Abstract
OBJECTIVE
Examine weight change in subjects receiving variable doses of transdermal nicotine replacement for smoking cessation.
DESIGN
Randomized, double-blind clinical trial.
SETTING
One-week inpatient treatment with outpatient follow-up through 1 year.
INTERVENTION
This report examines weight change after smoking cessation for 70 subjects randomized to placebo or to 11, 22, or 44 mg/d doses of transdermal nicotine. The study included 1 week of intensive inpatient treatment for nicotine dependence with active patch therapy continuing for another 7 weeks. Counseling sessions were provided weekly for the 8 weeks of patch therapy and with long-term follow-up visits at 3, 6, 9, and 12 months.
MEASUREMENTS AND MAIN RESULTS
Forty-two subjects were confirmed biochemically (i.e., by expired carbon monoxide) to be nonsmokers at all weekly visits during patch therapy. Their 8-week weight change from baseline was 3.0 ±2.0 kg. For these subjects, 8-week weight change was found to be negatively correlated with percentage of cotinine replacement (r=−.38, p=.012) and positively correlated with baseline weight (r=.48, p=.001), and age (r=.35, p=.025). Men had higher (p=.003) 8-week weight gain (4.0 ±1.8 kg) than women (2.1 ±1.7 kg). Of the 21 subjects who abstained continuously for the entire year, 20 had their weight measured at 1-year follow-up. Among these 20 subjects, 1-year weight change was not found to be associated with gender, baseline weight, baseline smoking rate, total dose of transdermal nicotine, or average percentage of cotinine replacement during the 8 weeks of patch therapy.
CONCLUSIONS
This study suggests that higher replacement levels of nicotine may delay postcessation weight gain. This effect is consistent for both men and women. We could not identify any factors that predict weight change with long-term abstinence from smoking.
Keywords: weight, smoking cessation, nicotine patch
Many studies have demonstrated that, for most smokers, cessation of smoking is accompanied by weight gain.1The acceptance of this consequence of smoking cessation is widespread, and many smokers report fear of weight gain as a reason for continuing to smoke or for their relapse to smoking after achieving abstinence.2,3,2–4Although there is little direct evidence that postcessation weight gain is a serious cause of smoking relapse,5the fact that fear of weight gain is a potential barrier to the initiation of cessation attempts makes the development of methods to control postcessation weight gain an important area of research for the treatment of nicotine dependence.
Postcessation weight gain is most likely influenced by changes in energy balance (caloric expenditure vs intake) that occur with smoking cessation. Klesges and coworkers have proposed a working model conceptualized as multiple variables moderating the energy balance equation, which in turn affects weight change.1An extensive discussion of potential biological and psychological mechanisms relevant to the relation between smoking, body weight, and energy balance can be found in the report of the task force from the National Working Conference on Smoking and Body Weight.6Finally, Perkins has proposed the possibility that modest weight gain after smoking cessation is largely unavoidable for most smokers because the intake of nicotine associated with smoking maintains an artificially reduced weight by increasing energy expenditure and thereby altering the regulation of body weight around a lower set point.5, 7Cessation removes this influence on energy expenditure, allowing body weight to return to “normal.”
If nicotine is an important variable influencing the energy balance equation, or the body weight set point, then nicotine replacement therapy should help control postcessation weight gain. Some studies involving nicotine gum have reported no difference in weight change associated with the use of gum during smoking cessation8,9,10,8–11; however, several studies report a significant weight-suppressing effect associated with gum use.2,3,4,5,6,7,12–18Similarly, a recent study of nicotine nasal spray found that, for subjects who abstained from smoking, the use of active spray effectively reduced weight gain.19Interestingly, most studies of transdermal nicotine replacement therapy have failed to demonstrate statistically significant effects on postcessation weight gain.10,1,2,3,20–24In a previous study using 22-mg transdermal nicotine patch therapy for 8 weeks, we observed that the median percentage of cotinine replacement in abstinent subjects receiving active patch therapy was 54%, with only 25.5% of the subjects achieving greater than 100% replacement.25Low maintenance levels of percentage of replacement may partially explain why previous patch studies have failed to detect significant weight-modifying effects of transdermal nicotine replacement therapy.5
The present study examined the effects of varying doses of transdermal nicotine replacement therapy on postcessation weight gain. For each individual, blood cotinine levels measured while smoking his or her usual number of cigarettes and while abstinent from smoking on active patch therapy were used to quantify nicotine replacement.
METHODS
This study was part of a randomized double-blind trial to determine the percentage of nicotine replacement achieved with transdermal nicotine doses of 11, 22, and 44 mg/d. The design of the study and outcomes relating to percentage of nicotine replacement and smoking cessation have been described in detail elsewhere.26
After an initial telephone screen, subjects came for an office visit, at which time the nature of the study was explained and informed consent was obtained. On the basis of their self-reported smoking rate obtained at the initial telephone screen, smokers were classified as follows: light smokers (10 –15 cigarettes per day [cpd]), moderate smokers (16 –30 cpd), and heavy smokers (more than 30 cpd). For each of these smoking rate categories, 24 subjects were recruited. After enrollment, the subjects were instructed to continue to smoke their usual number of cigarettes. Blood samples were collected, at either the subject's home or place of work, to determine the blood cotinine trough level (early morning before smoking) and peak level (6 hours after trough) while smoking their usual number of cigarettes. Within 2 weeks of baseline specimen collection, the subjects were admitted to a special inpatient unit at Saint Marys Hospital in eight groups with 6 to 11 subjects in each. Within each of the smoking rate categories, subjects were randomly assigned an initial dosage of placebo or 11, 22, or 44 mg/d of transdermal nicotine replacement. In all but 1 of the 12 subgroups defined by smoking rate and patch dose, we enrolled the requisite 6 subjects. We were able to recruit and enroll only 23 light smokers, which resulted in there being 5 rather than 6 light smokers randomly assigned to the 22-mg dose.
Baseline weight measurements were obtained on admission to the hospital, which was at about 3:00 pmon a Sunday. Subjects remained in the hospital until approximately 3:00 pmthe following Saturday. While subjects were in the hospital and on nicotine replacement, trough and peak blood samples for cotinine were collected each day. Measurements of weight, in street clothes without shoes, were obtained at approximately the same time each afternoon using a balance beam scale. During the hospital stay, all subjects received intensive nicotine dependence treatment based on our previous inpatient protocol.27
Following the hospital stay, all subjects continued active patch therapy for another 7 weeks. Subjects initially assigned to placebo patch for the inpatient phase were randomly assigned to either the 11 or 22 mg/d dose for the remaining 7 weeks of patch therapy. Subjects initially assigned to 11 or 22 mg/d continued on this dose for the remaining 7 weeks of patch therapy. After 4 weeks of patch therapy, those initially assigned to the 44 mg/d dose were reduced to 22 mg/d, which they continued for the remaining 4 weeks. No specific dietary intervention was prescribed, although prepared literature given to all subjects suggested ways to control weight. For the 7 weeks following hospital discharge, subjects returned weekly for group counseling sessions and then returned for long-term follow-up visits at 3, 6, 9, and 12 months. These weekly and monthly visits were scheduled for the early evening hours at times that remained consistent throughout the study for each group. At each visit, self-reported smoking status was obtained and carbon monoxide (CO) content of expired air was tested. Self-reported abstinence in the previous 7 days was considered biochemically confirmed if the expired air CO was ≤ 8 ppm. Weight was measured at weeks 2, 3, 4, 6, and 8, and at each of the long-term follow-up visits using a balance beam scale with subjects in street clothes and without shoes. At the week 8 follow-up visit, a blood sample was collected for cotinine analysis.
Data Analysis
For each subject, a steady state blood cotinine level while receiving transdermal nicotine at the dose assigned for the inpatient phase was determined from day 4 or 5 of the inpatient stay. Percentage of cotinine replacement for the inpatient phase was calculated by dividing the peak steady state blood cotinine level by the peak blood cotinine level obtained at baseline when the subject was smoking. As some subjects had a dose change during the 8 weeks of patch therapy (i.e., those initially assigned to placebo or 44 mg/d), the percentage of replacement achieved during the inpatient stay was not sustained for all 8 weeks. For each of these subjects, the average percentage of replacement for the 8 weeks of patch therapy was calculated using a weighted average of the inpatient and week 8 blood cotinine levels as the numerator.
Weight change from baseline to day 5 was used as the overall measure of weight change for the inpatient phase, and a two-way analysis of variance was used to determine whether weight change over the inpatient phase was associated with patch dose (placebo, 11 mg, 22 mg, 44 mg) or baseline smoking rate (light, moderate, heavy). The interaction term of dose by smoking level was included in this model to assess whether the effect of dose was specific to baseline smoking rate. Other statistical methods included Spearman rank correlation, one-sample signed-rank test, and two-sample rank-sum test. In all cases, two-sided tests were used with p values ≤ .05 used as evidence of findings not attributable to chance.
RESULTS
One subject, a light smoker assigned to the 44 mg/d dose, experienced symptoms of nicotine toxicity within 2 hours of the initial patch application. This subject was discontinued from patch therapy and excluded from all analyses. The baseline characteristics for the 70 subjects included in this report are summarized in Table 1). No significant differences were found between subjects assigned to placebo, 11 mg, 22 mg, or 44 mg with respect to the baseline characteristics of gender, age, weight, or smoking rate (cpd).
Table 1.
Baseline Patient Characteristics (n=70)

Weight Change at Completion of Inpatient Phase
All subjects abstained from smoking for the inpatient phase of the study. For placebo, 11 mg/d, and 22 mg/d doses, mean weight change from baseline was significantly greater than 0 on day 1 and remained so throughout the rest of the inpatient phase. For the 44 mg/d dose, weight change from baseline was not significantly greater than 0 until days 4 and 5 (Fig. 1). Weight change from baseline on day 5 was used as the overall measure of weight change for the inpatient phase. Overall, weight change was 1.3 ± 1.2 kg (mean ± SD), and 29% of subjects experienced a weight gain of more than 2 kg (Table 2 Weight change was not significantly associated with patch dose or baseline smoking rate. In addition, weight change was not significantly associated with gender, baseline blood cotinine level, baseline weight, age, or steady state percentage of cotinine replacement.
Figure 1.

Mean weight change from baseline during the inpatient phase according to patch dose. For placebo, 11-mg, and 22-mg doses, weight change was significantly greater than 0 on day 1 and remained so throughout the rest of the inpatient phase. For the 44-mg dose, weight change from baseline was significantly greater than 0 for days 4 and 5 of the inpatient phase.
Table 2.
Distributionof Weight Change According to Smoking Status*

Weight Change at Completion of Patch Therapy
Weight at the week 8 visit was obtained for 68 of the 70 subjects included in this report. Of these 68 subjects, 66 returned for their week 8 follow-up visit within 2 days of the 8-week anniversary of the start of their inpatient stay, while one returned at 9 weeks and one at 10 weeks. For subjects whose week 8 visit was not on their 8-week anniversary date, weight for week 8 was obtained via linear interpolation. For these 68 subjects, including those who relapsed to smoking, 8-week weight change was positively associated with baseline weight (r= .46, p < .001); and men gained more weight (3.7 ± 1.9 kg) than women (2.1 ± 1.7 kg, p < .001).
Of the 70 subjects included in this report, 42 remained abstinent from smoking for the entire 8 weeks. These continuous abstainers returned for all scheduled follow-up visits, reported no smoking for the entire 8 weeks, and had expired CO measurements ≤ 8 ppm at all visits. Although not significantly different, the 42 subjects who continuously abstained had a larger mean 8-week weight gain than the subjects who had relapsed to smoking (3.0 ± 2.0 kg vs 2.5 ± 1.9 kg) (Table 2). An analysis was performed to determine factors associated with 8-week weight change from baseline in the subjects with continuous abstinence. Of these 42 subjects, 16 received 44 mg for the first 4 weeks followed by 22 mg for the second 4 weeks, 8 received 22 mg for the entire 8 weeks, 7 received 11 mg for the entire 8 weeks, 6 received placebo for the first week followed by 11 mg for the last 7 weeks, and 5 received placebo for the first week followed by 22 mg for the last 7 weeks. The 8-week weight change from baseline was not associated with baseline smoking rate (cpd), or total dose of transdermal nicotine received over the 8 weeks; however, a significant negative correlation was found between 8-week weight change and average percentage of cotinine replacement over the 8 weeks of patch therapy (r= −.38, p= .012). Univariately, the 8-week weight change for subjects with continuous abstinence was also positively correlated with baseline weight (r= .48, p= .001) and age (r= .35, p= .025), and men had a significantly larger weight gain than women (4.0 ± 1.8 kg vs 2.1 ± 1.7 kg, p= .003) (Table 3)
Factors Univariately Associated with 8-Week Weight Change in 42 Subjects with Continuous Abstinence for 8 Weeks
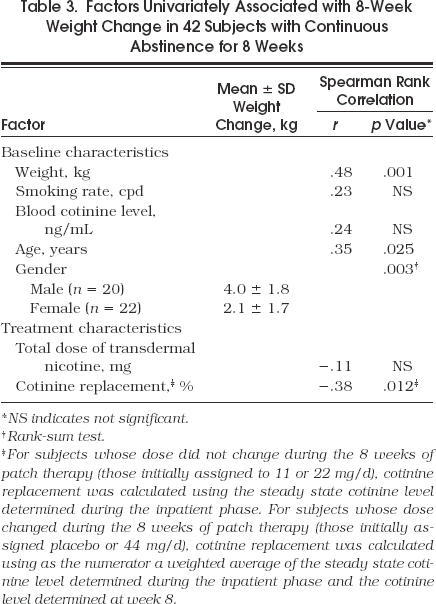
To determine whether percentage of cotinine replacement had an independent effect after adjusting for the other characteristics found to be univariately significant, a multiple linear regression model was used with 8-week weight change as the dependent variable and percentage of cotinine replacement, baseline weight, age, and gender as independent variables. In a model that included all univariate significant variables, a lower percentage of cotinine replacement (p= .038) and male gender (p= .013) were found to be independent predictors of larger 8-week weight gain. Furthermore, when nonsignificant variables were eliminated from this model using a backward stepwise approach, the only remaining simultaneously significant variables were percentage of cotinine replacement (p= .029) and gender (p = .001) (Fig. 2). From this analysis, the regression coefficient associated with percentage of cotinine replacement is consistent with an average decrease in 8-week weight gain of 0.15 kg for every 10 percentage point increase in percentage of cotinine replacement. In Figure 2, one data point appears to be a potential outlier; a light smoker assigned to the 44 mg/d dose achieved 228% cotinine replacement and gained 2.0 kg. The exclusion of this outlier from the analysis was not found to change the results.
Figure 2.
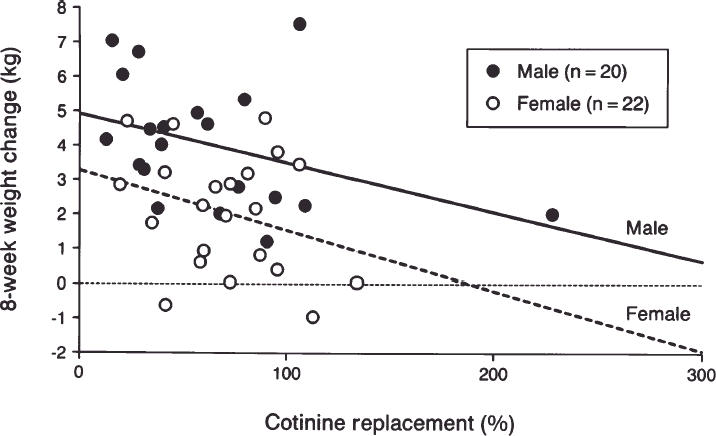
Scatterplot of 8-week weight change from baseline against percentage of cotinine replacement for 42 subjects who were continuous abstainers for the entire 8 weeks of patch therapy. Linear regression lines, calculated separately for each gender, are included.
To determine whether weight gain immediately following discontinuation of patch therapy was associated with dose of transdermal nicotine or level of replacement, we analyzed the weight change from week 8 to week 13 for 37 subjects who were continuous abstainers for the entire first 13 weeks. The weight change 5 weeks after patch therapy was not associated with percentage of cotinine replacement and not significantly different for subjects who had been assigned to the 22 mg/d dose for the last 4 weeks of patch therapy (n= 24, 1.8 ± 1.9 kg) versus those assigned to 11 mg/d for the last 4 weeks of patch therapy (n = 13, 1.4 ± 1.3 kg).
Weight Change at 1 Year
Twenty subjects were continuous abstainers for the entire year and had their weight recorded at the 1-year follow-up visit. Continuous abstainers had a significantly larger 1-year weight gain (6.2 ± 5.1 kg) than subjects who were smoking (2.9 ± 4.1 kg, p= .015) (Table 2). In subjects with continuous abstinence, no significant difference was found in 1-year weight change for women (n= 9, 7.3 ± 5.7 kg) versus men (n= 11, 5.2 ± 4.6 kg). Univariately the 1-year weight change in subjects with continuous abstinence was not significantly associated with baseline smoking rate (cpd), baseline blood cotinine level, baseline weight, total dose of transdermal nicotine, or the average percentage of cotinine replacement for the 8 weeks of patch therapy. There was some evidence of a negative correlation between 1-year weight change and age (r= −.46, p = .049).
DISCUSSION
This study provides important information for use in counseling smokers about the weight issues surrounding smoking cessation. First, it offers statistically significant evidence that transdermal nicotine replacement therapy may attenuate postcessation weight gain. Among those continuously abstinent from smoking over the 8 weeks of patch therapy, weight gain was inversely correlated with the percentage of cotinine replacement achieved using nicotine patches. Although other studies using transdermal nicotine patch have reported less weight gain in subjects treated with active versus placebo patch, most have not demonstrated statistically significant differences.10,1,2,3,20–24A likely explanation for the lack of significant findings in previous patch studies is the low level of replacement achieved with the various doses of transdermal nicotine used. The current study included four doses of transdermal nicotine (placebo to 44 mg/d) in a design stratified and balanced on baseline smoking level. This design produced a wide range of replacement levels with some subjects receiving 100% replacement or greater for the entire 8 weeks of patch therapy. The estimated mean percentage of cotinine replacement in participants receiving a 22-mg patch who had been smoking 20, 30, and 40 cpd at baseline was 62%, 51%, and 41%, respectively.26Previous patch studies that evaluated weight change used patch doses of 21 mg/d or less.1,2,3,20–24Thus most, if not all, previous studies probably produced replacement levels significantly lower than 100% in most subjects. Using high-dose transdermal nicotine therapy to achieve 100% replacement has been demonstrated to be safe and well tolerated and can result in less weight gain during patch use.26, 28, 29
Women gained less weight than men over the 8 weeks of patch use. Other studies using nicotine gum have also demonstrated this difference.1, 18The differential weight gain for men versus women was consistent over the observed range of cotinine replacement (Fig. 2). This indicates that the association of weight gain with percentage of cotinine replacement is not dependent on gender. This finding is important because women report substantially more concern than men about weight gain on quitting smoking, and are more likely than men to report using smoking as a weight control strategy.3, 11
Rapid weight gain may occur in the first week of cessation and is probably related to changes in body fluid. During the inpatient phase, subjects experienced significant weight gain within days after stopping smoking irrespective of dose of nicotine patch or percentage of cotinine replacement. In a prospective examination of tobacco withdrawal symptoms conducted in an inpatient environment, Hatsukami et al. found that subjects who abstained from smoking for 4 days experienced statistically significant increases in body weight and caloric intake. Although not statistically significant, an increase in fluid input and output was also observed.10,1,30–32A recent study reported that early, rapid weight gain following smoking cessation may include a larger proportion of water than that expected by changes in energy balance.33During the inpatient phase of our study, the average 24-hour urine volumes increased by more than 1,000 mL compared with baseline (data not shown). This suggests that some of the weight gain may have been due to increased fluid intake, and other changes in fluid balance, rather than gains in body mass. These findings may be important when counseling patients who experience rapid weight gain in the first week or so after stopping smoking.
Finally, two important caveats are evident. As Figure 2 illustrates, 100% replacement does not completely eliminate weight gain. This suggests that factors other than nicotine also affect postcessation weight gain. Our data also demonstrate that nicotine replacement therapy attenuates weight gain only during the period of use. For subjects who remained abstinent from smoking for an entire year, 1-year weight change was not associated with the level of cotinine replacement achieved during patch therapy. These findings are consistent with findings from trials using nicotine gum that have shown its use attenuates weight gain, but only during the period of use.5, 2,3,4,5,6,7,12–18
As with all investigations, our study has both strengths and limitations. We had nearly 100% follow-up on all subjects through 1 year with carefully monitored blood draws and consistent measurements over time. By randomizing to four levels of patch dose within three strata defined by baseline smoking rate, we were able to observe a wide range of replacement levels. However, because subjects randomized to 44 mg/d had their dose reduced to 22 mg/d after 4 weeks, we could not assess the full impact of long-term treatment with 44 mg/d on weight change. We did not control or measure changes in caloric intake or activity pattern, although our findings suggest that even when subjects were allowed to maintain their own diet and activity program, nicotine replacement moderated weight gain. Like most investigations of postcessation weight change, our analysis of potential predictors was restricted to subjects who abstained from smoking. Although continuous abstainers represented a high percentage of the study subjects at both 8 weeks and 1 year (60% and 30%, respectively), the actual number of subjects included in the analysis was small (42 at 8 weeks and 20 at 1 year). This limits the power of the study, especially with respect to factors associated with 1-year weight change.
In summary, this study suggests that more complete nicotine replacement levels achieved with transdermal nicotine are effective in delaying, but not preventing, some of the weight gain associated with smoking cessation. This and other studies have demonstrated the safety and tolerability of high-dose nicotine replacement.26, 28, 29How to most effectively integrate smoking cessation and weight control strategies remains unresolved. Instituting behavioral programs for weight control concurrent with smoking cessation programs has been shown to be counterproductive.4, 11Nonetheless, we believe that individual patients may benefit from the weight control aspects of high–dose nicotine replacement therapy. If a smoker (especially female) is resistant to or extremely concerned about stopping because of fear of weight gain, we suggest doses of transdermal nicotine that will achieve a target replacement level of 100% or greater (carefully monitored by serum cotinine levels). This will capitalize on transdermal nicotine's ability to help patients stop smoking, and at the same time control postcessation weight gain. Once comfortable with abstinence from smoking, dietary and activity changes to limit weight gain could be initiated as the nicotine replacement is tapered.
REFLECTIONS
Sensor
Is the pain you feel
Within sharp or dull
Steady or fleeting
Does it tighten the
Corners of your mouth
Ever so little
Or dilate an eye
Can it be measured
And interpreted
Reckoned and summed by
A machine or will
A lover's finger
As it runs from cheek
To neck to breast to
Abdomen draw the
Inwardness away
For just a moment
Just enough to tell
Plaint from alarm in
The unwilled clamor
Of tissue and bone
Emanuel E. Garcia, MD
Philadelphia, Pa.
REFERENCES
- 1.Klesges RC, Meyers AW, Klesges LM, Lavasque ME. Smoking, body weight, and their effects on smoking behavior: a comprehensive review of the literature. Psychol Bull. 1989;106:204–30. doi: 10.1037/0033-2909.106.2.204. [DOI] [PubMed] [Google Scholar]
- 2.Sorenson G, Pechacek TF. Attitudes toward smoking cessation among men and women. J Behav Med. 1987;10:129–37. doi: 10.1007/BF00846421. [DOI] [PubMed] [Google Scholar]
- 3.Klesges RC, Klesges LM. Cigarette smoking as a dieting strategy in a university population. Int J Eat Disord. 1988;7:413–19. [Google Scholar]
- 4.Gritz ER, St. Jeor ST, Bennett G, et al. Implications with respect to intervention and prevention. Health Psychol. 1992;11(suppl.):7–25. doi: 10.1037/h0090341. [DOI] [PubMed] [Google Scholar]
- 5.Perkins KA. Issues in the prevention of weight gain after smoking cessation. Ann Behav Med. 1994;16:46–52. [Google Scholar]
- 6.Grunberg NE, Greenwood MR, Collins F, et al. Mechanisms relevant to the relations between cigarette smoking and body weight. Health Psychol. 1992;11(suppl.):4–9. doi: 10.1037/h0090345. [DOI] [PubMed] [Google Scholar]
- 7.Perkins KA. Effects of tobacco smoking on caloric intake. Br J Addict. 1992;87:193–205. doi: 10.1111/j.1360-0443.1992.tb02693.x. [DOI] [PubMed] [Google Scholar]
- 8.Hjalmarson AIM. Effect of nicotine chewing gum in smoking cessation: a randomized, placebo-controlled double-blind study. JAMA. 1984;252:2835–8. [PubMed] [Google Scholar]
- 9.Hall SM, Ginsberg D, Jones RT. Smoking cessation and weight gain. J Consult Clin Psychol. 1986;54:342–6. doi: 10.1037//0022-006x.54.3.342. [DOI] [PubMed] [Google Scholar]
- 10.Tonnesen P, Fryd V, Hansen M, et al. Effect of nicotine chewing gum in combination with group counseling on the cessation of smoking. N Engl J Med. 1988;318:15–8. doi: 10.1056/NEJM198801073180104. [DOI] [PubMed] [Google Scholar]
- 11.Pirie PL, McBride CM, Hellerstedt W, et al. Smoking cessation in women concerned about weight. Am J Public Health. 1992;82:1238–43. doi: 10.2105/ajph.82.9.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emont SL, Cummings KM. Weight gain following smoking cessation: a possible role for nicotine replacement in weight management. Addict Behav. 1987;12:151–5. doi: 10.1016/0306-4603(87)90021-9. [DOI] [PubMed] [Google Scholar]
- 13.Fagerstrom KO. Reducing the weight gain after stopping smoking. Addict Behav. 1987;12:91–3. doi: 10.1016/0306-4603(87)90015-3. [DOI] [PubMed] [Google Scholar]
- 14.Hajek P, Jackson P, Belcher M. Long-term use of nicotine chewing gum: occurrence, determinants, and effects on weight gain. JAMA. 1988;260:1593–6. [PubMed] [Google Scholar]
- 15.Gross J, Stitzer ML, Malonado J. Nicotine replacement: effects on postcessation weight gain. J Consult Clin Psychol. 1989;57:87–92. doi: 10.1037//0022-006x.57.1.87. [DOI] [PubMed] [Google Scholar]
- 16.Killen JD, Fortmann SP, Newman B. Weight change among participants in a large sample minimal contact smoking relapse prevention trial. Addict Behav. 1990;15:323–32. doi: 10.1016/0306-4603(90)90042-v. [DOI] [PubMed] [Google Scholar]
- 17.Nides M, Rand C, Dolce J, et al. Weight gain as a function of smoking cessation and 2-mg nicotine gum use among middle-aged smokers with mild lung impairment in the first 2 years of the lung health study. Health Psychol. 1994;13:354–61. doi: 10.1037//0278-6133.13.4.354. [DOI] [PubMed] [Google Scholar]
- 18.Leischow SJ, Sachs DP, Bostrom AG, Hansen MD. Effects of differing nicotine-replacement doses on weight gain after smoking cessation. Arch Fam Med. 1992;1(2):233–7. doi: 10.1001/archfami.1.2.233. [DOI] [PubMed] [Google Scholar]
- 19.Sutherland G, Stapleton JA, Russell MA, et al. Randomized controlled trial of nasal nicotine spray in smoking cessation. Lancet. 1992;340:324–9. doi: 10.1016/0140-6736(92)91403-u. [DOI] [PubMed] [Google Scholar]
- 20.Abelin T, Muller P, Buehler A, Vesanen K, Imhof PR. Controlled trial of transdermal nicotine patch in tobacco withdrawal. Lancet. 1989;1:7–10. doi: 10.1016/s0140-6736(89)91671-1. [DOI] [PubMed] [Google Scholar]
- 21.Rose JE, Levin ED, Behm FM, Adivi C, Schur C. Transdermal nicotine facilitates smoking cessation. Clin Pharmacol Ther. 1990;47:323–30. doi: 10.1038/clpt.1990.35. [DOI] [PubMed] [Google Scholar]
- 22.Tonneson P, Norregaard J, Simonsen K, Sawe U. A double-blind trial of a 16-hour transdermal nicotine patch in smoking cessation. N Engl J Med. 1991;325:311–5. doi: 10.1056/NEJM199108013250503. [DOI] [PubMed] [Google Scholar]
- 23.Transdermal Nicotine Study Group. Transdermal nicotine for smoking cessation. JAMA. 1991;266:3133–8. [PubMed] [Google Scholar]
- 24.Sachs DPL, Sawe U, Leischow SJ. Effectiveness of a 16-hour transdermal nicotine patch in a medical setting, without intensive group counseling. Arch Intern Med. 1993;153:1881–90. [PubMed] [Google Scholar]
- 25.Hurt RD, Dale LC, Fredrickson PA, et al. Nicotone patch therapy for smoking cessation combined with physician advice and nurse follow-up. JAMA. 1994;271:595–600. [PubMed] [Google Scholar]
- 26.Dale LC, Hurt RD, Offord KP, Lawson GM, Croghan IT, Schroeder DR. High dose nicotine patch therapy: percentage of replacement and smoking cessation. JAMA. 1995;274:1353–8. [PubMed] [Google Scholar]
- 27.Hurt RD, Dale LC, Offord KP, Bruce BK, McClain LF. Inpatient treatment of severe nicotine dependence. Mayo Clin Proc. 1992;67:823–8. doi: 10.1016/s0025-6196(12)60819-2. [DOI] [PubMed] [Google Scholar]
- 28.Fredrickson PA, Hurt RD, Lee GM, et al. High dose transdermal nicotine therapy for heavy smokers: safety, tolerability and measurement of nicotine and cotinine levels. Psychopharmacology. 1995;122:215–22. doi: 10.1007/BF02246542. [DOI] [PubMed] [Google Scholar]
- 29.Jorenby DE, Smith SS, Fiore MC, et al. Varying nicotine patch dose and type of smoking cessation counseling. JAMA. 1995;274:1437–52. [PubMed] [Google Scholar]
- 30.Hatsukami D, LaBounty L, Jughes J, Laine D. Effects of tobacco abstinence on food intake among cigarette smokers. Health Psychol. 1993;12(6):499–502. doi: 10.1037//0278-6133.12.6.499. [DOI] [PubMed] [Google Scholar]
- 31.Hatsukami DK, Hughes JR, Pickens RW, Svikis D. Tobacco withdrawal symptoms: an experimental analysis. Psychopharmacology. 1984;84:231–6. doi: 10.1007/BF00427451. [DOI] [PubMed] [Google Scholar]
- 32.Hatsukami DK, Hughes JR, Pickens RW, Svikis D. Erratum: Tobacco withdrawal symptoms: an experimental analysis. Psycho-pharmacology. 1992;108:390. doi: 10.1007/BF00427451. [DOI] [PubMed] [Google Scholar]
- 33.Terry RB, Ward M, Swan G. Weight gain in early smoking cessation may include more water than fat. Addiction. 1996;91(1):144. . (Abstract). [Google Scholar]


