Abstract
OBJECTIVE
To determine the efficacy of the transdermal nicotine patch for smoking cessation in inner-city African Americans.
DESIGN
Double-blind, placebo-controlled, randomized trial.
SETTING
Outpatient in an inner-city hospital.
PATIENTS AND PARTICIPANTS
A computer-generated random numbers table with a block size set at 20 was used to randomize 410 patients to one of two study arms.
INTERVENTIONS
The transdermal nicotine patch for 10 weeks as an adjunct to brief counseling.
MEASUREMENTS AND MAIN RESULTS
Of the 410 patients randomized, mean age was 48 years, 65% were female, 41% had less than a high school education, 51% had an annual household income of less than $8,000, and the average number of cigarettes smoked per day was 20. Quit rates at 10 weeks were 21.5% (44/205) with the nicotine patch, and 13.7% (28/205) with the placebo patch (p=.03). At 6 months, quit rates were 17.1% (35/205) with the nicotine patch, and 11.7% (24/205) with the placebo patch (p=.08). After adjusting for baseline differences in age and educational attainment, differences remained significant at 10 weeks (p=.04), but were not significant at 6 months (p=.14). Compliance rates for return visits were 83%, 78%, 55%, and 52%, at 1, 2, 6, and 10 weeks, respectively.
CONCLUSIONS
The nicotine patch significantly improves short-term quit rates in inner-city African Americans who are interested in trying to quit smoking. Efforts should be made to reach underserved populations through smoking cessation programs, and to assist in maintaining abstinence.
Keywords: smoking cessation, African Americans, inner-city patients, nicotine patch
Tobacco use is the major contributor to cardiovascular disease, cancer, and cerebrovascular disease, the three leading causes of death in the United States.1 In 1993, approximately 46 million U.S. adults smoked,2 of whom more than 6 million were African American.3 Over the past three decades, smoking prevalence rates for African Americans and whites have both declined, from 46% to 26% for African Americans, and from 42% to 25% for whites.4 However, prevalence rates for inner-city African Americans remain high, ranging from 33% to 54%.5 6,5–7 In Harlem, prevalence rates reported for African–American men have been as high as 60% in health care settings and 52% in housing developments.7
Despite such high smoking rates among inner-city African Americans, research in smoking cessation has been conducted almost exclusively in white, middle-class populations. Moreover, whites and African Americans have significantly different smoking behaviors, so that findings from previous smoking cessation studies may not be generalizable to African-American smokers. When compared with whites, African Americans smoke fewer cigarettes,8 and are less likely to be heavy smokers and more likely to smoke mentholated and higher tar and nicotine brands.9 Although African Americans are more likely than white smokers to have quit for at least 1 day during the previous year,10 and to be confident that they will be abstinent at 1 year,11 African Americans who attempt to quit are significantly less likely than whites to remain abstinent for 1 year or more, even after adjustment for socioeconomic factors.10, 12
In U.S. smokers, without any smoking cessation intervention, the annual spontaneous quit rates range from 2% to 5%.13, 14 Quit rates can be significantly improved with the nicotine transdermal patch, which has been studied extensively in smoking cessation trials largely composed of white, middle-class smokers. The patch has been found to be both clinically effective,5,15–17 and cost-effective,14, 18 and has been recommended by the Agency for the Health Care Policy and Research Smoking Cessation Clinical Practice Guidelines.19 Owing to the lack of information on the nicotine patch in assisting African–American smokers to quit, we conducted a double-blind, placebo-controlled, randomized trial to evaluate its efficacy as an adjunct to brief counseling and education, in an inner-city population.
METHODS
Setting and Study Population
Enrollees were patients at a large inner-city hospital serving a low-income, predominantly African–American population. Patients were recruited by self-referral, referral by physicians, or active recruitment. Active recruitment was conducted from the nonappointment medical walk-in clinic and the lobby of the clinic building between the hours of 8 amand 5 pmon weekdays. Patients who met eligibility criteria at the screening visit were scheduled 1 to 2 weeks later for their randomization visit. Inclusion criteria included self-report of being African American, having smoked a minimum of 10 cigarettes a day continuously for at least the past year, at least one previous attempt to quit, a home address, a telephone number at which the patient could be reached, and weight more than 100 pounds. Subjects were enrolled if they reported being self-motivated to quit smoking; this was determined by an affirmative response to the question, “Are you motivated to quit smoking?”
Exclusion criteria included pregnancy, breast feeding, concurrent use of any other forms of tobacco or other nicotine-containing products, myocardial infarction within 3 months, unstable angina, serious arrhythmias, terminal illness, systemic dermatologic disorders such as psoriasis or eczema, and self-reported alcohol or drug dependency. Patients were also excluded if another member of the same household was enrolled in the trial.
The study protocol was approved by the Emory University School of Medicine Human Investigations Committee, and all study subjects gave written informed consent at the screening visit after the nature of the study had been fully explained. Owing to the prevalence of low literacy among our patients,20 the consent form was read aloud, and the research assistants administered the survey instrument.
Study Design
The study design was a double-blind, placebo-controlled, randomized trial of 10 weeks of patches. Patients returned at 1, 2, 6, and 10 weeks after the quit day, with a final visit at 6 months. The study began in November 1993, and all 6-month follow-up visits were completed by March 1995.
Patients were randomized to one of two study arms based on a computer-generated random numbers table with a block size set at 20. Both study staff and patients were blinded to patch treatment. All data collection, counseling, education, support, and brief follow-up counseling were performed by four trained research assistants who ranged in level of education from an undergraduate student to a research assistant who had a master's degree.
Transdermal Patches
Patients were instructed to use their placebo patches or transdermal nicotine patches (Nicoderm, Marion Merrell Dow) with the following schedule: 21 mg/d for 6 weeks, 14 mg/d for 2 weeks, and 7 mg/d for 2 weeks. At randomization, patients received 2 weeks of 21 mg/d patches and received the remaining 4 weeks of 21 mg/d at the 1-week visit. At the 2-week follow-up visit, they received 2 weeks each of 14 mg/d and 7 mg/d. The three doses of transdermal nicotine patches provide an average steady-state plasma nicotine concentration of 17, 12, and 6 ng/mL, respectively.21 Placebo systems contained a pharmacologically irrelevant amount of nicotine in the drug reservoir to mimic the odor of active systems but delivered less than 1 mg of nicotine in 24 hours. Patients were instructed to apply a new patch system each morning to a dry skin site on the upper torso, upper back, or upper, outer arm on a 7-day cycle. All patches were packaged in unlabeled boxes that held a 2-week supply.
Patients who relapsed to smoking cigarettes were advised to discontinue using the patch and set another quit date. Adherence with the patch protocol was assessed by having patients document their use of the patch and the number of cigarettes smoked that day on a daily calendar diary. At follow-up visits, after 1, 2, 6, and 10 weeks, patients were asked about adherence to use of the patch since their previous visit. Side effects and adverse effects of patch use were also recorded.
Behavioral Support Program and Follow-up Visits
At the screening visit, all eligible patients received Pathways to Freedom: Winning the Fight Against Tobacco,22 a culturally sensitive smoking cessation guide that is written at the sixth grade reading level. This 8 1/2-by-11-inch manual is printed on glossy paper and includes a number of color photographs and line drawings. The manual has three parts: a presentation of the characteristics of cigarette smoking among African Americans; instructions on how to quit smoking; and suggestions for how communities can combat tobacco dependence by working collaboratively. Patients also received an instructional 34-minute audiocassette titled “Nicoderm: Behavioral Support and Proper Use Information.” The audiocassette covers issues surrounding nicotine patch use for 8 minutes, and for the remaining 26 minutes it covers coping techniques, relaxation techniques, and behavioral change issues. To be enrolled in the study, patients were then scheduled for their randomization visit and quit date.
At randomization, patients were seen in groups of two to four. At this visit, patients received their initial 2 weeks of 21 mg/d patches, and had a 1-hour visit with a counselor for instructions on the use of the patch, brief education about the risks of smoking and the benefits of quitting, and general medical advice. No psychological counseling was done. The actual quit date, when the first patch was applied, was the day after randomization. All patients also received a small red duffle bag with the program logo and clinic telephone number.
The bag contained a folder with a copy of the patient's informed consent, a 1-page nicotine patch tip sheet (created by the investigators), a Quit for Good Prescription signed by the enrollee, and a sheet listing the health benefits of quitting smoking. The 1-page patch tip sheet was written at the sixth grade reading level, and pictures were created to match the text for those with lower literacy skills. A blank daily diary for the patient to complete each day was also enclosed. This calendar requested patients to list the number of cigarettes they smoked each day, and circle yes or no in response to whether they had applied the patch that day. In addition, patients received a written guide titled “The 6-2-2 Committed Quitters' Program: How to Quit Smoking Using Nicoderm”.23 This guide, produced by the manufacturer of the transdermal nicotine patches used in this trial, was assessed to be at the seventh grade literacy level by the Flesh-Kincaid scale.
Measurements at randomization included weight and exhaled carbon monoxide (CO) with personalized feedback. Patients received a written report that highlighted their CO level compared with that of a nonsmoker, and that of a light, moderate, and heavy smoker. The CO measurement was performed using a hand-held, portable CO monitor (Bedfont Micro Smokerlyzer, Kent, U.K.).
Return visits lasted about 10 minutes. Patients were reimbursed $5 at each of these follow-up visits for transportation costs. Return visit schedules were flexible, allowing a window of 3 days on each side for the visits at weeks 1 and 2. A window of 1 week was allowed for the visits at weeks 6 and 10, and at 6 months. At these brief visits, study staff reviewed the patient's progress, discussed issues related to relapse, encouraged compliance with the treatment regimen, determined if there were any adverse reactions, and reviewed information entered by the patient into the daily diary. At these follow-up visits, patients were briefly asked about patch side effects, adherence to use of the patch, and usefulness of the two written guides. A telephone call was made to encourage all patients who did not keep their scheduled appointments to come in. For patients who did not return for their 10-week and 6-month follow-up appointment, three telephone calls were attempted to encourage follow-up and to obtain smoking status over the telephone.
Outcome Measure
Smoking cessation was defined as self-reported abstinence (not even a puff) since the last visit. The primary outcome measure used to measure success at 6 months was continuous abstinence from the end of patch treatment. A secondary outcome was 30-day abstinence at 10 weeks.
Survey Instrument
Age, gender, educational level, household income, marital status, and employment status were obtained by patient self-report. Insurance status was obtained from hospital administrative records. Six questions measured self-reported prevalence of diabetes, angina, hypertension, asthma, chronic obstructive pulmonary disease, and stroke.
Questions about smoking and tobacco use were taken from the 1988 National Health Interview Survey,24 as well as other instruments used in previous research studies.25 These included age at initiation of smoking, average number of cigarettes smoked a day, brand of cigarettes, use of menthol cigarettes, amount of cigarettes smoked, level of inhalation, parental history of smoking, and the number of current smokers living in the same household. Nicotine dependence was assessed with the Fagerstrom Test for Nicotine Dependence.26, 27 This 6-item instrument is summed to yield an overall dependence score that ranges from 0 to 10 (severe nicotine dependence). A score of 7 or higher is generally interpreted as a high degree of dependence. History about quitting, the number of serious quit attempts, longest duration of abstinence, likelihood of success, and methods used (cold turkey, group program, meditation or prayer, or a product from a drugstore) were also asked.
Statistical Methods
Data editing and review preceded double-entry verification into a database using EpiInfo Version 5.01 (USD Inc., Stone Mountain, Ga., 1990). Additional quality control procedures, database management, and statistical analyses were performed using SAS software (SAS User's Guide: Statistics, 6th ed., SAS Institute Inc., Cary, NC, 1990). Any disparities were resolved by referring back to the original forms.
The active and placebo groups were compared to determine whether randomization was successful in creating similar groups with regard to demographic variables and other baseline patient characteristics. Continuous measures such as age, number of years smoking, and Fagerstrom score were compared using independent group Student's t tests. Dichotomous characteristics were compared between the two groups using χ2 statistics or Fisher's Exact Tests. Two-sided p values <.05 were considered significant.
Separate one-sided Fisher's Exact Tests were used to compare the rates of smoking abstinence in the two groups at 10 weeks and 6 months. One-sided significance was chosen because there is no evidence that using nicotine patches would result in lower quit rates when compared with placebo patches. Two outcome analyses were conducted. First, we analyzed the data assuming that subjects lost to follow-up or in violation of the study protocol were smokers. Second, we analyzed the data on the basis of the information we had on patients whom we could contact at 10 weeks and 6 months.
To explore potential confounding of the association between the treatment group and quit rate, continuous demographic and smoking history variables (age, weight, Fagerstrom score, years smoking, cigarettes per day, number of previous quit attempts, and baseline CO levels) were compared between the quitters and relapsers using independent group t tests or rank-sum tests as appropriate. Similarly, contingency table analyses were used to evaluate the potential confounding effect of categorical variables such as gender. Reported p values are unadjusted for the number of comparisons, but conclusions regarding associations are based on adjustment for this multiplicity. Assessment of the patch's ability to promote smoking cessation while controlling for baseline differences was carried out using logistic regression.28
RESULTS
Subjects
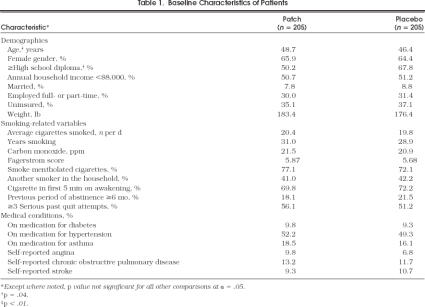
For the study, 833 patients were screened, and 586 were eligible, of whom 410 returned for randomization. Of the 410 patients who returned, 205 were randomized into each of two arms of the study. Demographics and smoking history for the 410 African–American patients are shown in Table 1). The active and placebo groups were comparable except that the active group was older (48.7 vs 46.4 years;p= .04) and had received less formal education (49.3% vs 32.2% with less than a high school diploma;p < .01). There were no other statistically significant differences between the two groups. At randomization, all 410 patients stated they would be successful in quitting.
Table 1.
Baseline Characteristics of Patients
Abstinence Rates
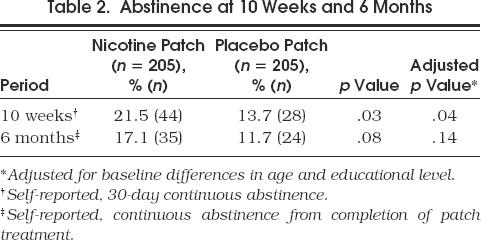
At 10 weeks, quit rates were 21.5% (44/205) in the nicotine patch group and 13.7% (28/205) in the placebo patch group (p= .03)(Table 2 Abstinence was defined as no cigarettes in the previous 30 days. The 42 patients who were unable to be contacted at 10 weeks were labeled as smokers. At 6 months, the self-reported quit rates, defined as no cigarettes from the end of treatment, were 17.1% (35/205) in the nicotine patch group, and 11.7% (24/205) in the placebo patch group (p= .08). The 111 patients who were unable to be contacted were labeled as smokers. After adjusting for baseline differences in age and educational level, differences remained significant at 10 weeks (p= .04), but were not significant at 6 months (p= .14) (Table 2).
Table 2.
Abstinence at 10 Weeks and 6 Months
In a secondary analysis, we excluded the patients whom we were unable to contact and performed the same outcomes analysis. After adjusting for baseline differences, age, and educational attainment, at 10 weeks, the self-reported quit rates were 27.2% (44/162) in the nicotine patch group, and 16.9% (28/166) in the placebo patch group (p= .02). At 6 months, the self-reported quit rates were 23.0% (35/152) in the nicotine patch group, and 16.3% (24/147) in the placebo patch group (p= .13).
Adherence
Despite aggressive retention efforts, including three telephone calls and open appointments, the adherence rate for return visits was 83% (342/410), 78% (319/410), 55% (226/410), and 52% (212/410), at 1, 2, 6, and 10 weeks, respectively, and 31% (128/410) at 6 months (Fig. 1). For patients who did not return for the 10-week and 6-month scheduled follow-up visits, attempts were made to reach patients by telephone. At 10 weeks, 149 patients were reached by telephone, resulting in a patient contact rate of 80% (328/410). At 6 months, 171 patients were reached by telephone, for a contact rate of 73% (299/410). Reasons for not being able to reach the 111 patients who did not return included disconnected telephone, patient no longer at the telephone number, telephone number changed to an unpublished number, and the wrong telephone number.
FIGURE 1.
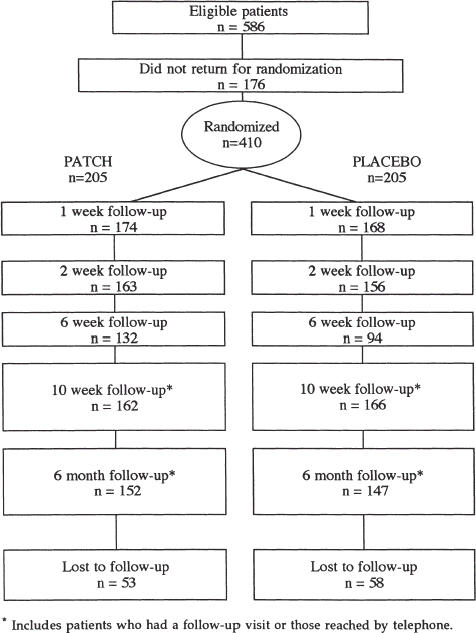
Flow chart for randomized trial of smoking cessation.
Use of the daily diary to assess the number of cigarettes smoked per day and adherence with patch use was not possible because only 49% of the patients had fully completed their 10-week diary. Adherence with the patch was determined by adherence to the follow-up visits at 1 and 2 weeks, since the remaining 21 mg/d patches were dispensed at the 1-week visit, and the 14 mg/d and 7 mg/d patches were dispensed at the 2-week visit. At the 1-week visit, 17% of patients did not pick up their remaining 21 mg/d patches, and at the 2-week visit, 22% did not pick up the 2 weeks each of 14 mg/d and 7 mg/d patches. There were no significant differences in follow-up rates between the two groups.
Educational Materials
At the 1-week follow-up visit, 70% (240/342) of those patients who returned stated that they found the Pathways to Freedom guide useful, compared with 65% (222/342) who found the “6-2-2 Quitters' Guide” useful (p= .14).
Blinding
At 6 months, subjects were asked if they could tell whether they were on the nicotine or placebo patch. Sixty-three percent (95/152) of the patients on nicotine patches, and 44% (65/147) of the patients on placebo patches correctly identified the patch they were on (p < .01). In the placebo group, nine patients either did not know or did not use the patch, compared with 10 patients in the nicotine patch group.
Side Effects
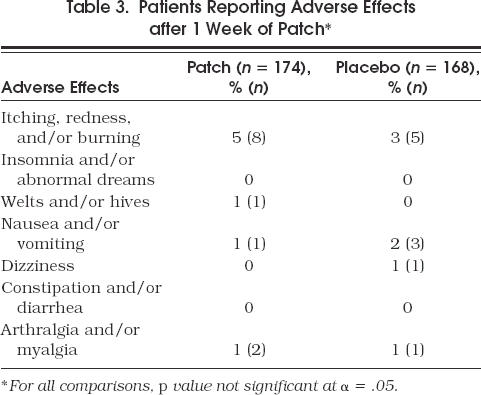
Adverse effects were documented by asking patients at the 1-week visit if they were having any problems with the patch (Table 3) There was no overall difference in adverse events between the nicotine patch and placebo patch groups (6.9% vs 6.0%). At 2, 6, and 10 weeks, the overall and specific side effect rate continued to be no different between the two study groups, and there was no increase in the rate of events over the 10 weeks. During the patch phase, one patient became pregnant, and her nicotine patch therapy was discontinued. At no time during the trial was it necessary to break the randomization code.
Table 3.
Patients Reporting Adverse Effects after 1 Week of Patch
DISCUSSION
Transdermal nicotine patch significantly improved short-term smoking cessation rates in inner-city African Americans, despite poor return for follow-up. To our knowledge, this is the first study to assess the efficacy of nicotine replacement in either African–American or low-income smokers. At 10 weeks, the quit rates in patients on patches and on placebo patches were 21.5% and 13.7%, respectively, and at 6 months, 17.1% and 11.7%, respectively. These quit rates are somewhat lower than those found in previous studies conducted in predominantly white middle-class populations.5,6,15–17 One meta-analysis of 17 studies reported abstinence rates of 27% for the active patch versus 13% for the placebo patch at the end of treatment, and 22% versus 9%, respectively, at 6 months. Another meta-analysis found similar quit rates at 6 months—20.5% in active patch patients, and 10.8% in placebo patients.16
There are a number of possible explanations for the lower quit rates in our study. When compared with previous patch studies,29–32 we used less intensive behavioral support. Second, defining the 111 patients whom we were unable to track at 6 months as smokers probably further lowered the true quit rates. In hindsight, our study was underpowered, as shown by an effect that was clinically significant, yet only approached statistical significance. Third, the lower quit rate may also be influenced by a number of factors that we did not measure including the surrounding environment, the increased presence of daily life hassles,33, 34 and the heavy use of billboard and magazine advertising.35 Fourth, poor access to primary care may be especially important,36 since African Americans are more likely to report that they would follow a physician's advice to reduce cancer risks than whites.37 Fifth, there are also data to suggest that African Americans generally have lower success in quit attempts. In fact, the quit ratio (former smokers divided by ever smokers), a population measure for success in quitting over time, is 31.5% for African Americans and 46.4% for whites.10 This concerning relation holds true even after adjustment for socioeconomic factors.12 Sixth, a number of patients did not adhere to the follow-up appointments and therefore were not in adherence with the nicotine patch therapy or with the brief counseling at each encounter. Seventh, the possibility does exist that African American smokers are less nicotine dependent, and therefore quit rates would not be expected to improve considerably with the addition of nicotine replacement. A crude measure of nicotine dependence, the Fagerstrom score was significantly lower at baseline in our study population than in many other clinical trials with largely white populations.30, 32, 8,9,38–40 Finally, our quit rates may be lower because our study did not have a relapse prevention component occasionally used in other studies.40
A second finding of this study was the quit rate among patients randomized to placebo patch. Compared with spontaneous quit rates of 3% to 5%, the 11.7% quit rate in the placebo arm is respectable and, in fact, the highest reported for an inner-city population. However, the placebo quit rate was achieved in a self-selected group of patients who were interested in a smoking cessation program and in quitting smoking. The behavioral support provided was of minimal intensity and less than that found in many previously conducted nicotine patch studies, and visits occurred at longer intervals than in many other studies.15, 30–32, 38, 39, 41 Thus, our program could easily be replicated in a number of inner-city settings, including physician practices.
In this trial, we attempted to tailor our smoking cessation interventions to the population we served with use of Pathways to Freedom, a guide specifically designed for low literate African Americans.22 When we asked patients who returned at 1 week if they found this guide useful, 70% replied in the affirmative. Interestingly, about the same number found the manufacturer's product guide useful as well. Some authors have felt that providing culturally sensitive materials will enhance adoption of suggestions and subsequently enhance quit rates.42, 43
Our follow-up rate was low. In our conservative method of analysis, we labeled all subjects who we could not locate as smokers. This strict method may underreport actual quit rates. Therefore, we conducted a secondary analysis of patients for whom smoking status was available. In this secondary analysis, quit rates were higher. It is possible that in a largely disenfranchised population, one in which 51% have a household income of less than $8,000 and 36% are uninsured, follow-up in a smoking cessation clinic may not be feasible. Future methods to determine smoking status may have to rely on home visits using outreach research assistants.
Some limitations of this study should be noted. One is the lack of biochemical verification of smoking cessation. Prior to starting the study, we had decided not to use biochemical verification because of expected low follow-up rates in our study population. However, self-report in low-pressure and minimal-intervention smoking cessation studies is felt to be quite reflective of the truth.10 In addition, biochemical testing has its own limitations; for example, it is often only reflective of smoking up to the past 72 hours. A second limitation was the choice of a 6-month interval for follow-up, rather than 1 year. We chose 6-month follow-up based on data suggesting that smoking status at 6 months is an acceptable marker for long-term abstinence.44 Third, we did not assess the literacy level of our patients or their ability to comprehend the intervention materials. Fourth, our use of one-sided statistical analysis, decided prior to initiating the study, limits the reporting of risk ratios with 95% confidence intervals. A final limitation of the study was that the only outcome measured was total abstinence. In other areas of medicine it is recognized that most treatments have relative rather than complete efficacy. For example, the partial reduction of angina is very important to the patient, even though there may not be a total remission of symptoms. Similarly, smoking cessation also should be viewed as a dynamic process rather than a discrete event.45, 46 For example, any reduction in the number of cigarettes smoked may create an opportunity for the patient to be more receptive to counseling, to learn constructive alternatives to use of tobacco, and to reduce harm caused by tobacco.
This study highlights that poor, inner-city, African–American smokers are interested in smoking cessation. The transdermal nicotine patch significantly improved 10-week smoking cessation rates, but more relapse prevention is needed to ensure abstinence to 6 months and beyond. Further research is also needed to better understand nicotine dependence in African–American populations. Even in those patients using the placebo patch, 10-week quit rates were two to three times higher than national quit rates when no intervention is used. If we are to be successful in lowering the prevalence of cigarette smoking to 20% in African Americans, a Healthy People 2000 goal,47 we must take advantage of pharmacologic modalities and the high desire to quit expressed by inner-city African–American smokers.11 Even though the nicotine patch is now available over-the-counter, some consideration by Medicaid programs should be given to covering the cost of smoking cessation programs and over-the-counter nicotine replacement products, and at the very least, other smoking cessation prescription products.
Acknowledgments
The authors express their sincere appreciation to George Cotsonis, Barbara Gibbs-Hodge, and Mary Swierzynski for assistance with data collection and manuscript preparation.
REFERENCES
- 1.McGinnis JM, Foege WH. Actual causes of death in the United States. JAMA. 1993;270:2207–12. [PubMed] [Google Scholar]
- 2.Centers for Disease Control. Cigarette smoking among adults—United States. MMWR 1994. 1993;43:925–30. [PubMed] [Google Scholar]
- 3.Centers for Disease Control. African Americans and smoking. Centers for Disease Control—At A Glance
- 4.Giovino GA, Henningfield JE, Tomar SL, Escobedo LG, Slade J. Epidemiology of tobacco use and dependence. Epidemiol Rev. 1995;17:48–65. doi: 10.1093/oxfordjournals.epirev.a036185. [DOI] [PubMed] [Google Scholar]
- 5.Hahn LP, Folsom AR, Sprafka JM, Norsted SW. Cigarette smoking and cessation behaviors among urban blacks and whites. Public Health Rep. 1990;105:290–5. [PMC free article] [PubMed] [Google Scholar]
- 6.Ahluwalia JS, McNagny SE. Smoking prevalence and desire to quit in inner-city African-American walk-in clinic patients. Clin Res. 1993;41:752A. [Google Scholar]
- 7.Resnicow K, Futterman R, Weston RW, et al. Smoking prevalence of Harlem residents: baseline results from the Harlem Health Connection Project. Am J Health Promot. 1996;10:343–6. doi: 10.4278/0890-1171-10.5.343. [DOI] [PubMed] [Google Scholar]
- 8.Office on Smoking and Health. Washington, DC: U.S. Dept. of Health and Human Services, Public Health Service PHS publication; 1989. Adult Use of Tobacco. 90-2004. [Google Scholar]
- 9.U.S. Dept. of Health and Human Services. A Report of the Surgeon General. Washington, DC: U.S. Dept. of Health and Human Services, Public Health Service, Centers for Disease Control, Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health DHHS publication (CDC); 1989. Reducing the Health Consequences of Smoking: 25 years of Progress; pp. 89–8411. [Google Scholar]
- 10.U.S. Dept. Health and Human Services. The Health Benefits of Smoking Cessation. U.S. Dept. Health and Human Services, Public Health Service, Centers for Disease Control, Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health DHHS publication (CDC); 1990. pp. 90–8416. [Google Scholar]
- 11.Martin R, Cummings SR, Coates TJ. Ethnicity and smoking: differences in white, black, Hispanic and Asian. Am J Prev Med. 1990;6:194–9. [PubMed] [Google Scholar]
- 12.Novotny TE, Warner KE, Kendrick JS, Remington PL. Smoking by blacks and whites: socioeconomic and demographic differences. Am J Public Health. 1988;78:1187–9. doi: 10.2105/ajph.78.9.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control. MMWR. Vol. 42. 1993. Smoking cessation during previous year among adults—United States, 1990 and 1991; pp. 504–7. [PubMed] [Google Scholar]
- 14.Fiscella K, Franks P. Cost-effectiveness of the transdermal nicotine patch as an adjunct to physician's smoking cessation counseling. JAMA. 1996;275:1247–51. [PubMed] [Google Scholar]
- 15.Fiore MC, Smith SS, Jorenby DE, Baker TB. The effectiveness of the nicotine patch for smoking cessation. JAMA. 1994;271:1940–7. [PubMed] [Google Scholar]
- 16.Silagy C, Mant D, Fowler G, Lodge M. Meta-analysis on efficacy of nicotine replacement therapies in smoking cessation. Lancet. 1994;343:139–42. doi: 10.1016/s0140-6736(94)90933-4. [DOI] [PubMed] [Google Scholar]
- 17.Tang JL, Law M, Wald N. How effective is nicotine replacement therapy in helping people to stop smoking? BMJ. 1994;308:21–6. doi: 10.1136/bmj.308.6920.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wasley MA, McNagny SE, Ahluwalia JS, Phillips VL. The cost-effectiveness of the nicotine transdermal patch for smoking cessation. Prev Med. 1997;26:264–70. doi: 10.1006/pmed.1996.0127. [DOI] [PubMed] [Google Scholar]
- 19.Flore MC, Bailey WC, Cohen SJ, et al. Quick Reference Guide for Smoking Cessation Specialists. No. 18. Rockville, Md: U.S. Dept. of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research, Centers for Disease Control and Prevention; 1996. Smoking Cessation: Information for Specialists. Clinical Practice Guideline. [Google Scholar]
- 20.Williams MV, Parker RM, Baker DW. Inadequate functional health literacy among patients at two public hospitals. JAMA. 1995;274:1677–82. et al. [PubMed] [Google Scholar]
- 21.Gorsline J, Gupta SK, Dye D, Rolf CN. Nicotine dose relationship for Nicoderm (nicotine transdermal system) at steady state. Pharmacol Res. 1991;10(suppl):S299. doi: 10.1002/j.1552-4604.1993.tb03938.x. [DOI] [PubMed] [Google Scholar]
- 22.Robinson RG`, Orleans CT, James DA, Sutton CD. Philadelphia, Pa: Fox Chase Cancer Center; 1992. Pathways to Freedom: Winning the Fight Against Tobacco. [Google Scholar]
- 23.Marion Merrill Dow Inc. Kansas City, Mo: Marion Merrill Dow Inc; 1993. The 6-2-2 Committed Quitter's Program: Nicoderm (Nicotine Transdermal System) [Google Scholar]
- 24.Duelberg SI. Preventive health behavior among black and white women in urban and rural areas. Soc Sci Med. 1992;34:191–8. doi: 10.1016/0277-9536(92)90096-9. [DOI] [PubMed] [Google Scholar]
- 25.Orleans CT, Schoenbach VJ, Salmon MA, et al. A survey of smoking and quitting patterns among black Americans. Am J Public Health. 1989;79:176–81. doi: 10.2105/ajph.79.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fagerstrom KO, Schneider NG. Measuring nicotine dependence: a review of the Fagerstrom Tolerance Questionnaire. J Behav Med. 1989;12:159–82. doi: 10.1007/BF00846549. [DOI] [PubMed] [Google Scholar]
- 27.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom K. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 28.Hosmer DW, Lemeshow S. Applied Logistic Regression. New York, NY: John Wiley and Sons; 1989. [Google Scholar]
- 29.Abelin T, Ehrsam R, Buhler-Reichert A, Imhof PR, Muller P, Thommen A. Effectiveness of a transdermal nicotine system in smoking cessation studies. Methods Find Exp Clin Pharmacol. 1989;11:205–14. [PubMed] [Google Scholar]
- 30.Fiore MC, Kenford SL, Jorenby DE, Wetter DW, Smith SS, Baker TB. Two studies of the clinical effectiveness of the nicotine patch with different counseling treatments. Chest. 1994;105:524–33. doi: 10.1378/chest.105.2.524. [DOI] [PubMed] [Google Scholar]
- 31.Richmond RL, Harris K, Neto A. The transdermal nicotine patch: results of a randomised placebo-controlled trial. Med J Aust. 1994;161:130–5. doi: 10.5694/j.1326-5377.1994.tb127344.x. [DOI] [PubMed] [Google Scholar]
- 32.Tonnesen P, Norregaard J, Simonsen K, Sawe U. A double-blind trial of a 16-hour transdermal nicotine patch in smoking cessation. N Engl J Med. 1991;325:311–5. doi: 10.1056/NEJM199108013250503. [DOI] [PubMed] [Google Scholar]
- 33.Romano PS, Bloom J, Syme SL. Smoking, social support, and hassles in an urban African-American community. Am J Public Health. 1991;81:1415–22. doi: 10.2105/ajph.81.11.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Delongis A, Coyne J, Dakof G, Folkman S, Lazarus R. Relationship of daily hassles, uplifts, and major life events to health status. Health Psychol. 1982;1:119–36. [Google Scholar]
- 35.Robinson RG, Pertschuk JD, Sutton C. Smoking and African Americans. In: Smauels SE, Smith MD, editors. Improving the Health of the Poor—Strategies for Prevention. Menlo Park, Calif: The Henry J. Kaiser Family Foundation; 1992. [Google Scholar]
- 36.Short PF, Cornelius L j, Goldstone DE. Health insurance of minorities in the United States. J Health Care Poor Underserved. 1990;1:9–24. doi: 10.1353/hpu.2010.0484. [DOI] [PubMed] [Google Scholar]
- 37.National Cancer Institute. Cancer Prevention Awareness Survey Wave II: Management Summary. Bethesda, Md: Office of Cancer Communications, National Cancer Institute, National Institutes of Health; 1987. [Google Scholar]
- 38.Daughton DM, Heatley SA, Prendergast JJ, et al. Effect of transdermal nicotine delivery as an adjunct to low-intervention smoking cessation therapy—a randomized, placebo-controlled, double-blind study. Arch Intern Med. 1991;151:749–52. [PubMed] [Google Scholar]
- 39.Transdermal Nicotine Study Group. Transdermal nicotine for smoking cessation—six-month results from two multicenter controlled clinical trials. JAMA. 1991;266:3133–8. [PubMed] [Google Scholar]
- 40.Hurt RD, Dale LC, Frederickson PA, et al. Nicotine patch therapy for smoking cessation combined with physician advice and nurse follow-up. JAMA. 1994;271:595–600. [PubMed] [Google Scholar]
- 41.Fiore MC, Jorenby DE, Baker TB, Kenford SL. Tobacco dependence and the nicotine patch. JAMA. 1992;268:2687–94. [PubMed] [Google Scholar]
- 42.Glynn TJ, Boyd GM, Gruman JC. Essential elements of self-help/minimal intervention strategies for smoking cessation. Health Educ Q. 1990;17:329–45. doi: 10.1177/109019819001700308. [DOI] [PubMed] [Google Scholar]
- 43.Glynn TJ, Boyd GM, Gruman JC. Bethesda, Md: Smoking and Tobacco Control Program, National Center Institute, USDHHS; 1990. Self-Guided Strategies for Smoking Cessation: A Program Planner's Guide. [Google Scholar]
- 44.U.S. Dept. of Health and Human Services. A Report of the Surgeon General. Washington, DC: U.S. Dept. of Health and Human Services DHHS publication (CDC); 1988. The Health Consequences of Smoking: Nicotine Addiction; pp. 88–8406. [Google Scholar]
- 45.Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol. 1983;51:390–5. doi: 10.1037//0022-006x.51.3.390. [DOI] [PubMed] [Google Scholar]
- 46.Voorhees CC, Stillman FA, Swank RT, Heagerty PJ, Levine DM, Becker DM. Heart, body and soul: impact of church-based smoking cessation interventions on readiness to quit. Prev Med. 1996;25:277–85. doi: 10.1006/pmed.1996.0057. [DOI] [PubMed] [Google Scholar]
- 47.U.S. Dept of Health and Human Services. Washington, DC: Government Printing Office; 1995. Healthy People 2000: Midcourse Review and 1995 Revisions. [Google Scholar]