Abstract
Apophysomyces elegans was considered a rare but medically important zygomycete. We analyzed the clinical records of eight patients from a single center in whom zygomycosis due to A. elegans was diagnosed over a span of 25 months. We also attempted a DNA-based method for rapid identification of the fungi and looked for interstrain polymorphism using microsattelite primers. Three patients had cutaneous and subcutaneous infections, three had isolated renal involvement, one had rhino-orbital tissue infection, and the final patient had a disseminated infection involving the spleen and kidney. Underlying illnesses were found in two patients, one with diabetes mellitus and the other with chronic alcoholism. A history of traumatic implantation was available for three patients. All except two of the patients responded to surgical and/or medical therapy; the diagnosis for the two exceptions was made at the terminal stage of infection. Restriction enzyme (MboI, MspI, HinfI) digestion of the PCR-amplified internal transcribed spacer region helped with the rapid and specific identification of A. elegans. The strains could be divided into two groups according to their patterns, with clustering into one pattern obtained by using microsatellite [(GTG)5 and (GAC)5] PCR fingerprinting. The study highlights the epidemiology, clinical spectrum, and diagnosis of emerging A. elegans infections.
Zygomycosis is a serious and often rapidly fatal infection especially in immunocompromised patients. It is caused by sparsely septate filamentous, saprophytic fungi belonging to the class Zygomycetes and the order Mucorales. The species of the genera Absidia, Rhizopus, Rhizomucor, Mucor, Apophysomyces, Saksenaea, Cunnighamella, Cokeromyces, and Syncephalastrum have been reported to cause invasive infections. However, species of the genera Rhizopus, Absidia, and Rhizomucor are the more commonly reported pathogens (10, 19, 31). Apophysomyces elegans is a relatively newer agent in this order, being first isolated from the soil in India in 1979 (23). It has been a rather infrequent causative agent of zygomycosis, and about 26 cases have been reported so far, mostly from cutaneous and subcutaneous infections (12, 13, 15, 28, 30). In our center we diagnosed eight cases of zygomycosis due to A. elegans between August 1999 and September 2001. This report highlights the sudden increase in cases due to this uncommon pathogen in India.
MATERIALS AND METHODS
Patients.
All patients diagnosed with a zygomycosis due to A. elegans from August 1999 through September 2001 at Nehru Hospital, which is a tertiary-care center with major superspecialties of medical sciences and which is affiliated with the Postgraduate Institute of Medical Education and Research, Chandigarh, India, were included in the present study. Zygomycosis was suspected on the basis of clinical and/or radiological findings. The diagnosis was established by direct microscopic evidence of broad, aseptate, or sparsely septate ribbon-like hyphae with right-angled branching in stained sections of tissue specimens or aspirated pus and culture isolation of A. elegans. The detailed clinical history of each patient including presentation, site of involvement, underlying illness, risk factors, diagnosis, and outcome of therapy was analyzed.
Fungal strains.
Nineteen zygomycete isolates, as detailed in Table 1, were included in the present study. All strains were stored at −80°C in our Mycology Culture Collection Laboratory (MCCL).
TABLE 1.
Fungal strains used for molecular study
| Fungi | No. of strains | Source | Straina |
|---|---|---|---|
| Apophysomyces elegans | 8b | Human | MCCL 102008, MCCL 102009, MCCL 102012, MCCL 102014, MCCL 102015, MCCL 102017, MCCL 102018, MCCL 102019 |
| Apophysomyces elegans | 3c | Human | MCCL 102001, MCCL 102002, MCCL 102005 |
| Apophysomyces elegans | 1 | Dolphin | CDC B5656 |
| Absidia corymbifera | 2 | Human | MCCL 700003, CDC B 5792 (MCCL 700002) |
| Saksenaea vasiformis | 1 | Human | MCCL 111004 |
| Rhizopus arrhizus | 1 | Human | MCCL 710010 |
| Mucor circinelloides | 1 | Air | MCCL 690001 (MTCC 1294) |
| Basidiobolus ranarum | 1 | Human | MCCL W6 |
| Rhizomucor pusillus | 1 | Environment | MCCL 720006 |
MCCL, Mycology Culture Collection Laboratory; CDC, Centers for Disease Control and Prevention; MTCC, Microbial Type Culture Collection, Institute of Microbial Technology, Chandigarh, India.
From the present series.
Isolated earlier.
Isolation of whole-cell DNA.
Whole-cell DNA from each isolate was extracted with the Scotlab Nucleon kit (Scotlab Bioscience, Coatbridge, United Kingdom) according to the direction of the manufacturer. Each isolate was grown on Sabouraud dextrose agar slopes for 3 to 5 days. Mycelia from the slope were reinoculated in Sabouraud dextrose broth and incubated at 30°C in a rotary shaker (150 rpm) for 7 days. The mycelial mat was recovered by filtration and washed with normal saline. About 0.2 to 0.3 g of the mycelial mat was placed in a clean mortar, liquid nitrogen was added, and the mat was ground quickly with a pestle to make a fine powder. The resultant powder was then transferred to a clean sterile 1.5-ml microcentrifuge tube, and 340 μl of reagent B (400 mM Tris-HCl [pH 8.0], 60 mM EDTA, 150 mM NaCl, 1% sodium dodecyl sulfate) was added to the ground material. Then, 100 μl of 5 M sodium perchlorate was added and the tube was incubated at 65°C for 20 min with occasional shaking. Cold (−20°C) chloroform (580 μl) was added to the tube, and the tube was incubated for 20 min at room temperature. The tube was then spun and the upper layer was removed and placed in a clean and fresh tube. This aqueous layer was treated twice with phenol-chloroform-isoamylalcohol (25:24:1), and finally, the DNA was precipitated with cold absolute alcohol. The pellet was washed with 70% alcohol, treated with 100 μl of TER (10 mM Tris HCl [pH 7.5], 1 mM EDTA, 50 μg of RNase per ml) at 37°C for 2 h, reprecipitated with absolute alcohol, and finally, dissolved in 100 μl of TE (10 mM Tris HCl [pH 7.5], 1 mM EDTA) and stored at −20°C for further use.
PCR.
The internal transcribed spacer (ITS) regions were amplified with primers pITS1F (TCCGTAGGTGAACCTGCGG) and pITS4R (TCCTCCGCTTATTGATATGC), whose sequences were designed from the conserved regions of the 18S and 26S rRNA genes, respectively (36). The primers were obtained from Integrated DNA Technologies, Inc., Coralville, Iowa. The PCRs were performed in a final reaction mixture (50 μl) containing 50 ng of genomic DNA; 25 pmol of each primer (pITS1F and pITS4R); 200 mM each dATP, dTTP, dGTP, and dCTP (Promega Corporation, Madison, Wis.); 2.5 mM MgCl2; 2.0 U of Taq polymerase (Promega); and 5 μl of 10× reaction buffer (Promega). The amplification reactions were performed in a PTS 100 Mini Cycler (MJ Research, Waltham, Mass.) with the following cycling parameters: for amplification of the ITS region, initial denaturation for 5 min at 94°C, followed by 30 cycles of 30 s at 94°C, 30 s at 55°C, and 1.0 min at 72°C, with a final extension for 10 min at 72°C and cooling to 4°C. The amplified products were separated on a 1.2% agarose (Sisco Research Laboratories, Mumbai, Maharashtra, India) gel by electrophoresis and visualized by staining with ethidium bromide (0.5 μg/ml). Gel photographs were taken with a VDS Image Master (Pharmacia Biotech, Piscataway, N.J.).
Restriction fragment length polymorphism (RFLP) analysis.
The restriction enzymes MboI, MspI, HinfI, BamHI, and BglII were used for digestion of the amplified products of the ITS regions. The restriction mixture (30 μl), which contained 3 μl of 10× buffer, 1 μl (10 to 12 U) of restriction enzymes, 10 μl of amplified product, and 16 μl of double-distilled water, was incubated at 37°C for 3 h; and 15 μl of each digested product was electrophoresed in a 1.5% agarose gel for 12 h. The bands were visualized by ethidium bromide staining for 1 h in the dark and destaining in 1× TAE (Tris-acetate-EDTA) buffer for 1 h. The bands were photographed with a VDS Image Master (Pharmacia Biotech).
Microsatellite fingerprinting.
For microsatellite fingerprinting, a single primer, pMS1 [(GTG)5] or pMS2 [(GAC)5], was used; and all other components of the PCR mixture were same as those used for amplification of the ITS regions. The following cycling parameters were used for microsatellite fingerprinting: initial denaturation for 5 min at 94°C, followed by 40 cycles of 1 min at 94°C, 2 min at 45°C (for pMS1) or 55°C (for pMS2), and 3 min at 72°C, with a final extension for 10 min at 72°C and cooling to 4°C. The amplified product was separated, visualized, and photographed as described above.
RESULTS
The clinical and laboratory details for the eight patients with zygomycosis due to A. elegans are presented in Table 2. Three patients had cutaneous and subcutaneous infections, three had isolated renal tissue involvement, one had rhino-orbital tissue infection due to A. elegans, and one had a possible extension of infection from subcutaneous tissue to the kidney and spleen. Underlying illness could be determined in two patients: one had diabetes mellitus and the other was a chronic alcoholic. A history of trauma as a risk factor was available for three patients; two had zygomycosis affecting the intramuscular injection sites and the third patient had a history of a blunt injury on his back 5 months earlier. All except two patients responded well to surgical and/or medical therapy; the diagnoses for the two exceptions were made very late in the course of infection, and the patients succumbed to their illness before proper therapy could be instituted.
TABLE 2.
Clinical and laboratory details for patients with zygomycosis due to A. elegansa
| Patient no. | Mo and yr of isolation | Age (yr)/sex | Strain isolated no. (MCCL) | Patient presentation | Underlying illness | Risk factor | Radiologic findings | Diagnosis | Therapy | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | August 1999 | 65/M | 102008 | Progressive painful cutaneous swelling with blackish discoloration (12 by 15 cm) in rt. lumbar region (5 days) | Nil | Unknown | Not done | Histopathology and culture of described tissue | Local debridement and amphotericin B (50 mg/day) | LAMA after 6 days of therapy |
| 2 | August 1999 | 20/F | 102009 | Pain, redness, swelling, and protrusion of rt. eye along with rhinitis, headache, and fever (7 days) | Nil | Unknown | On CT, a mass extending across the nasal septum, displacing the rt. orbital tissue with retro-orbital extension | Histopathology and culture of debrided tissue | Local debridement and exteneration of rt. side and amphotericin B (total dose, 1.5 g) | Recovery after 1 mo of therapy |
| 3 | December 1999 | 35/M | 102012 | Abdominal pain in both flanks, dysuria and pyuria (3 mo), fever (1 day) | Chronic alcoholic | Unknown | On USG, a bilateral hydronephrosis and rt. pyonephrosis | Microscopy of pus drained by pig tail, catheter from upper calyx of it. kidney and culture | Percutaneous nephrostomy of both kidneys and amphotericin B (total dose, 1.8 g) | Recovery after 1.5 mo of therapy |
| 4 | March 2000 | 10/M | 102014 | Fever, bilateral flank pain and pyuria (2 mo); Escherichia coli was grown twice from urine, but the infection was adequately treated with antibiotics | Nil | Unknown | On USG, bilateral multiple hypodense cortical lesions, pyonephrosis, and rt. hydronephrosis; on CT, acute pyelonephritis and an abscess in both kidneys | Microscopy of ultrasound-guided pus drained from lt. kidney and culture | Surgical drainage of pus and amphotericin B (total dose, 1.06 g) | Recovery |
| 5 | September 2000 | 45/M | 102015 | Fever, weight loss, and pain in lt. flank; progressively increasing red patch (14 by 12 cm) on the same area (1 mo); dyspnea (10 days) | Nil | H/O trauma (a brick wall, fell on his back) 5 mo earlier | On USG, multiple splenic abscesses with loculated lt. pleural effusion; on CT, collapse of lt. lung, pleural effusion, infracts in lower pole of spleen and upper pole of lt. kidney | Microscopy of ultrasound-guided aspirate material from spleen and histopathology of debrided material from superficial area of lt. flank and culture | Local debridement and amphotericin B (0.5 mg/kg/day) | Death within 1 day of therapy |
| 6 | October 2000 | 25/F | 102017 | Progressive cutaneous swelling with black discoloration on rt. gluteal region and fever (25 days) | Diabetes mellitus | i. m. injection at same site (6 days prior) | Not done | Histopathology and culture of described tissue | Local debridement, antibiotic, and no antifungal | Recovery within 15 days |
| 7 | August 2001 | 28/M | 102018 | Swelling and pain on rt. gluteal region (35 days), unremitting fever (1 mo), diarrhea, vomiting (10 days) | Nil | i. m. injection earlier at same site (7 days prior) | Not done | Histopathology and culture of described tissue | Local debridement and itraconazole (400 mg/day × 1 mo) | Recovery within 40 days |
| 8 | September 2001 | 70/M | 102019 | Fever (1.5 mo), jaundice, pain in lt. flank (1 mo), abdominal distension and pedal edema (15 days) | Nil | Unknown | On chest X ray, lt. pleural effusion; on USG and CT, a perinephric abscess, lt. pyonephrosis and renal vein thrombosis | Microscopy of drained pus from retroperitoneal area and culture; at partial medical autospy, the lt. kidney had infraction, abscesses, and necrosis; ulcerated pelvis; thrombosed and occluded smaller vein and arteries; broad aseptate hyphae in necrosed area including blood vessels | Local debridement | Death before initiation of therapy |
Abbreviations: lt., left; rt., right; LAMA, left against medical advice; i. m., intramuscular; USG, ultrasonography; CT, computerized tomography; F, female; M, male; H/O, history of.
Fast-growing, creamy white, and cottony colonies were grown from biopsy tissue, aspirated material, or pus from all eight patients. Microscopically, the growth presented as broad, hyaline, infrequently septate, thin-walled hyphae without sporulation. Five 2-cm-square agar blocks of mycelial growth on Sabouraud dextrose agar were cut aseptically from the culture of each isolate and transferred to plates containing 20 ml of distilled water and 0.2 ml of 10% filter-sterilized yeast extract. The plates were incubated at 37°C in the dark for 7 days (27). Numerous sporangia with prominent apophyses borne at the tips of sporangiophores were seen. Sporangiophores generally developed singly, arising at the ends of stolon-like hyphae, and were dark grayish brown and thick walled below the apophyses. The apophyses were dark, campanulate, or champagne glass shaped. Sporangia were borne at the tips of sporangiophores and were pyriform and multispored. The sporangiospores were oblong, subhyaline, and smooth and measured 5 to 8 by 4 to 5 μm. The isolates grew well up to 42°C and were identified as A. elegans. They are deposited in the Postgraduate Institute of Medical Education and Research of MCCL in Chandigarh as isolates MCCL 102008, MCCL 102009, MCCL 102012, MCCL 102014, MCCL 102015, MCCL 102017, MCCL 102018, and MCCL 102019.
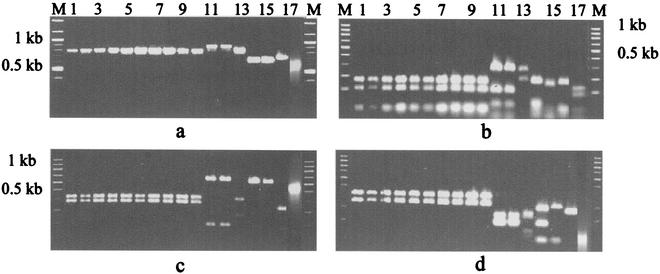
A. elegans strains were analyzed by amplification of the ITS region. The primers amplified a 760-bp fragment from all A. elegans isolates, whereas the same set of primers amplified an 860-bp fragment of Absidia corymbifera, an 800-bp fragment of Saksenaea vasiformis, a 620-bp fragment Rhizopus arrhizus, a 630-bp fragment of Rhizomucor pusillus, a 680-bp fragment of Basidiobolus ranarum, and a 590-bp fragment of Mucor circinelloides (Fig. 1a). The specificity of the 760-bp fragment was further confirmed by restriction enzyme (MboI, MspI, HinfI) digestion, which showed patterns distinct from those for the other zygomycetes evaluated (Fig. 1b to d). The results for strains MCCL 102018 and MCCL 102019 are not shown in Fig. 1, as those two strains were isolated later. When those strains were analyzed, a 760-bp fragment was amplified from both strains and their RFLP patterns were exactly the same as those for the other A. elegans strains tested. There was no restriction site for restriction enzymes BamHI and BglII in a 760-bp fragment of the A. elegans isolates (data not shown).
FIG. 1.
(a) PCR amplification of ITS regions of different zygomycetes. (b) Restriction digestion patterns of products amplified from the ITS region after digestion with MboI. (c) Restriction digestion patterns of products amplified from the ITS region after digestion with MspI. (d) Restriction digestion patterns of products amplified from the ITS region after digestion with HinfI. (a to d) Lanes: M, marker; 1, A. elegans (CDC B5656); 2 to 10, A. elegans isolated from clinical samples (strains MCCL 102001, MCCL 102002, MCCL 102005, MCCL 102008, MCCL 102009, MCCL 102014, MCCL 102015, MCCL 102017, and MCCL 102012, respectively); 11, A. corymbifera (CDC 5792); 12, A. corymbifera (MCCL 700003); 13, S. vasiformis (MCCL 1110004); 14, R. arrhizus (MCCL 710010); 15, R. pusillus (MCCL 720006); 16, B. ranarum (MCCL W6); and 17, M. circinelloides (MCCL 690001).
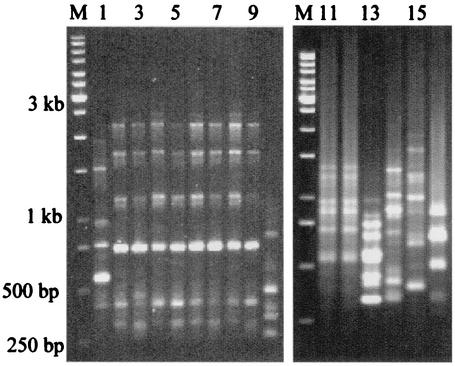
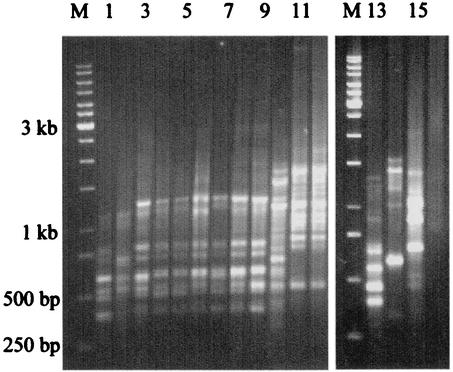
Only two patterns were seen among all Indian A. elegans isolates by PCR fingerprinting with repetitive primers (GTG)5, and (GAC)5, and both these patterns were different from that for the isolate from the Centers for Disease Control and Prevention, Atlanta, Ga. (Fig. 2 and 3). Ten Indian strains had similar patterns. However, the strain isolated from a patient with renal zygomycosis in December 1999 was distinctly different from those of the other A. elegans isolates with both primers. The PCR fingerprinting patterns of the A. elegans isolates further differentiated those strains from other zygomycetes.
FIG. 2.
PCR fingerprinting with microsatellite oligonucleotide primer (GTG)5 of different zygomycetes. Lanes: M, marker; 1, A. elegans (CDC B5656); 2 to 10, A. elegans isolated from clinical samples (strains MCCL 102001, MCCL 102002, MCCL 102005, MCCL 102008, MCCL 102009, MCCL 102014, MCCL 102015, MCCL 102017, and MCCL 102012, respectively); 11, A. corymbifera (CDC 5792); 12, A. corymbifera (MCCL 700003); 13, S. vasiformis (MCCL 1110004); 14, R. arrhizus (MCCL 710010); 15, R. pusillus (MCCL 720006); and 16, B. ranarum (MCCL W6).
FIG. 3.
PCR fingerprinting with microsatellite oligonucleotide primer (GAC)5 of different zygomycetes. Lanes: M, marker; 1, A. elegans (CDC B5656); 2 to 10, A. elegans isolated from clinical samples (strains MCCL 102001, MCCL 102002, MCCL 102005, MCCL 102008, MCCL 102009, MCCL 102014, MCCL 102015, MCCL 102017, and MCCL 102012, respectively); 11, A. corymbifera (CDC 5792); 12, A. corymbifera (MCCL 700003); 13, S. vasiformis (MCCL 1110004); 14, R. arrhizus (MCCL 710010); 15, R. pusillus (MCCL 720006); and 16, B. ranarum (MCCL W6).
DISCUSSION
A. elegans was first isolated by Misra et al. (23) in 1979 from soil samples collected from a mango orchard in northern India. Subsequently, this agent was isolated from soil samples and samples of air filter dust in north Australia in association with human infections (8, 32). Its distribution in tropical and subtropical climates is further substantiated by the occurrence of most human cases in such climates (3, 5-7, 9, 11, 14, 16, 17, 20, 22, 24, 25, 29, 34, 35). However, infection due to A. elegans was considered rare, as about 26 cases have been reported to date (12, 13, 15, 28, 30). Interestingly, eight patients with A. elegans infection were reported from MCCL from 1990 to 1999, and the agent made up 32% of all zygomycetes isolated during the same period (4). The present report of eight cases from August 1999 to September 2001 (25 months) from a single center emphasizes further that A. elegans is possibly an emerging zygomycete in this part of the world. However, in general, a lack of awareness about fungal infections in most centers of developing countries underestimates its importance. Although all our patients came to the hospital with infection acquired in the community or other health centers, an attempt was made during an earlier study from our center to isolate A. elegans from the hospital environment. A. elegans could not be isolated from the hospital premises (4). Nevertheless, a thorough environmental sampling of the community and the hospital environment may help provide a further understanding of the epidemiology of this infection.
A. elegans is known to cause cutaneous, subcutaneous, and soft tissue infections following trauma, burns, or invasive procedures in apparently healthy hosts (1, 8, 9, 21, 24, 25, 29, 34). However, in the present series, besides four (50%) cases of cutaneous or subcutaneous tissue involvement, three (32%) patients had definite renal involvement due to A. elegans. In addition, one patient had a possible renal infection. In a previous study we reported on one patient with renal zygomycosis due to A. elegans (7). One patient in the present series had rhino-orbitocerebral infection with A. elegans, and four similar cases were reported earlier (6, 12, 13, 29). Thus, it can reasonably be concluded that A. elegans infection is not restricted only to cutaneous or subcutaneous sites; it can frequently cause renal and rhino-orbitocerebral infections.
By far, A. elegans most commonly causes infections in apparently healthy individuals (3, 7, 20, 29, 30, 32, 35). In the present series, similarly, only two patients had underlying conditions (diabetes mellitus and chronic alcoholism). Infection of cutaneous and subcutaneous tissues with A. elegans is predominantly the result of the introduction of spore-containing soil and vegetation into wounds arising from trauma or surgery (16, 20), burns (8), injection (5), or an insect bite (35). In two of our patients with subcutaneous tissue involvement, the infection occurred at the injection site, and in another patient the infection was possibly acquired from a site of trauma. However, the route of infection in renal tissue is not known; except that it may have been due to possible contiguous spread in one patient. Similarly, we could not ascertain the route of infection in our patient with renal zygomycosis due to A. elegans that we reported on earlier (7). In rhino-orbitocerebral zygomycosis, the infection probably resulted from inhalation of spores into the sinus.
Aggressive management including surgical intervention with or without medical therapy saved most of the patients with A. elegans infections (30). Surgical removal of infected tissue provides a more definitive treatment, while the response to amphotericin B treatment is variable at best. All except two of our patients responded well to therapy; the diagnoses for the two exceptions were made late in the course of infection. For superficial zygomycosis, the importance of appropriate surgical intervention is stressed further by the outcomes for one patient in the present series and another patient reported on earlier (5), who recovered only after surgical debridement of the lesion.
Although excellent mycelial growth is seen on standard culture media, A. elegans, unlike other zygomycetes, does not readily produce asexual spores. A special nutrient-deficient growth medium, a high temperature of incubation, and prolonged incubation can be used to induce A. elegans isolates to sporulate. Padhye and Ajello (27) demonstrated a simpler method of sporulation in water with yeast extract at 37°C after 7 to 10 days of incubation, and Lombardi et al. (18) developed a rapid exoantigen test for specific identification of this fungus. Still, given this problem of delay in identification, DNA-based molecular typing techniques show enormous potential for rapid and accurate identification of the etiological agents of infections caused by some of the zygomycetes (26). Recently, 13 taxon-specific PCR primer pairs that specifically amplify DNA for most commonly reported zygomycetes were designed by using aligned 28S rRNA gene sequences (33). In the present study we found that a simple molecular method for identification of A. elegans was use of the MboI, MspI, or HinfI restriction enzyme patterns of PCR-amplified ITS regions of ribosomal DNA. This method specifically distinguished this fungus from A. corymbifera, S. vasiformis, R. arrhizus, R. pusillus, M. circinelloides, and B. ranarum. This method is so useful that when we used this technique with all isolates of zygomycetes stored in our culture collection, two strains that had been misidentified earlier as A. elegans were correctly identified as A. corymbifera (data not shown).
For strain typing, although sequencing of suitable sites and development of an ideal probe may help mostly in strain differentiation, we initially attempted to type our A. elegans strains by RFLP analysis of the ITS region, but that method failed to differentiate the strains. Subsequently, we used microsattelite primers pMS1 [(GTG)5] and pMS2 [(GAC)5] to demonstrate interstrain polymorphisms among A. elegans strains. Microsatellite oligonucleotide primers have been used for molecular fingerprinting of Saccharomyces cerevisiae strains (2). Although this method has shown two patterns for our A. elegans strains and those patterns were distinct from that for an unrelated strain from the United States, the method is not very discriminatory, as 10 strains isolated at different times had same pattern. At present, we are sequencing the ITS spacer region to find a species-specific signature sequence that may be useful for the rapid identification of A. elegans and related species.
Thus, the present series highlights the importance of emerging A. elegans infections, A. elegans-infected host characteristics, the newer clinical spectrum, the need for rapid diagnosis, and treatment outcomes. Molecular identification of A. elegans, although expensive compared to the costs of available conventional procedures, especially in a developing country like India, may be performed in reference laboratories for accurate identification of this fungus.
Acknowledgments
We thank P. Roy and S. Ghoshal Sushmita for helping us to prepare the manuscript and the Indian Council of Medical Research for partial financial assistance.
REFERENCES
- 1.Adam, R. D., G. Hunter, J. DiTomasso, and G. Comerci, Jr. 1994. Mucormycosis; emerging prominence of cutaneous infections. Clin. Infect. Dis. 19:67-76. [DOI] [PubMed] [Google Scholar]
- 2.Balerias Conto, M. M., B. Eijsma, H. Hotstra, J. H. Huis in't Veld, and J. M. van der Vassen. 1996. Evaluation of molecular typing techniques to assign genetic diversity among strains of Saccharomyces cerevisiae. Appl. Environ. Microbiol. 62:41-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caceres, A. M., C. Sardina, C. Marcano, R. Guevara, J. Barros, G. Bianchi, V. Rosario, R. Balza, M. Silva, M. C. Redondo, and M. Nunez. 1997. Apophysomyces elegans limb infection with a favorable outcome: case report and review. Clin. Infect. Dis. 25:331-332. [DOI] [PubMed] [Google Scholar]
- 4.Chakrabarti, A., A. Das, A. Sharma, N. Panda, S. Das, K. L. Gupta, and V. Sakhuja. 2001. Ten years' experience in zygomycosis at a tertiary care centre in India. J. Infect. 42:261-266. [DOI] [PubMed] [Google Scholar]
- 5.Chakrabarti, A., P. Kumar, A. A. Padhye, L. Chatha, S. K. Singh, A. Das, J. D. Wig, and R. N. Kataria. 1997. Primary cutaneous zygomycosis due to Saksenaea vasiformis and Apophysomyces elegans. Clin. Infect. Dis. 24:580-583. [DOI] [PubMed] [Google Scholar]
- 6.Chakrabarti, A., N. Panda, S. C. Varma, K. Singh, A. Das, S. C. Sharma, and A. A. Padhye. 1997. Craniofacial zygomycosis caused by Apophysomyces elegans. Mycoses 40:419-421. [DOI] [PubMed] [Google Scholar]
- 7.Chugh, K. S., A. A. Padhye, A. Chakrabarti, V. Sakhuja, K. L. Gupta, and P. Kathuria. 1996. Renal zygomycosis in otherwise healthy hosts. J. Mycol. Med. 6:22-25. [Google Scholar]
- 8.Cooter, R. D., I. S. Lim, D. H. Ellis, and I. O. W. Leitch. 1990. Burn wound zygomycosis caused by Apophysomyces elegans. J. Clin. Microbiol. 28:2151-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eaton, M. E., A. A. Padhye, D. A. Schwartz, and J. P. Steinberg. 1994. Osteomyelitis of the sternum caused by Apophysomyces elegans. J. Clin. Microbiol. 32:2827-2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edwards, J. E. 1980. Clinical aspects of mucormycosis. Ann. Intern. Med. 93:96-99. [Google Scholar]
- 11.Ellis, J. J., and L. Ajello. 1982. An unusual source for Apophysomyces elegans and a method for stimulating sporulation of Saksenaea vasiformis. Mycologia 74:144-145. [Google Scholar]
- 12.Fairley, C., T. J. Sullivan, P. Bartley, T. Allworth, and R. Lewandowski. 2000. Survival after rhino-orbito-cerebral mucormycosis in an immunocompetent patient. Ophthalmology 107:555-558. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Covarrubias, L., R. Barlett, D. M. Barratt, and R. J. Wassermannn. 2001. Rhino-orbito-cerebral mucormycosis attributable to Apophysomyces elegans in an immunocompromised individual: case report and review of literature. J. Trauma 50:353-357. [DOI] [PubMed] [Google Scholar]
- 14.Huffnagle, K. E., P. M. Southern, C. T. Byrd, and R. M. Gander. 1992. Apophysomyces elegans as an agent of zygomycosis is a patient following trauma. J. Med. Vet. Mycol. 30:83-86. [DOI] [PubMed] [Google Scholar]
- 15.Kimura, M., M. B. Smith, and M. R. McGinnis. 1999. Zygomycosis due to Apophysomyces elegans: report of 2 cases and review of the literature. Arch. Pathol. Lab. Med. 123:386-390. [DOI] [PubMed] [Google Scholar]
- 16.Lakshmi, V., T. Suda Rani, S. Sharma, V. S. Mohan, C. Sundaram, R. R. Rao, and G. Satyanarayana. 1993. Zygomycotic necrotising fasciitis caused by Apophysomyces elegans. J. Clin. Microbiol. 31:1368-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lawrence, R. M., W. T. Snodgrass, G. Reichel, A. A. Padhye, L. Ajello, and F. W. Chandler. 1986. Systemic zygomycosis caused by Apophysomyces elegans. J. Med. Vet. Mycol. 24:57-65. [PubMed] [Google Scholar]
- 18.Lombardi, G., A. A. Padhye, P. G. Standard, L. Kaufman, and L. Ajello. 1989. Exoantigen tests for rapid and specific identification of Apophysomyces elegans and Saksenaea vasiformis. J. Med. Vet. Mycol. 27:113-120. [PubMed] [Google Scholar]
- 19.Marchevsky, A. M., E. J. Bottone, S. A. Geller, and D. K. Giger. 1980. The changing spectrum of disease, etiology and diagnosis of mucormycosis. Hum. Pathol. 11:457-464. [DOI] [PubMed] [Google Scholar]
- 20.Mathews, M. S., A. Raman, and A. Nair. 1997. Nosocomial zygomycotic post-surgical necrotising fasciitis in a healthy adult caused by Apophysomyces elegans in south India. J. Med. Vet. Mycol. 35:61-63. [DOI] [PubMed] [Google Scholar]
- 21.McGinnis, M. R., J. Mindez, L. Pasarell, and A. Haque. 1993. Necrotizing fasciitis caused by Apophysomyces elegans. J. Mycol. Med. 3:175-179. [Google Scholar]
- 22.Meis, J. F. G. M., B. J. Kullberg, M. Pruszczynski, and R. P. H. Veth. 1994. Severe osteomyelitis due to the zygomycete Apophysomyces elegans. J. Clin. Microbiol. 32:3078-3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Misra, P. C., K. J. Srivastava, and K. Lata. 1979. Apophysomyces, a new genus of the Mucorales. Mycotaxon 8:377-382. [Google Scholar]
- 24.Naguib, M. T., M. M. Huycke, J. A. Pederson, L. R. Pennington, M. E. Burton, and R. A. Greenfield. 1995. Apophysomyces elegans infection in a renal transplant recipient. Am. J. Kidney Dis. 26:381-384. [DOI] [PubMed] [Google Scholar]
- 25.Okhuysen, P. C., J. H. Rex, M. Kapusta, and C. Fife. 1994. Successful treatment of extensive post traumatic soft-tissue and renal infections due to Apophysomyces elegans. Clin. Infect. Dis. 19:329-331. [DOI] [PubMed] [Google Scholar]
- 26.Oliver, M. R., W. C. Van Voorhis, M. Boeckh, D. Mattson, and R. A. Bowden. 1996. Hepatic mucormycosis in a bone marrow transplant recipient who ingested naturopathic medicine. Clin. Infect. Dis. 22:521-524. [DOI] [PubMed] [Google Scholar]
- 27.Padhye, A. A., and L. Ajello. 1988. Simple method of inducing sporulation by Apophysomyces elegans and Saksenaea vasiformis. J. Clin. Microbiol. 26:1862-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Page, R., D. J. Gardam, and C. H. Heath. 2001. Severe cutaneous mucormycosis (zygomycosis) due to Apophysomyces elegans. Aust. N. Z. J. Surg. 71:184-186. [DOI] [PubMed] [Google Scholar]
- 29.Radner, A. B., M. D. Witt, and J. E. Edwards. 1995. Acute invasive rhinocerebral zygomycosis in an otherwise healthy patient: case report and review. Clin. Infect. Dis. 20:163-166. [DOI] [PubMed] [Google Scholar]
- 30.Ribes, J. A., C. L. Vanover-Sams, and D. J. Baker. 2000. Zygomycetes in human disease. Clin. Microbiol. Rev. 13:236-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rinaldi, M. G. 1989. Zygomycosis. Infect. Dis. Clin. N. Am. 3:19-41. [PubMed] [Google Scholar]
- 32.Sedralis, T., S. Krishnan, and J. Holland. 1997. Martini glass mucormycosis: Apophysomyces elegans infection in an immunocompetent host. Aust. J. Otolaryngol. 2:600. [Google Scholar]
- 33.Voigt, K., E. Cigelnik, and K. O'Donnell. 1999. Phylogeny and PCR identification of clinically important zygomycetes based on nuclear ribosomal DNA sequence data. J. Clin. Microbiol. 37:3957-3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiden, M. A., K. K. Steinbronn, A. A. Padhye, L. Ajello, and F. W. Chandler. 1985. Zygomycosis caused by Apophysomyces elegans. J. Clin. Microbiol. 22:522-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weinberg, W. G., B. H. Wade, G. Cierny III, D. Stacy, and M. G. Rinaldi. 1993. Invasive infection due to Apophysomyces elegans in immunocompetent hosts. Clin. Infect. Dis. 17:881-884. [DOI] [PubMed] [Google Scholar]
- 36.White, T. J., T. Burns, S. Lee, and J. W. Taylor. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p. 315-322. In M. A. Iunis, D. H. Geffand, J. J. Suinsky, and T. J. White (ed.), PCR protocols: a guide to methods and applications. Academic Press, Inc., San Diego, Calif.