl. 2):323-324. [DOI] [PubMed] [Google Scholar]
- 6.Graf, P., and L. Bader. 2000. Gastroenteritis-Ausbruch durch Clostridium perfringens. Epidemiol. Bull. 41:327-329. [Google Scholar]
- 7.Grimont, F., and P. A. D. Grimont. 1986. Ribosomal ribonucleic acid gene restriction patterns as potential taxonomic tools. Ann. Inst. Pasteur/Microbiol. (Paris) 137:165-175. [DOI] [PubMed] [Google Scholar]
- 8.Hielm, S., J. Björkroth, E. Hyytiä, and H. Korkeala. 1998. Genomic analysis of Clostridium botulinum group II by pulsed-field gel electrophoresis. Appl. Environ. Microbiol. 64:703-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kato, H., N. Kato, K. Watanabe, T. Yamamotot, K. Suzuki, S. Ishigo, S. Kunihiro, K. Nakamura, G. E. Killigore, and S. Nakamura. 2001. Analysis of Clostridium difficile isolates from nosocomial outbreaks at three hospitals in diverse areas of Japan. J. Clin. Microbiol. 39:1391-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9a.Klein, G., A. Pack, H. Eisgruber, and A. Stolle. 1996. Mögliche Zusammenhänge zwischen Clostridium perfringens-Isolaten aus Lebensmitteln und Clostridium perfringens Erkrankungen, p. 248-255. In Proceedings der 37. Arbeitstagung des Arbeitsgebietes Lebensmittelhygiene, Garmisch- Partenkirchen, 3.09.-02.10.1996. Arbeitstagung des Arbeitsgebietes Lebensmittelhygiene der Deutschen Veterinärmedizinischen Gesellschaft, Giessen, Germany.
- 10.Kristijansson, M., M. H. Samore, D. N. Gerding, P. C. DeGirolami, K. M. Bettin, A. W. Karchmer, and R. D. Arbeit. 1994. Comparison of restriction endonuclease analysis, ribotyping, and pulsed-field gel electrophoresis for molecular differentiation of Clostridium difficile strains. J. Clin. Microbiol. 32:1963-1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahony, D. E., G. A. Clark, M. F. Stringer, M. C. MacDonald, D. R. Duchesne, and J. A. Marder. 1986. Rapid extraction of plasmids from Clostridium perfringens. Appl. Environ. Microbiol. 51:521-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maslanka, S. E., J. G. Kerr, G. Willians, J. M. Barbaree, L. A. Carson, J. M. Miller, and B. Swaminathan. 1999. Molecular subtyping of Clostridium perfringens by pulsed-field gel electrophoresis to facilitate food-borne-disease outbreak investigations. J. Clin. Microbiol. 37:2209-2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matushek, M. G., M. J. M. Bonten, and M. K. Hayden. 1996. Rapid preparation of bacterial DNA for pulsed-field gel electrophoresis. J. Clin. Microbiol. 34:2598-2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDonel, J. L. 1979. The molecular mode of action of Clostridium perfringens enterotoxin. Am. J. Clin. Nutr. 32:210-218. [DOI] [PubMed] [Google Scholar]
- 15.Pitcher, D. G., N. A. Saunders, and R. J. Owen. 1989. Rapid extraction of bacterial genomic DNA with guanidium thiocyanate. Lett. Appl. Microbiol. 8:151-156. [Google Scholar]
- 16.Schalch, B., B. Sperner, H. Eisgruber, and A. Stolle. 1999. Molecular methods for the analysis of Clostridium perfringens relevant to food hygiene. FEMS Immunol. Med. Microbiol. 24:281-286. [DOI] [PubMed] [Google Scholar]
- 17.Songer, J. G. 1996. Clostridial enteric diseases of domestic animals. Clin. Microbiol. Rev. 9:216-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Southern, E. M. 1975. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J. Mol. Biol. 98:503-517. [DOI] [PubMed] [Google Scholar]
- 19.Sperner, B., B. Schalch, H. Eisgruber, and A. Stolle. 1999. Short protocol for pulsed-field gel electrophoresis of a variety of Clostridium species. FEMS Immunol. Med. Microbiol. 24:287-292. [DOI] [PubMed] [Google Scholar]
- 20.Wada, A., Y. Masuda, M. Fukayama, T. Hatakeyama, Y. Yanagawa, H. Watanabe, and T. Inamatusu. 1996. Nosocomial diarrhea in the elderly due to enterotoxigenic Clostridium perfringens. Microbiol. Immunol. 40:767-771. [DOI] [PubMed] [Google Scholar]