Abstract
Dmrt1 is a recently described gene that is expressed exclusively in the testis and is required for postnatal testis differentiation. Here we describe the expression of Dmrt1 in postnatal rat testis and Sertoli cells. RNase protection analysis was used to examine Dmrt1 messenger RNA (mRNA) levels in intact testis during postnatal development and in primary cultures of Sertoli cells under various culture conditions. We show that Dmrt1 mRNA levels rise significantly beginning approximately 10 days after birth and remain elevated until after the third postnatal week. Thereafter, mRNA levels drop coincident with the proliferation of germ cells in the testis. In freshly isolated Sertoli cells, Dmrt1 mRNA levels were robust but decreased significantly when the cells were placed in culture for 24 h. Treatment of Sertoli cells with either FSH or 8-bromo-cAMP resulted in a significant rise in Dmrt1 mRNA levels. This cAMP response was sensitive to treatment with the transcriptional inhibitor actinomycin D but not to the translational inhibitor cycloheximide. The cAMP-dependent rise in Dmrt1 mRNA also required activation of protein kinase A, as mRNA induction was sensitive to the inhibitor H89. Studies also show that Dmrt1 expression was inhibited by phorbol esters (PMA) but only modestly effected by serum.
IN MAMMALS, the gonad develops from the genital ridge, a bipotential primordium that has the capacity to form either an ovary or a testis, a decision ultimately determined by the chromosomal composition of the individual (reviewed in Ref. 1). Under normal circumstances, the presence of a Y chromosome induces formation of a testis, which, in turn, directs subsequent sexual differentiation programs down the male pathway (2). A single gene on the Y chromosome, Sry, is responsible for initiating events that direct formation of the testis and thus male sexual differentiation, whereas its absence results in development of ovaries and female sexual differentiation (3–5). In addition to Sry, several other genes important for gonadogenesis and sex determination have been identified. These include the genes encoding SF-1, Dax1, WT1, Lim1 Emx2, and SOX9 (6–11). However, despite progress in understanding the mechanisms controlling gonad development and sex determination, only a minority of human XY sex reversal cases can be attributed to currently identified genes. In particular, deletions in chromosomes 9p and 10q have been associated with 46,XY sex reversal, indicating that these regions contain genes important for testicular development (12, 13).
Recently, a strong candidate for a sex determination gene on chromosome 9 was identified (14–16). This gene, DMRT1, was initially recognized through a database search for proteins containing a unique DNA-binding motif called the DM domain, a motif first revealed as a region of similarity between proteins encoded by the male sexual regulatory genes mab-3 of Caenorhabditis elegans and doublesex of Drosophila melanogaster (16). Identification of this common structural feature suggested that DM-containing proteins play a role in aspects of sex determination that are conserved across phyla and implicated DMRT1 as a candidate sex determination gene. Importantly, chromosome mapping studies revealed that DMRT1 was located within a short interval on chromosome 9 associated with XY sex reversal (14–16).
Characterization of Dmrt1 in different species has provided additional support for its involvement in testis development and sex determination. Thus, Dmrt1 messenger RNA (mRNA) is present only in the gonads in humans and mice and is expressed in a sexually dimorphic manner during gonadal development in mammals, birds, and reptiles (14, 17, 18). In mice, Dmrt1 mRNA was present only in the genital ridge of early XX and XY embryos (14). However, as development proceeded, Dmrt1 expression was almost entirely restricted to the XY gonad, where it was found in Sertoli cells and primordial germs cells of the seminiferous cords (14, 19). While studies have predominantly focused on Dmrt1 in the embryo, investigations in humans and mice revealed testis-specific expression in the adult, with no detectable ovarian expression (16, 19).
A role for Dmrt1 in testis differentiation was recently confirmed in mice by studies demonstrating severe testicular defects upon ablation of the gene (20). Like humans missing portions of chromosome 9, mice lacking Dmrt1 had a failure in testis differentiation accompanied by germ cell death. In addition, the defects observed from the Dmrt1 deletion were male specific but only apparent in postnatal animals. Further analysis of the Dmrt1−/− animals revealed that the germ cells failed to enter meiosis and that the Sertoli cells over proliferated, failed to adopt a differentiated phenotype, and eventually died (20). The observed phenotype in the Dmrt1−/− mice was consistent with a role for Dmrt1 in the differentiation of Sertoli cells. These cells play a vital role in the development and function of the testis. In the embryo, Sertoli cells, under the direction of Sry, are the first somatic cells to differentiate in the gonad and are thought to orchestrate subsequent events in testis formation and sex determination (1, 21–25). Sertoli cells also play a critical role in testis function after birth, as they support the differentiation of germ cells into viable sperm. During early postnatal and pubertal development, Sertoli cells exhibit remarkable structural and functional changes that are required for the initiation and maintenance of spermatogenesis (26). Many of these changes are regulated by hormones and growth factors, one of which is the pituitary glycoprotein hormone FSH. This hormone acts specifically on Sertoli cells and causes a number of biochemical changes that impact proliferation and maturation of this cell (27–29). Identifying the molecular events that are required for these observed changes in Sertoli cell and testis function will extend our understanding of the factors necessary for spermatogenesis and fertility.
Here we describe the expression of Dmrt1 mRNA in the postnatal testis and Sertoli cells and its regulation by FSH, 8-bromo-cAMP, and phorbol 12-myristate 13-acetate (PMA). We have found that Dmrt1 expression depends on the post-natal age of the animal and correlates with important functional changes in the testis after birth. In cultured Sertoli cells, Dmrt1 mRNA levels rose dramatically in response to FSH stimulation, suggesting it is responsible for regulating some of the effects of this hormone. In contrast, PMA reduced Dmrt1 mRNA levels and blocked induction by 8-bromo-cAMP.
Materials and Methods
Cell preparation
Sertoli cells were cultured as described previously except 2.5% FBS was used in place of 5% FBS (30, 31). Unless indicated, 40 h after plating, cells were treated with a hypotonic solution of 10 mm Tris (7.4) for 2 min to remove germ cells. Following hypotonic shock, cells were fed media containing 2.5% FBS. Approximately 12 h later, cells were re-fed with media lacking FBS and treatments were initiated 4 h later. Treatments were added to the cultures as follows; 8-bromo-cAMP (1 mm), human recombinant FSH (150 ng/ml), PMA (10−7 m), FBS (15%). PMA was resuspended in dimethyl sulfoxide (DMSO) and in experiments with PMA, DMSO, was added to control samples (vehicle). For PMA treatment, cells were cultured in the absence of added serum before the addition of PMA. For studies using PMA alone, cells were serum-starved for 16 h before treatment. For PMA studies done in conjunction with cAMP, cells were serum starved for 3 h before the addition of treatment. For treatment with the PKA inhibitor H89 cells were pretreated with 15 μm H89 for 40 min and then treated with 8-bromo-cAMP for the indicated amount of time. A similar treatment paradigm was used for PD98059 (50 μm, 1 h pretreatment), wortmannin (1 μm, 1 h pretreatment), and SB203580 (1 μm, 30 min pretreatment). Cycloheximide was added at a concentration of 50 μg/ml and added to media 30 min before the addition of cAMP or FSH. Actinomycin D was added at a final concentration of 5 μg/ml with a pretreatment time of 30 min.
Peritubular myoid cells, a testicular cell type that surrounds the seminiferous tubules, were obtained from the supernatant fraction after tubules from Sertoli cell preparations were sedimented following collagenase treatment. Peritubular myoid cells were cultured in Ham’s F12 media supplemented with 10% FBS and antibiotics. Culture conditions for MSC-1 cells, a mouse Sertoli cell line, are described elsewhere (30, 31). TM4 cells, also a Sertoli cell line, were isolated from two different sources, one of which was purchased from the American Type Culture Collection [ATCC (Manassas, VA) no. CRL-1715 (32)], and cultured according to ATCC recommendations.
RNase protection assay
Total RNA was isolated from cells and tissues using TRIZOL reagent according to manufacturer’s procedures (Life Technologies, Inc., Grand Island, NY). A Dmrt1 5′RACE complementary DNA (cDNA) subclone containing a 151- bp fragment beginning 500-bp downstream from the translational start site was cloned into the HindIII and SalI sites of pGEM4Z. Antisense RNA probe was generated by linearizing the plasmid with EcoRI followed by in vitro transcription using T7 polymerase according to the manufacturer’s recommendations (Promega Corp., Madison, WI). This generated a 227 base probe that protects 151 bases of Dmrt1 mRNA. The actin RNA probe was generated by in vitro transcription with T7 polymerase and the pTR1-β-actin template from Ambion, Inc. (Austin, TX) linearized with EcoRI. This generates a 188 base transcript that protects 126 bases of actin mRNA. The actin probe was generated to have approximately 50-fold lower specific activity than the Dmrt1 probe. An antisense probe for c-fos was generated by digestion of the cDNA with EcoRI to linearize the plasmid and in vitro transcription using T7 polymerase according to the manufacturer’s recommendations (Promega Corp., Madison, WI).
The RNase protection assay was adapted from a previous published protocol (33). Unless otherwise noted, assays were performed using 15 μg of total RNA. Briefly, RNA samples were precipitated, air dried, and resuspended in 30 μl of hybridization buffer [40 mm Pipes (6.4), 1 mm EDTA (pH 8.0), 0.4 m NaCl, 80% formamide] containing 5 × 105 cpm Dmrt1 probe and 1 × 104 cpm β-actin probe. Samples were incubated at 85 C for 10 min and then to 45 C overnight. Samples were cooled to room temperature and 300 μl of RNase digestion buffer (10 mm Tris (7.5), 300 mm NaCl, 5 mm EDTA, 900 U/ml RNase T1) was added and incubated for 1 h at 30 C. Next, 20 μl of 10% SDS and 10 μl Proteinase K (10 mg/ml) were added to each sample and incubated for 30 min at 37 C. Samples were extracted with 400 μl of phenol:chloroform and precipitated. RNA pellets were resuspended in 10 μl formamide loading buffer (80% formamide, 10 mm EDTA, 1 mg/ml xylene cyanol, 1 mg/ml bromophenol blue), heated to 95 C for 5 min, loaded on a 5% denaturing gel, and run at 250 V for 2.25 h. Samples were visualized by autoradiography. For quantitative analysis, optical densities from the autoradiograms were quantified using Gel-Pro Analyzer image analysis software (Media Cybernetics, Baltimore, MD). Optical densities for Dmrt1 and c-fos were normalized to those of actin and the relative densitometric units reported.
DNA clones
Complementary DNA was generated using Superscript reverse transcriptase (Life Technologies, Inc., Grand Island, NY), 0.25 μg oligo-dT, and 2 μg of total RNA isolated from rat testes. The cDNA was used as a template in a PCR with Dmrt1 primers selected from alignment of the mouse and human Dmrt1 cDNA sequences (14, 16, 19). The upstream primer, Dmrt1.5b 5′-CCCAAGCTTCCATGCCGAACGACGA-3′ contained an introduced HindIII site at the 5′ end (underlined) whereas the downstream primer, Dmrt1.11 5′-CCCGTCGACCTTGCAGATGG-TAGTC-3′ contained an introduced SalI site at the 5′ end (underlined) to facilitate cloning. Amplified DNA was digested with HindIII and SalI and cloned into these sites in pGEM4Z.
The rat c-fos cDNA clone was amplified from cDNA generated as described above using RNA isolated from rat Sertoli cells stimulated with cAMP. Primers used for amplification were; upstream rat-c-fos1 5′-ACTCCCCACCCCGTCGACC and downstream primer rat-c-fos2 CCCCAAGCTTGCTCCCTCCTCCGATTCCG. The amplified product was digested with SalI (internal site) and HindIII (introduced into the primer) and cloned into the respective sites in pGEM4Z.
Results
Cloning of the rat Dmrt1 cDNA
The cDNA for rat Dmrt1 was cloned by RT-PCR using primers selected by alignment of the human, mouse, and chicken cDNA sequences (14) Fig. 1A). Comparison of the putative amino acid sequence of rat with that of pig, human, mouse, chicken, and a teleost fish (tilapia) revealed several regions having high sequence conservation (Fig. 1B, shaded regions). Consistent with previous reports that compared Dmrt1 sequence between different vertebrate species and that of mab-3 and dsx, the highest level of conservation was within the DM domain of the proteins (14, 16). In addition, two other regions (Fig. 1B, regions 1 and 2) showed significant sequence conservation. Although the DM-domain has a proposed role in DNA binding, the functions of the other conserved regions are unknown. Interestingly, region 2 was recently proposed to represent a male-specific motif as it was absent in an ovarian DM-domain cDNA from talapia that has significant sequence similarity to Dmrt1 (34).
FIG. 1.
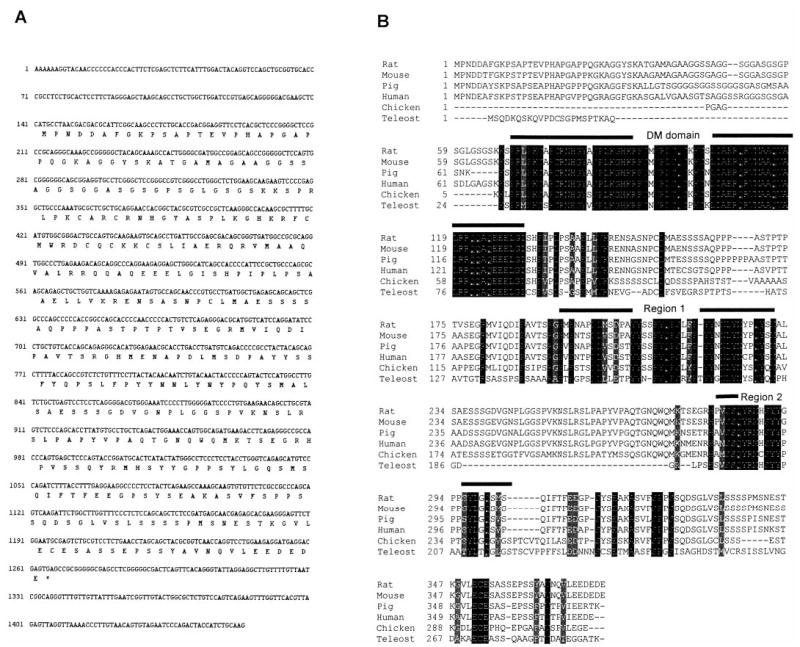
Sequence of rat Dmrt1 cDNA. A, DNA and putative protein sequence of the rat Dmrt1 cDNA cloned from primary cultures of Sertoli cells. B, Alignment of the rat mouse, pig, human, chicken, and fish (teleost) Dmrt1 proteins. The DNA binding domain (DM domain) and two other conserved regions (Regions 1 and 2) are indicated by bars over the sequence.
Dmrt1 mRNA levels depend on the age of the postnatal testis
Although Dmrt1 expression has been described in developing embryo, little is known about its expression the post-natal testis. After birth, the testis undergoes significant structural and functional changes, where it develops from an immature organ with underdeveloped cords and undifferentiated germ cells into one that produces viable sperm and steroid hormones. To help determine if Dmrt1 plays a role in the regulation of postnatal testis function and development, we examined its expression at different times after birth. Using RNase protection analysis (RPA), we measured its mRNA levels in testes of rats ranging in age from embryonic day 18 (E18) to postnatal day 60 (p60). In addition to Dmrt1, actin mRNA levels were similarly measured to account for nonspecific changes in mRNA. Dmrt1 expression was similar in testes from embryonic day 18 and postnatal day 5 rats and showed a marked increase (approximately 9-fold) by post-natal day 10 (Fig. 2). Note that the low actin signal at p5 was an abnormality with this gel and additional studies confirmed the relative change in Dmrt1 mRNA from p5 to p10. Thereafter, mRNA levels increase slightly (~1.5×) from p10 to p15 and were similar between p15 and p20. However, as the age of the animal increased from p20 to p40 there was a large drop (~5×) in Dmrt1 transcript levels.
FIG. 2.
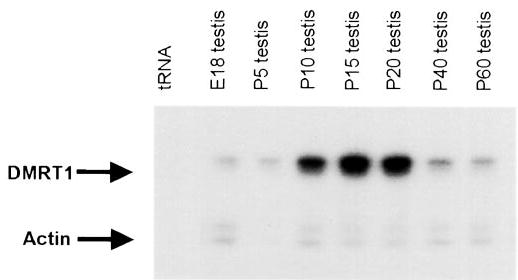
Dmrt1 mRNA levels change during postnatal development of the testes. RNA was isolated from testes of different aged rats, including E18 (embryonic day 18) and postnatal days 5 (P5), 10 (P10), 15 (P15), 20 (P20), 40 (P40), and 60 (P60). Fifteen micrograms of total testis RNA was assayed for both Dmrt1 and actin mRNA levels using RNase protection analysis with approximately 5 × 105 cpm of the Dmrt1 antisense probe and 1 × 104 cpm of the antisense actin probe (see Materials and Methods). Following overnight hybridization and digestion with RNase the samples were run on a 5% denaturing gel to resolve protected fragments of 151 bp (Dmrt1) and 126 bp (actin). Arrows mark the protected fragments. Transfer RNA (tRNA) is added as a negative control for the formation of protected fragments.
Dmrt1 is expressed in primary cultures of Sertoli cells but not in Sertoli cell lines MSC-1 and TM4
Previous studies using in situ hybridization observed Dmrt1 mRNA within the seminiferous tubules of the testis and identified both Sertoli cells and germ cells as sites of Dmrt1 expression (14, 19). To explore the mechanisms regulating Dmrt1 in the testis, we have evaluated its expression in primary cultures of Sertoli cells and two Sertoli cell lines, MSC-1 and TM4. Sertoli cells were isolated from 15-day-old rats and cultured for various times and under different conditions. Total RNA was isolated and Dmrt1 mRNA levels, together with that of actin, were assayed by RPA. RNA isolated from Sertoli cells before being placed in culture had significantly higher levels of Dmrt1 mRNA than RNA isolated from cells placed in culture for 24 h (Fig. 3A, compare fresh d15 SC and 24 h culture). Notably, actin mRNA levels did not change significantly. An additional, less substantial, decrease in Dmrt1 mRNA was observed when the cells were cultured for an additional 24 h (Fig. 3A, 48 h culture), whereas levels were unchanged between 48 and 72 h of culture (Fig. 3A). In addition, Dmrt1 mRNA levels were not significantly influenced by treatment of the cultures with an isotonic shock (+) to remove any contaminating germ cells (Fig. 3A, compare 48 h culture and 48 h culture +). Peritubule myoid cells, testicular cells that surround the seminiferous epithelium, were negative for Dmrt1 mRNA.
FIG. 3.
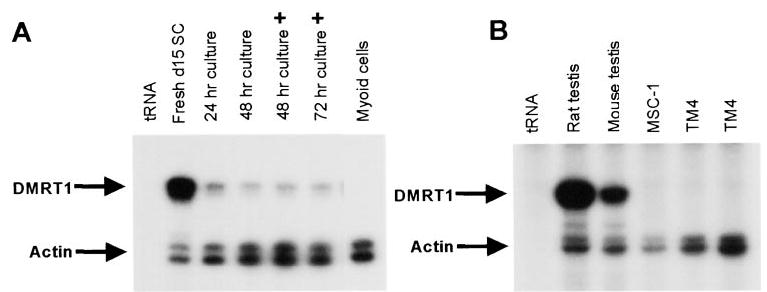
Dmrt1 mRNA is expressed in testicular Sertoli cells. Dmrt1 and actin mRNA levels were measured by RPA as described in Fig. 2. A, Sertoli cells were isolated from testes of 15 day-old rats as described in Materials and Methods and RNA was isolated either immediately following completion of the preparation (Fresh d15 SC) or after various times in culture (24, 48, or 72 h) and in cells that either did (+) or did not receive a hypotonic shock to remove contaminating germ cells. RNA isolated from myoid cells, a testicular cell type that surrounds the seminiferous epithelium, was also assayed for Dmrt1. Dmrt1 and actin mRNA levels were also measured in intact tubule fragments either immediately following isolation (fresh d15 tubulutes) or after 24 h in culture (cultures tubules 24 h). B, Dmrt1 and acting mRNAs were measured by RPA in intact rat (20 days of age) and mouse (25 days of age) testes and in two mouse Sertoli cell lines, MSC-1 and TM4. Two separate isolates of the TM4 cell line were characterized. Arrows mark the protected fragments. tRNA was added as a negative control for the formation of protected fragments.
In addition to primary cultures of Sertoli cells, RNA was isolated from two different mouse Sertoli cell lines, TM4 and MSC-1, and analyzed for Dmrt1 transcripts (32, 35, 36). Dmrt1 mRNA was not detected in either MSC-1 cells or TM4 cells prepared from two different sources (Fig. 3B). Longer exposures also failed to detect Dmrt1 transcripts in these cells. The inability to observe Dmrt1 mRNA in MSC-1 and TM4 cells cannot be explained by failure of the rat probe to cross-hybridize with mouse Dmrt1 mRNA, as Dmrt1 message was clearly observed in RNA isolated from mouse testes (Fig. 3B).
8-bromo-cAMP and FSH induce expression of Dmrt1 mRNA
The decrease in Dmrt1 mRNA when cells were placed in culture suggested factors important for Dmrt1 expression are lost when Sertoli cells are removed from their natural environment. In addition, the temporal rise in Dmrt1 mRNA correlates with the increase in the testicular response to pituitary gonadotropins that occurs after birth in the rat (37–40). The pituitary hormone FSH regulates Sertoli cell function by binding a cell-surface receptor and initiating an intracellular signaling cascade that results in increased cAMP production. To determine if Dmrt1 expression was regulated by either FSH or its intracellular second messenger, Sertoli cell cultures were treated with either 8-bromo-cAMP, a cAMP analog, or recombinant human FSH (hFSH) and RNA was collected at various time points and examined for Dmrt1 mRNA by RPA. When Sertoli cells were cultured in the presence of 8-bromo-cAMP, an increase in the steady-state levels of Dmrt1 message was observed as early as 1 h after treatment and reached a peak after 4 h (Figs. 4, A and D). Remarkably, Dmrt1 mRNA levels increased nearly 20-fold when 8-bromo-cAMP was present in the culture media. Treatment of the cells with hFSH resulted in a similar response but mRNA induction was less substantial and not sustained as long as when cells were treated with 8-bromo-cAMP (Figs. 4, B and D).
FIG. 4.
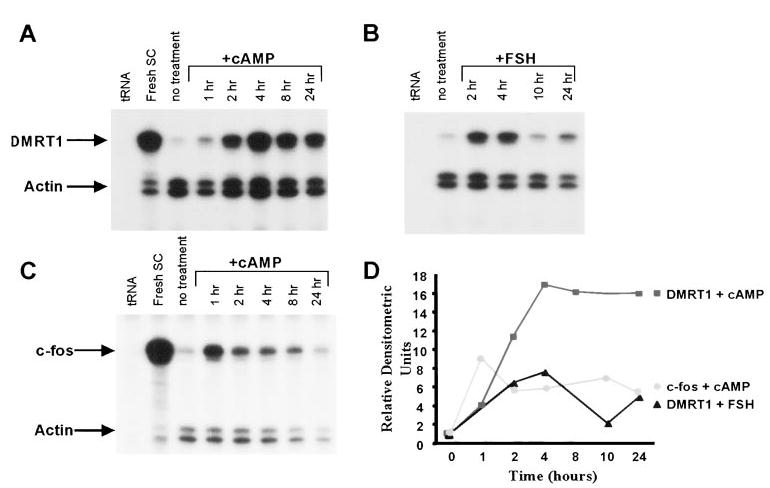
Dmrt1 mRNA is induced by FSH and 8-bromo-cAMP A, Analysis of Dmrt1 and actin mRNA levels in Sertoli cells treated with 8-bromo-cAMP. Sertoli cells were cultured in absence (no treatment) or the presence of 8-bromo-cAMP (1 mm) as described in Materials and Methods and RNA isolated from the cells at the designated times. Dmrt1 and actin mRNAs were measured by RPA as described in the legend for Fig. 2. For the no treatment sample, RNA was isolated at the zero time point. B, Analysis of Dmrt1 and actin mRNA levels in Sertoli cells treated with FSH. Sertoli cells were cultured in the absence (no treatment) or presence of human recombinant FSH (150 ng/ml) for the designated times. RNA was isolated and Dmrt1 and actin mRNA levels were measured by RPA. C, Analysis of c-fos and actin mRNA levels in Sertoli cells treated with 8-bromo-cAMP. c-fos and actin mRNA levels were measured in the RNA samples described in A. For A–C, arrows mark the protected fragments and tRNA and RNA from freshly isolated Sertoli cells were added as negative and positive controls, respectively. D, The optical densities the protected bands in A–C were quantified from the autoradiograms using Gel-Pro Analyzer image analysis software (Media Cybernetics). Optical densities for Dmrt1 and c-fos were normalized to those of actin. The relative densitometric units represent the mRNA/actin ratio from each treatment time point made relative to the mRNA/actin ratio from the no treatment sample.
Previous studies have shown that the protooncogene c-fos is regulated by FSH and cAMP in cultured Sertoli cells and identified it as an immediate-early gene to FSH stimulation (41, 42). Examination of the RNA samples for c-fos transcripts revealed that Dmrt1 and c-fos followed different kinetics in response to 8-bromo-cAMP (Figs. 4, C and D). Thus, Dmrt1 mRNA reached a maximum after 4 h of 8-bromo-cAMP treatment and the induced mRNA levels were maintained for 24 h. In contrast, c-fos mRNA reached a maximum after only 1 h and expression was more transient, as a marked reduction in induced c-fos mRNA was observed after only 2 h.
Induction of Dmrt1 mRNA by cAMP required ongoing transcription but not new protein synthesis
The above studies demonstrated that steady-state levels of Dmrt1 mRNA increased in response to FSH or 8-bromo-cAMP. To better define the mechanism by which cAMP regulates Dmrt1, the translational inhibitor cycloheximide (CHX) and the transcriptional inhibitor actinomycin D (Act D) were evaluated for their impact on cAMP-induced Dmrt1 mRNA. Treatment of Sertoli cells with CHX alone resulted in a small increase in both Dmrt1 and actin mRNA (Fig. 5A). Importantly, CHX had no significant effect on the ability of 8-bromo-cAMP to induce Dmrt1 mRNA, revealing that new protein synthesis was not required for the cAMP-induced increase in Dmrt1 expression (Fig. 5A; compare CHX, cAMP, and cAMP + CHX lanes). Treatment of Sertoli cells with actinomycin D alone resulted in a small decrease in Dmrt1 mRNA relative to that of actin (Fig. 5B). Furthermore, cAMP induction of Dmrt1 mRNA was completely blocked by treatment with actinomycin D, indicating that ongoing transcription is required for cAMP-induced expression of Dmrt1. These studies support the hypothesis that the Dmrt1 gene is a direct downstream target of the FSH signal transduction pathway in the testis.
FIG. 5.
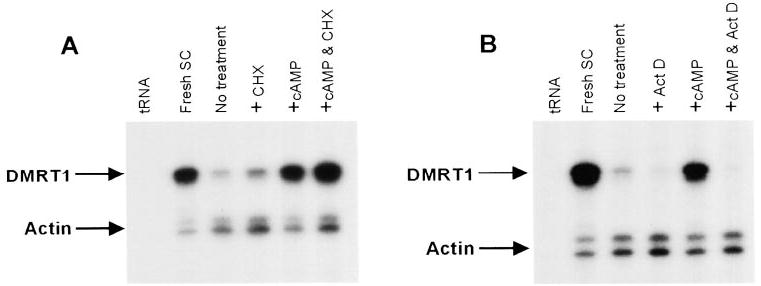
Induction of Dmrt1 by 8-bromo-cAMP requires ongoing transcription but not translation. A, Sertoli cells were treated with the protein synthesis inhibitor cycloheximide in the presence and absence of 8-bromo-cAMP and RNA levels for Dmrt1 and actin were measured by RPA as described in the legend for Fig. 2. Where indicated Sertoli cells were pretreated with cylcohexamide (CHX) for 30 min. The CHX treated cells were either left to incubate an additional 4 h (+CHX) or treated with 8-bromo-cAMP (1 mm) for the same time period (+cAMP & CHX). The other samples included cells cultured in the absence of addition treatments (no treatment) for the same period of time or ones treated with 8-bromo-cAMP alone for 4 h (+cAMP). B, Sertoli cells were treated with the transcriptional inhibitor actinomycin D in the presence and absence of 8-bromo-cAMP and RNA levels for Dmrt1 and actin were measured by RPA. Where indicated Sertoli cells were pretreated with actinomycin D (ActD) for 30 min. The Act D treated cells were either left to incubate an addition 4 h (+ActD) or treated with 8-bromo-cAMP (1 mm) for the same time period (+cAMP & ActD). The other samples included cells cultured in the absence of addition treatments (no treatment) for the same period of time or ones treated with 8-bromo-cAMP alone for 4 h (+cAMP). Arrows mark the protected fragments and tRNA and RNA from freshly isolated Sertoli cells were added as negative and positive controls, respectively.
Activation of protein kinase A is required for cAMP induction of Dmrt1 mRNA
The classic intracellular target of cAMP is cAMP-dependent protein kinase, or protein kinase A (PKA), which is known to mediate many of the events induced by this intracellular second messenger (43). More recently, PKA-independent pathways have been described that mediate response to elevated cAMP (44–47). Of particular interest is a study in rat ovarian Granulosa cells that provides evidence for a PKA-independent pathway stimulated by FSH and cAMP (47). To help distinguish between potential pathways, we employed pharmacological agents that inhibit various signal transduction pathways to determine their impact on cAMP induction of Dmrt1. Sertoli cells were pretreated with the inhibitors for 20–40 min before addition of 8-bromo-cAMP and RNA was isolated from all treatment groups after either one or four hours in the presence of cAMP. By itself, H89, an inhibitor of PKA, had little effect on Dmrt1 mRNA levels after four hours in culture (Fig. 6, compare no treatment and +H89). However, in the presence of cAMP, H89 blocked induction of Dmrt1 mRNA, indicating that PKA activity was necessary for full cAMP induction of Dmrt1 (Fig. 6). In contrast, little or no effect was observed on cAMP induction by inhibitors of PI3 kinase (wortmannin), MEK1/2 (PD98059), or p38 kinase (SB203580, Fig. 6, B and C). Wortmannin did, however, decrease basal levels of Dmrt1 mRNA.
FIG. 6.
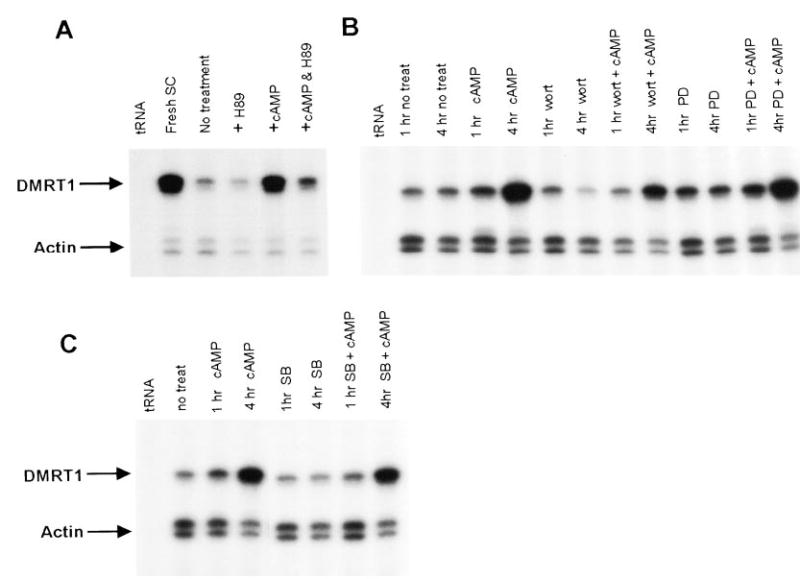
Induction of Dmrt1 mRNA by cAMP requires activation of protein kinase A. Sertoli cells were treated with the inhibitor of protein kinase A, H89, in the presence and absence of 8-bromo-cAMP and RNA assayed for Dmrt1 and actin transcripts by RPA. H89 treated cells were incubated for 4 h in the absence (+H89) or presence 8-bromo-cAMP (+cAMP & H89). Also included are cells cultured in the absence of additional treatments (no treatment) for the same period of time or ones treated with 8-bromo-cAMP alone for 4 h (+cAMP). B, Sertoli cells were treated with the inhibitors for PI3 kinase (wortmannin, 1 μm) and MEK1 (PD98059 50 μm) in the presence and absence of 8-bromo-cAMP and RNA assayed for Dmrt1 and actin transcripts by RPA. Cells were incubated with the inhibitors for either 1 or 4 h in the absence (1 h and 4 h inhibitor) or presence 8-bromo-cAMP (1 h and 4 h inhibitor + cAMP). Also included are cells cultured in the absence of additional treatments (no treatment) for the same period of time or ones treated with 8-bromo-cAMP alone for 1 and 4 h (1 h and 4 h cAMP). C, Sertoli cells were treated with the p38 kinase inhibitor SB203580 (1 μm) in the presence and absence of 8-bromo-cAMP and Dmrt1 and actin mRNA was measured using RPA. Cells were incubated with SB203580 for either 1 or 4 h in the absence (1 h and 4 h SB) or presence 8-bromo-cAMP (1 h and 4 h SB +cAMP). Also included are cells cultured in the absence of additional treatments (no treatment) for the same period of time or ones treated with 8-bromo-cAMP alone for 1 and 4 h 1 h and 4 h cAMP). Arrows mark the protected fragments and tRNA and RNA from freshly isolated Sertoli cells were added as negative and positive controls, respectively.
The phorbol ester PMA decreased Dmrt1 mRNA and blocked induction by cAMP
To further evaluate the signal transduction pathways important for Dmrt1 expression, we examined the potential role of protein kinase C in the regulation of Dmrt1 mRNA. Protein kinase C, like PKA, has been extensively studied for its role in mediating the effects of second messengers, namely di-acylglycerol. Several PKC isoforms, including α, β, and γ, have been described in Sertoli cells but their role in testis function is not well understood (48). Treatment of Sertoli cells with the phorbol ester phorbol 12-myristate 13-acetate (PMA) to activate PKC resulted in a significant decrease in Dmrt1 mRNA (Fig. 7, A and B). A small decrease in mRNA was first observed after treatment of the cells for 1 h and the largest decrease observed 4–8 h posttreatment. The effects of PMA appeared to diminish after twelve hours as a slight rise in mRNA levels was observed. After 24 h of PMA treatment, Dmrt1 mRNA levels were similar to that in the untreated cells (data not shown).
FIG. 7.
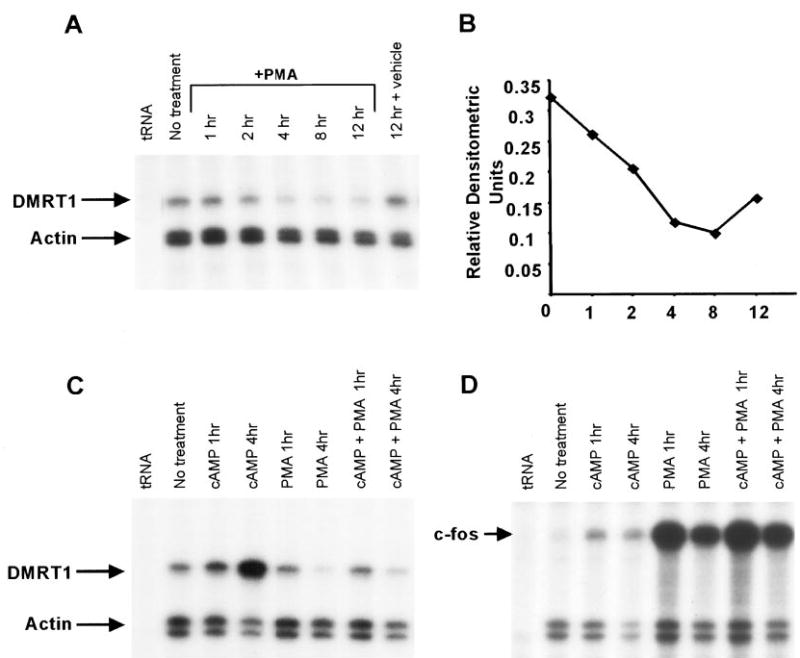
Phorbol 12-myristate 13-acetate (PMA) decreases expression of Dmrt1 and blocks Dmrt1 induction by cAMP. A, Analysis of Dmrt1 and actin mRNA levels in Sertoli cells treated with PMA. Sertoli cells were cultured in absence (no treatment) or the presence of PMA (10−7 m) as described in Materials and Methods. RNA isolated from the cells at the designated times after the addition of PMA. Dmrt1 and actin mRNAs were measured by RPA as described (see Fig. 2 legend). For the no treatment sample, RNA was isolated at the zero time point. B, The optical density the protected fragments for Dmrt1 and actin were quantified from the autoradiogram using Gel-Pro Analyzer image analysis software. The optical density of Dmrt1 was normalized to that for actin and the relative densitometries were plotted in the graph. C, Analysis of Dmrt1 and actin mRNA levels in Sertoli cells treated with PMA and cAMP. Sertoli cells were cultured for 1 and 4 h in the presence of PMA alone (PMA 1 h and 4 h), 8-bromo-cAMP alone (cAMP 1 h and 4 h), or PMA plus 8-bromo-cAMP (cAMP+PMA 1 h and 4 h). The no treatment sample was harvested at the zero hour time point. RNA isolated from the cells and assayed for Dmrt1 and actin. D, Analysis of c-fos and actin mRNA levels in Sertoli cells treated with PMA and cAMP. Samples described in C were assayed for c-fos expression as described above.
To determine the impact of simultaneously activating PKA and PKC, we examined Dmrt1 mRNA levels in Sertoli cells cultured in the presence of both 8-bromo-cAMP and PMA and compared it to levels in cells cultured with each substance alone. After either 1 or 4 h of treatment, RNA was isolated and Dmrt1 mRNA assayed by RPA. As previously observed, 8-bromo-cAMP increased and PMA decreased Dmrt1 mRNA levels (Fig. 7C). Importantly, in the presence of both 8-bromo-cAMP and PMA, little or no induction of Dmrt1 mRNA was observed at the 4-h time point. Thus PMA appeared to block the ability of cAMP to induce Dmrt1, indicating that PKC antagonizes the actions of PKA on Dmrt1 expression. For c-fos, an increase in mRNA was observed when cells were treated with PMA or 8-bromo-cAMP alone and transcript levels remained elevated in the presence of both (Fig. 7D).
To help elucidate the pathways involved in PMA regulation of Dmrt1, we examined the effects of PMA on Dmrt1 expression in the presence and absence of the MAP kinase (MEK1/2) inhibitor PD98059. PD98059 alone had no significant effect on Dmrt1 mRNA levels nor did it affect the ability of cAMP to induce Dmrt1 expression (Fig. 8A). Interestingly, in the presence of both PMA and cAMP, PD98059 was able to partially release the PMA block on cAMP induction of Dmrt1. This indicated that activation of MEK1/2 antagonizes the cAMP effect on Dmrt1 expression. In addition, PD98059 partially blocked the PMA-induced decrease in Dmrt1 message and the PMA-induced increase in c-fos mRNA (~50% for each, Fig. 8, A and B).
FIG. 8.
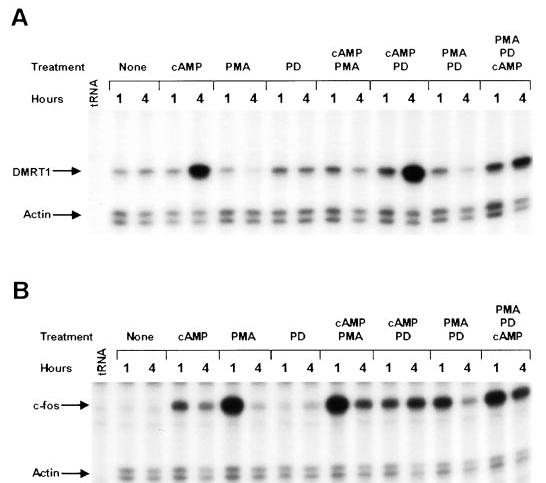
The MAP kinase inhibitor PD98059 releases the PMA-induced block on cAMP activation of Dmrt1. Analysis of Dmrt1, c-fos, and actin mRNA levels in Sertoli cells treated with PMA, cAMP, and the MEK1/2 inhibitor PD98059. Sertoli cells were cultured as described in the material and methods either without any added treatment (None), in the presence of 8-bromo-cAMP, in the presence of PMA, in the presence of the MEK1/2 inhibitor PD98059 (PD) or in the presence of a combination of each of these treatments. Cells were cultured for either 1 or 4 h with each treatment. RNase protection assays were used to examine mRNA levels for Dmrt1 (A) and c-fos (B). Actin mRNA was measured to control for general RNA changes and sample loading. Arrows mark the protected fragments and tRNA represents the negative control.
Serum induces c-fos expression but only modestly affects Dmrt1
The large drop in Dmrt1 and c-fos mRNAs when cells were placed in culture (Figs. 3 and 4) suggested that factors critical for their expression are absent in the cell culture system. Although loss of stimulation from FSH may be responsible for this drop, it seems likely that other factors contribute to this as well. Serum, which was present in only low amounts (2.5%) in the Sertoli cell cultures, contains a number of important growth factors known to influence gene expression. Therefore to determine if serum influences the expression of Dmrt1 or c-fos, cultured Sertoli cells were maintained in serum-free media for 3 h and then stimulated with media containing 15% FBS. RNA was collected at various times after stimulation and RPA was used to examine Dmrt1 and c-fos mRNA. Transcription of the c-fos gene has been shown to be transiently activated in response to either polypeptide mitogens or whole serum (49, 50). In Sertoli cells, c-fos mRNA levels were transiently induced by serum, with the highest mRNA levels observed after 2 h poststimulation (Fig. 9, A and C). By 24 h, c-fos mRNA levels were similar to those observed in unstimulated Sertoli cells (Fig. 9, A and C). In contrast, Dmrt1 mRNA levels were only modestly induced (~1.5×) by serum at the 2-h time point and levels subsequently declined to approximately 60% of the unstimulated levels and remained low for the duration of the experiment. Thus, serum significantly induced c-fos expression but only modestly effected Dmrt1.
FIG. 9.
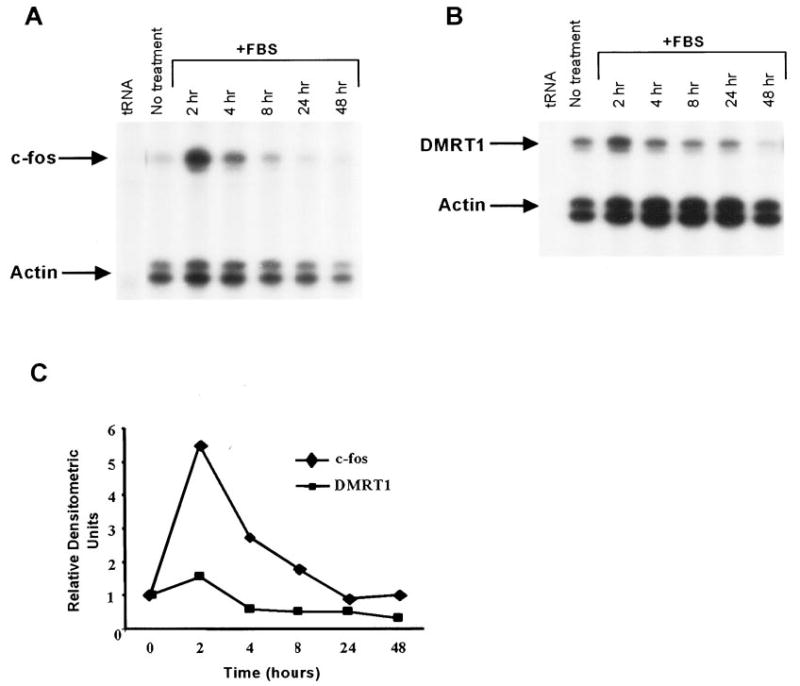
Serum has little impact on Dmrt1 mRNA levels but induces c-fos expression. Analysis of Dmrt1, c-fos, and actin mRNA levels in Sertoli cells treated with FBS. Sertoli cells were cultured in absence (no treatment) or the presence of 15% FBS as described in Materials and Methods and RNA isolated from the cells at the designated times. Dmrt1 (A) and c-fos (B) mRNAs were measured together with actin mRNA by RPA as described (2). For the no treatment sample, RNA was isolated at the zero time point. C, The optical densities of the protected fragment for each RNA (A and B) was quantified from the autoradiograms and the optical densities of Dmrt1 or c-fos were normalized to that for actin. The Dmrt1/actin or c-fos/actin ratio from each treatment was made relative to the no treatment ratio for the respective mRNA and the relative densitometric units plotted in the graph.
Discussion
Over the past few years, studies have suggested that DM-containing proteins are involved in the regulation of sexual development across phyla and implicated Dmrt1 in the regulation of testis development and sex determination in mammals (14–19, 51–53). More recently, gene knockout studies revealed Dmrt1’s importance in postnatal testis differentiation in mice and suggested that it is involved in the regulation of Sertoli cell function (20). Dmrt1 is expressed only in the gonads during embryogenesis and this expression becomes restricted to the testis at later stages of development and remains testis-specific in the postnatal animal (14, 16, 17, 19, 53). The cell-specific properties and functional importance of Dmrt1 prompted us to examine its expression in postnatal testis and Sertoli cells, to elucidate its role in testis function and to help unravel the mechanisms important for Dmrt1 expression. Our studies indicate that Dmrt1 mRNA levels are regulated with respect to testis age and are sensitive to variations in PKA and PKC activity as well as other environmental cues that have yet to be discovered.
In the male rat, postnatal development is marked by significant changes in the morphology and function of the testes as well as the endocrine state of the animal. Dmrt1 mRNA was observed to undergo dramatic changes with respect to testis age. Similar fluctuations in Dmrt1 protein levels were recently reported in studies on the mouse testis (20). In the early postnatal period (first week after birth), little or no change in Dmrt1 mRNA levels was observed when compared with testes from e18 embryos. During this period, gonadotropin and testosterone levels are low and testis function is marked by the reinitiation of mitotic division of gonocytes in the seminiferous tubules (54). By the time the animal is 10 days old, Dmrt1 mRNA levels have risen significantly with an increase of approximately 10-fold when compared with levels at day 5. The mRNA remains elevated through postnatal day 20. Interestingly, this is the period in which many changes take place in the testis with respect to gonadotropin stimulation and structural development of the seminiferous epithelium, and importantly, the Sertoli cells play a central role in the orchestration of these changes (27, 55). During this period, Sertoli cells stop dividing and form tight junctional complexes between adjacent cells to form the blood-testis barrier (~d16–20). Closely related to this event is the formation of the lumen in the seminiferous tubules. In addition, there is a significant increase in the testicular response to FSH, where there is both an increase in FSH receptors and FSH stimulated cAMP in Sertoli cells (37–40).
As the animal enters puberty and approaches sexual maturity (i.e. days 40 and 60 days, respectively), the testicular levels of Dmrt1 mRNA decreased significantly. Although this drop in Dmrt1 correlates with a decrease in the ability of the testis to respond to FSH, there is also a large increase in the number of spermatogenic cells at this time (40, 56). These additional cells contribute substantially to the RNA pool and dilute mRNAs from cells whose numbers are not changing (i.e. Sertoli cells). Thus, whereas actual changes in Dmrt1 expression may account for some of the observed drop in Dmrt1 expression at 40 and 60 days, this change most likely predominantly reflects a dilution of Dmrt1 mRNA with RNA produced by increasing numbers of nonexpressing spermatogenic cells.
Studies with cultured Sertoli cells revealed that FSH significantly elevated Dmrt1 mRNA, implicating it in the regulation of FSH-stimulated events in Sertoli cells. In prenatal and newborn rats, FSH is critical for the stimulation of Sertoli cell proliferation, which, in turn, is an important determinant of the spermatogenic output of the testis (reviewed in Ref. 27). However, at these times, Dmrt1 mRNA is low, suggesting its role is minimal in expansion of the Sertoli cell population. However, Dmrt1 expression increased at a time when Sertoli cells cease to divide and FSH action is thought to induce many of the final maturation events in these cells such as formation of the tight junctional complexes and initiation of the first wave of spermatogenesis (28, 29). Importantly, the above studies revealed a strong correlation between Dmrt1 expression and several significant changes that occur in Sertoli cells during postnatal development; most notably FSH response, cessation of mitotic activity, formation of tight junctional complexes, and lumen formation. This correlation was recently substantiated with the finding that mice lacking Dmrt1 had testes containing abundant, immature Sertoli cells that failed to form a lumen, indicating that Sertoli cell differentiation and cessation of mitosis was impaired (20).
FSH and/or cAMP has been shown to induce mRNA expression of several genes in Sertoli cells, including c-fos, jun-B, inhibin α, the subunits for protein kinase A (RIα, RIIβ. RIIα. Cα), tissue plasminogen activator (tPA), c-myc, phos-phodiesterase, and PKIα (41, 42, 57–59). Interestingly, response to FSH or cAMP exhibits a variety of different kinetics with respect to induction of these genes. Both c-fos and jun-B mRNA levels increased rapidly, with changes observed within 30 min and maximal response at 1–2 h posttreatment (42). Changes in c-fos and jun-B closely mimicked the induction of cAMP in response to FSH (41). Inhibinα and tPA exhibited somewhat slower kinetics with mRNA levels reaching a maximum 4–6 h after stimulation. In contrast, c-myc induction was not observed until 18 h after FSH treatment (60).
Studies on Dmrt1 revealed that cAMP stimulation follows a time course similar to that observed for tPA and inhibinα. Thus Dmrt1 mRNA reached a maximum 4 h after treatment. In addition, Dmrt1 was much more highly induced by the cAMP analog 8-bromo-cAMP than by FSH, suggesting that other aspects in FSH signaling (i.e. receptor down-regulation or increased Ca+2) impact Dmrt1 expression. Analysis of c-fos mRNA revealed similar kinetics and level of induction in our studies to those previous reported, with an approximate 9-fold change occurring within 1 h of treatment (42). Comparison of the 8-bromo-cAMP induction for these two messages underscores the kinetic differences observed in Sertoli cells in response to FSH. Thus whereas c-fos was induced early and mRNA levels were more transient, Dmrt1 mRNA levels were highest at the four-hour time point and sustained for at least 24 h (Fig. 4A).
In general, treatment of Sertoli cells with FSH results in a complexity of cellular and biochemical changes. Considerable evidence supports the view that stimulation of Sertoli cells with FSH activates the classical pathway in which cAMP acts as a second messenger to activate PKA (38, 61, 62). PKA is known to phosphorylate and increase the transcriptional activity of several closely related transcription factors in the bZIP family, namely cAMP response element binding protein (CREB), cAMP response element modulator (CREM), and activating transcription factor-1 (ATF-1) (reviewed in Ref. 63). Both CREB and CREM expression have been described in the testis and show remarkable changes in expression and splicing in response to FSH (64–73). Although the mechanism of Dmrt1 activation is not yet known, our evidence indicates that it requires activation of PKA and ongoing transcription but not protein synthesis. These observations are consistent with the hypothesis that the Dmrt1 gene is activated by resident transcription factors whose activity is induced by FSH or cAMP. CREB and CREM represent good candidates for such a transcription factor. Additional studies focused on the Dmrt1 gene and its promoter will help unravel the molecular mechanism by which cAMP regulates Dmrt1 expression.
Our studies also revealed that Dmrt1 mRNA is regulated by factors lost when cells are placed in culture. Thus a large drop in Dmrt1 transcript levels was observed after cells were placed in culture when compared with freshly isolated cells. Although the loss of FSH stimulation may account for the decreased message, contributions by other factors should also be considered. To this end, we evaluated the role of another prominent protein kinase (PKC) and the influence of serum factors. Our results showed that activation of PKC decreased Dmrt1 mRNA, whereas serum had little impact on its expression. PKC was also shown to antagonize the positive effects of PKA on Dmrt1 expression in a manner that, at least partially, required the activation of MEK1/2. Additional studies to determine how PKC and PKA regulate Dmrt1 and the identification of additional factors regulating transcription of the Dmrt1 gene will provide insight into the mechanisms needed for cell-specific gene regulation and hormone response in Sertoli cells.
Acknowledgments
Human recombinant FSH (AFP8468A) was obtained through the NHPP, NIDDK & Dr. A. F. Parlow. We thank Dr. Michael Wolfe for his generosity and helpful suggestions.
Footnotes
This work was supported in part by the Madison and Lila Self Graduate Fellowship.
References
- 1.Capel B. The battle of the sexes. Mech Dev. 2000;92:89–103. doi: 10.1016/s0925-4773(99)00327-5. [DOI] [PubMed] [Google Scholar]
- 2.Jost A. Recherches sur la differenciation sexuelle de l’embryon de lapin. Arch Anat Microsc Morphol Exp. 1947;36:271–315. [Google Scholar]
- 3.Sinclair AH, Berta P, Palmer MS, Hawkins JR, Griffiths BL, Smith MJ, Foster JW, Frischauf AM, Lovell-Badge R, Goodfellow PN. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature. 1990;346:240–244. doi: 10.1038/346240a0. [DOI] [PubMed] [Google Scholar]
- 4.Gubbay J, Collignon J, Koopman P, Capel B, Economou A, Munsterberg A, Vivian N, Goodfellow P, Lovell-Badge R. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature. 1990;346:245–250. doi: 10.1038/346245a0. [DOI] [PubMed] [Google Scholar]
- 5.Lovell-Badge R, Robertson E. XY female mice resulting from a heritable mutation in the primary testis-determining gene, Tdy. Development. 1990;109:635–646. doi: 10.1242/dev.109.3.635. [DOI] [PubMed] [Google Scholar]
- 6.Kreidberg JA, Sariola H, Loring JM, Maeda M, Pelletier J, Housman D, Jaenisch R. WT-1 is required for early kidney development. Cell. 1993;74:679–691. doi: 10.1016/0092-8674(93)90515-r. [DOI] [PubMed] [Google Scholar]
- 7.Kent J, Wheatley SC, Andrews JE, Sinclair AH, Koopman P. A male-specific role for SOX9 in vertebrate sex determination. Development. 1996;122:2813–2822. doi: 10.1242/dev.122.9.2813. [DOI] [PubMed] [Google Scholar]
- 8.Morais-da-Silva S, Hacker A, Harley V, Goodfellow P, Swain A, Lovell-Badge R. Sox9 expression during gonadal development implies a conserved role for the gene in testis differentiation in mammals and birds. Nat Genet. 1996;14:62–68. doi: 10.1038/ng0996-62. [DOI] [PubMed] [Google Scholar]
- 9.Swain A, Narvaez V, Burgoyne P, Camerino G, Lovell-Badge R. Dax1 antagonizes Sry action in mammalian sex determination. Nature. 1998;391:761–767. doi: 10.1038/35799. [DOI] [PubMed] [Google Scholar]
- 10.Shawlot W, Behringer RR. Requirement for Lim1 in head-organizer function. Nature. 1995;374:425–430. doi: 10.1038/374425a0. [DOI] [PubMed] [Google Scholar]
- 11.Miyamoto N, Yoshida M, Kuratani S, Matsuo I, Aizawa S. Defects of urogenital development in mice lacking Emx2. Development. 1997;124:1653–1664. doi: 10.1242/dev.124.9.1653. [DOI] [PubMed] [Google Scholar]
- 12.Bennett CP, Docherty Z, Robb SA, Ramani P, Hawkins JR, Grant D. Deletion 9p and sex reversal. J Med Genet. 1993;30:518–520. doi: 10.1136/jmg.30.6.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilkie AO, Campbell FM, Daubeney P, Grant DB, Daniels RJ, Mullarkey M, Affara NA, Fitchett M, Huson SM. Complete and partial XY sex reversal associated with terminal deletion of 10q: report of 2 cases and literature review. Am J Med Genet. 1993;46:597–600. doi: 10.1002/ajmg.1320460527. [DOI] [PubMed] [Google Scholar]
- 14.Raymond CS, Kettlewell JR, Hirsch B, Bardwell VJ, Zarkower D. Expression of Dmrt1 in the genital ridge of mouse and chicken embryos suggests a role in vertebrate sexual development. Dev Biol. 1999;215:208–220. doi: 10.1006/dbio.1999.9461. [DOI] [PubMed] [Google Scholar]
- 15.Raymond CS, Parker ED, Kettlewell JR, Brown LG, Page DC, Kusz K, Jaruzelska J, Reinberg Y, Flejter WL, Bardwell VJ, Hirsch B, Zarkower D. A region of human chromosome 9p required for testis development contains two genes related to known sexual regulators. Hum Mol Genet. 1999;8:989–996. doi: 10.1093/hmg/8.6.989. [DOI] [PubMed] [Google Scholar]
- 16.Raymond CS, Shamu CE, Shen MM, Seifert KJ, Hirsch B, Hodgkin J, Zarkower D. Evidence for evolutionary conservation of sex-determining genes. Nature. 1998;391:691–695. doi: 10.1038/35618. [DOI] [PubMed] [Google Scholar]
- 17.Smith CA, McClive PJ, Western PS, Reed KJ, Sinclair AH. Conservation of a sex-determining gene. Nature. 1999;402:601–602. doi: 10.1038/45130. [DOI] [PubMed] [Google Scholar]
- 18.Kettlewell JR, Raymond CS, Zarkower D. Temperature-dependent expression of turtle Dmrt1 prior to sexual differentiation. Genesis. 2000;26:174–178. [PubMed] [Google Scholar]
- 19.De Grandi A, Calvari V, Bertini V, Bulfone A, Peverali G, Camerino G, Borsani G, Guioli S. The expression pattern of a mouse doublesex-related gene is consistent with a role in gonadal differentiation. Mech Dev. 2000;90:323–326. doi: 10.1016/s0925-4773(99)00282-8. [DOI] [PubMed] [Google Scholar]
- 20.Raymond CS, Murphy MW, O’Sullivan MG, Bardwell VJ, Zarkower D. Dmrt1, a gene related to worm and fly sexual regulators, is required for mammalian testis differentiation. Genes Dev. 2000;14:2587–2595. doi: 10.1101/gad.834100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magre S, Jost A. The initial phases of testicular organogenesis in the rat. An electron microscopy study. Arch Anat Microsc Morphol Exp. 1980;69:297–318. [PubMed] [Google Scholar]
- 22.McLaren A. Development of the mammalian gonad: the fate of the supporting cell lineage. Bioessays. 1991;13:151–156. doi: 10.1002/bies.950130402. [DOI] [PubMed] [Google Scholar]
- 23.McLaren A. Gonad development: assembling the mammalian testis. Curr Biol. 1998;8:R175–R177. doi: 10.1016/s0960-9822(98)70104-6. [DOI] [PubMed] [Google Scholar]
- 24.Karl J, Capel B. Sertoli cells of the mouse testis originate from the coelomic epithelium. Dev Biol. 1998;203:323–333. doi: 10.1006/dbio.1998.9068. [DOI] [PubMed] [Google Scholar]
- 25.Capel B. Sex in the 90s: SRY and the switch to the male pathway. Annu Rev Physiol. 1998;60:497–523. doi: 10.1146/annurev.physiol.60.1.497. [DOI] [PubMed] [Google Scholar]
- 26.Gondos B, Berndston WE. Postnatal and pubertal development. In: Knobil E, Neill JD (eds) The Physiology of Reproduction, ed 2. Raven Press, New York, vol. 1994;1:116–154. [Google Scholar]
- 27.Griswold MD 1993 Actions of FSH on mammalian Sertoli cells. In: Russell LD, Griswold MD (eds) The Sertoli Cell. Cache River Press, Clearwater, pp 493–508
- 28.Solari AJ, Fritz IB. The ultrastructure of immature Sertoli cells. Maturation-like changes during culture and the maintenance of mitotic potentiality. Biol Reprod. 1978;18:329–345. doi: 10.1095/biolreprod18.3.329. [DOI] [PubMed] [Google Scholar]
- 29.Posalaky Z, Meyer R, McGinley D. The effects of follicle-stimulating hormone (FSH) on Sertoli cell junctions in vitro: a freeze-fracture study. J Ultrastruct Res. 1981;74:241–254. doi: 10.1016/s0022-5320(81)80115-3. [DOI] [PubMed] [Google Scholar]
- 30.Karl AF, Griswold MD. Sertoli cells of the testis: preparation of cell cultures and effects of retinoids. Methods Enzymol. 1990;190:71–75. doi: 10.1016/0076-6879(90)90010-x. [DOI] [PubMed] [Google Scholar]
- 31.Heckert LL, Daggett MA, Chen J. Multiple promoter elements contribute to activity of the follicle-stimulating hormone receptor (FSHR) gene in testicular Sertoli cells. Mol Endocrinol. 1998;12:1499–1512. doi: 10.1210/mend.12.10.0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mather JP. Establishment and characterization of two distinct mouse testicular epithelial cell lines. Biol Reprod. 1980;23:243–252. doi: 10.1095/biolreprod23.1.243. [DOI] [PubMed] [Google Scholar]
- 33.Gilman M 1994 Ribonuclease protection assay. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K (eds) Current Protocols in Molecular Biology. Greene and Wiley Inter-Science, New York, pp 4.7.1–4.7.8
- 34.Guan G, Kobayashi T, Nagahama Y. Sexually dimorphic expression of two types of DM (doublesex/Mab-3)-domain genes in a teleost fish, the tilapia (Oreochromis niloticus) Biochem Biophys Res Commun. 2000;272:662–666. doi: 10.1006/bbrc.2000.2840. [DOI] [PubMed] [Google Scholar]
- 35.McGuinness MP, Linder CC, Morales CR, Heckert LL, Pikus P, Griswold MD. Relationship of a mouse Sertoli cell line (MSC-1) to normal Sertoli cells. Biol Reprod. 1994;51:116–124. doi: 10.1095/biolreprod51.1.116. [DOI] [PubMed] [Google Scholar]
- 36.Peschon JJ, Behringer RR, Cate RL, Harwood KA, Idzerda RL, Brinster R, Palmiter RD. Directed expression of an oncogene to Sertoli cells in transgenic mice using Müllerian inhibiting substance regulatory sequence. Mol Endocrinol. 1992;6:1403–1411. doi: 10.1210/mend.6.9.1331774. [DOI] [PubMed] [Google Scholar]
- 37.Heckert LL, Griswold MD. Expression of the FSH receptor in the testis. Recent Prog Horm Res. 1993;48:61–77. doi: 10.1016/b978-0-12-571148-7.50006-3. [DOI] [PubMed] [Google Scholar]
- 38.Means AR, Dedman JR, Tash JR, Tindall DJ, van Sickle M, Welsh MJ. Regulation of the testis Sertoli cell by follicle-stimulating hormone. Annu Rev Physiol. 1980;42:58–71. doi: 10.1146/annurev.ph.42.030180.000423. [DOI] [PubMed] [Google Scholar]
- 39.Ketelslegers JM, Hetzel WD, Sherins RJ, Catt KJ. Developmental changes in testicular gonadotropin receptors: plasma gonadotropins and plasma testosterone in the rat. Endocrinology. 1978;103:212–222. doi: 10.1210/endo-103-1-212. [DOI] [PubMed] [Google Scholar]
- 40.Eskola V, Nikula H, Huhtaniemi I. Age-related variation of follicle-stimulating hormone-stimulated cAMP production, protein kinase C activity and their interactions in the rat testis. Mol Cell Endocrinol. 1993;93:143–148. doi: 10.1016/0303-7207(93)90117-3. [DOI] [PubMed] [Google Scholar]
- 41.Hall SH, Joseph DR, French FS, Conti M. Follicle-stimulating hormone induces transient expression of the protooncogene c-fos in primary Sertoli cell cultures. Mol Endocrinol. 1988;2:55–61. doi: 10.1210/mend-2-1-55. [DOI] [PubMed] [Google Scholar]
- 42.Hamil KG, Conti M, Shimasaki S, Hall SH. Follicle-stimulating hormone regulation of AP-1: inhibition of c-jun and stimulation of jun B gene transcription in the rat Sertoli cell. Mol Cell Endocrinol. 1994;99:269–277. doi: 10.1016/0303-7207(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 43.Sutherland EW. Studies on the mechanism of hormone action. Science. 1972;177:401–408. doi: 10.1126/science.177.4047.401. [DOI] [PubMed] [Google Scholar]
- 44.de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Witting-hofer A, Bos JL. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396:474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- 45.Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman DE, Graybiel AM. A family of cAMP-binding proteins that directly activate Rap1. Science. 1998;282:2275–2279. doi: 10.1126/science.282.5397.2275. [DOI] [PubMed] [Google Scholar]
- 46.Zwartkruis FJ, Bos JL. Ras and Rap1: two highly related small GTPases with distinct function. Exp Cell Res. 1999;253:157–165. doi: 10.1006/excr.1999.4695. [DOI] [PubMed] [Google Scholar]
- 47.Gonzalez-Robayna IJ, Falender AE, Ochsner S, Firestone GL, Richards JS. Follicle-stimulating hormone (FSH) stimulates phosphorylation and activation of protein kinase B (PKB/Akt) and serum and glucocorticoid-lnduced kinase (Sgk): evidence for a kinase-independent signaling by FSH in granulosa cells. Mol Endocrinol. 2000;14:1283–1300. doi: 10.1210/mend.14.8.0500. [DOI] [PubMed] [Google Scholar]
- 48.Ree AH, Hansson V, Walaas SI, Eskild W, Tasken KA. Calcium/phospholipid-dependent protein kinases in rat Sertoli cells: regulation of androgen receptor messenger ribonucleic acid. Biol Reprod. 1999;60:1257–1262. doi: 10.1095/biolreprod60.5.1257. [DOI] [PubMed] [Google Scholar]
- 49.Treisman R. Identification of a protein-binding site that mediates transcriptional response of the c-fos gene to serum factors. Cell. 1986;46:567–574. doi: 10.1016/0092-8674(86)90882-2. [DOI] [PubMed] [Google Scholar]
- 50.Treisman R. Transient accumulation of c-fos RNA following serum stimulation requires a conserved 5′ element and c-fos 3′ sequences. Cell. 1985;42:889–902. doi: 10.1016/0092-8674(85)90285-5. [DOI] [PubMed] [Google Scholar]
- 51.Calvari V, Bertini V, De Grandi A, Peverali G, Zuffardi O, Ferguson-Smith M, Knudtzon J, Camerino G, Borsani G, Guioli S. A new submicroscopic deletion that refines the 9p region for sex reversal. Genomics. 2000;65:203–212. doi: 10.1006/geno.2000.6160. [DOI] [PubMed] [Google Scholar]
- 52.Guioli S, Schmitt K, Critcher R, Bouzyk M, Spurr NK, Ogata T, Hoo JJ, Pinsky L, Gimelli G, Pasztor L, Goodfellow PN. Molecular analysis of 9p deletions associated with XY sex reversal: refining the localization of a sex-determining gene to the tip of the chromosome. Am J Hum Genet. 1998;63:905–908. doi: 10.1086/302017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moniot B, Berta P, Scherer G, Sudbeck P, Poulat F. Male specific expression suggests role of DMRT1 in human sex determination. Mech Dev. 2000;91:323–325. doi: 10.1016/s0925-4773(99)00267-1. [DOI] [PubMed] [Google Scholar]
- 54.Ojeda SR, Andrews WW, Advis JP, White SS. Recent advances in the endocrinology of puberty. Endocr Rev. 1980;1:228–257. doi: 10.1210/edrv-1-3-228. [DOI] [PubMed] [Google Scholar]
- 55.Mathews DM, Andrews WW, Parker R, Jr, Ojeda SR. A role for aromatizable androgens in female rat puberty. Biol Reprod. 1987;36:836–843. doi: 10.1095/biolreprod36.4.836. [DOI] [PubMed] [Google Scholar]
- 56.Steinberger A, Hintz M, Heindel JJ. Changes in cyclic AMP responses to FSH in isolated rat Sertoli cells during sexual maturation. Biol Reprod. 1978;19:566–572. doi: 10.1095/biolreprod19.3.566. [DOI] [PubMed] [Google Scholar]
- 57.Canipari R, Galdieri M. Retinoid modulation of plasminogen activator production in rat sertoli cells. Biol Reprod. 2000;63:544–550. doi: 10.1095/biolreprod63.2.544. [DOI] [PubMed] [Google Scholar]
- 58.Tasken KA, Knutsen HK, Attramadal H, Tasken K, Jahnsen T, Hansson V, Eskild W. Different mechanisms are involved in cAMP-mediated induction of mRNAs for subunits of cAMP-dependent protein kinases. Mol Endocrinol. 1991;5:21–28. doi: 10.1210/mend-5-1-21. [DOI] [PubMed] [Google Scholar]
- 59.Van Patten SM, Donaldson LF, McGuinness MP, Kumar P, Alizadeh A, Griswold MD, Walsh DA. Specific testicular cellular localization and hormonal regulation of the PKIalpha and PKIbeta isoforms of the inhibitor protein of the cAMP-dependent protein kinase. J Biol Chem. 1997;272:20021–20029. doi: 10.1074/jbc.272.32.20021. [DOI] [PubMed] [Google Scholar]
- 60.Lim K, Hwang BD. Follicle-stimulating hormone transiently induces expression of protooncogene c-myc in primary Sertoli cell cultures of early pubertal and prepubertal rat. Mol Cell Endocrinol. 1995;111:51–56. doi: 10.1016/0303-7207(95)03543-g. [DOI] [PubMed] [Google Scholar]
- 61.Means AR 1975 Biochemical effects of follicle-stimulating hormone on the testis. In: Hamilton DW, Greep RO (eds) Endocrinology. Waverly Inc., Baltimore, vol V:203–218
- 62.Fakunding JL, Tindall DJ, Dedman JR, Mena CR, Means AR. Biochemical actions of follice-stimulating hormone in the sertoli cell of the rat testis. Endocrinology. 1976;98:392–402. doi: 10.1210/endo-98-2-392. [DOI] [PubMed] [Google Scholar]
- 63.Sassone-Corsi P. Transcription factors responsive to cAMP. Annu Rev Cell Dev Biol. 1995;11:355–377. doi: 10.1146/annurev.cb.11.110195.002035. [DOI] [PubMed] [Google Scholar]
- 64.Waeber G, Meyer TE, LeSieur M, Hermann HL, Gerard N, Habener JF. Developmental stage-specific expression of cyclic adenosine 3′,5′-monophosphate response element-binding protein CREB during spermatogenesis involves alternative exon splicing. Mol Endocrinol. 1991;5:1418–1430. doi: 10.1210/mend-5-10-1418. [DOI] [PubMed] [Google Scholar]
- 65.Daniel PB, Habener JF. Cyclical alternative exon splicing of transcription factor cyclic adenosine monophosphate response element-binding protein (CREB) messenger ribonucleic acid during rat spermatogenesis. Endocrinology. 1998;139:3721–3729. doi: 10.1210/endo.139.9.6174. [DOI] [PubMed] [Google Scholar]
- 66.Delmas V, van der Hoorn F, Mellstrom B, Jegou B, Sassone-Corsi P. Induction of CREM activator proteins in spermatids: down-stream targets and implications for haploid germ cell differentiation. Mol Endocrinol. 1993;7:1502–1514. doi: 10.1210/mend.7.11.8114765. [DOI] [PubMed] [Google Scholar]
- 67.Lee CQ, Yun Y, Hoeffler JP, Habener JF. Cyclic-AMP-responsive transcriptional activation of CREB-327 involves interdependent phosphorylated subdomains. EMBO J. 1990;9:4455–4465. doi: 10.1002/j.1460-2075.1990.tb07896.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 68.Meyer TE, Habener JF. Cyclic adenosine 3′,5′-monophosphate response element binding protein (CREB) and related transcription-activating deoxyribonucleic acid-binding proteins. Endocr Rev. 1993;14:269–290. doi: 10.1210/edrv-14-3-269. [DOI] [PubMed] [Google Scholar]
- 69.Walker WH, Daniel PB, Habener JF. Inducible cAMP early repressor ICER down-regulation of CREB gene expression in Sertoli cells. Mol Cell Endocrinol. 1998;143:167–178. doi: 10.1016/s0303-7207(98)00082-3. [DOI] [PubMed] [Google Scholar]
- 70.Walker WH, Sanborn BM, Habener JF. An isoform of transcription factor CREM expressed during spermatogenesis lacks the phosphorylation domain and represses cAMP-induced transcription. Proc Natl Acad Sci USA. 1994;91:12423–12427. doi: 10.1073/pnas.91.26.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Delmas V, Sassone-Corsi P. The key role of CREM in the cAMP signaling pathway in the testis. Mol Cell Endocrinol. 1994;100:121–124. doi: 10.1016/0303-7207(94)90290-9. [DOI] [PubMed] [Google Scholar]
- 72.Foulkes NS, Mellstrom B, Benusiglio E, Sassone-Corsi P. Developmental switch of CREM function during spermatogenesis: from antagonist to activator. Nature. 1992;355:80–84. doi: 10.1038/355080a0. [DOI] [PubMed] [Google Scholar]
- 73.Sassone-Corsi P. CREM: a master-switch regulating the balance between differentiation and apoptosis in male germ cells. Mol Reprod Dev. 2000;56:228 –229. doi: 10.1002/(SICI)1098-2795(200006)56:2+<228::AID-MRD2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]


