Abstract
OBJECTIVE
To examine the association of clinic HIV-focused features and advanced HIV care experience with Pneumocystis carinii pneumonia (PCP) prophylaxis and development of PCP as the initial AIDS diagnosis.
DESIGN
Nonconcurrent prospective study.
SETTING
New York State Medicaid Program.
PARTICIPANTS
Medicaid enrollees diagnosed with AIDS in 1990–1992.
MEASUREMENTS AND MAIN RESULTS
We collected patient clinical and health care data from Medicaid files, conducted telephone interviews of directors of 125 clinics serving as the usual source of care for study patients, and measured AIDS experience as the cumulative number of AIDS patients treated by the study clinics since 1986. Pneumocystis carinii pneumonia prophylaxis in the 6 months before AIDS diagnosis and PCP at AIDS diagnosis were the main outcome measures. Bivariate and multivariate analyses adjusted for clustering of patients within clinics. Of 1,876 HIV-infected persons, 44% had PCP prophylaxis and 38% had primary PCP. Persons on prophylaxis had 20% lower adjusted odds of developing PCP (95% confidence interval [CI] 0.64, 0.99). The adjusted odds of receiving prophylaxis rose monotonically with the number of HIV-focused features offered by the clinic, with threefold higher odds (95% CI 1.6, 5.7) for six versus two or fewer such features. Patients in clinics with three HIV-focused features had 36% lower adjusted odds of PCP than those in clinics with one or none. Neither clinic experience nor specialty had a significant association with prophylaxis or PCP.
CONCLUSIONS
PCP prevention in our study cohort appears to be more successful in clinics offering an array of HIV-focused features.
Keywords: Pneumocystis carinii pneumonia (PCP), AIDS, clinical competence, ambulatory care, case management
From the earliest years of the human immunodeficiency virus type 1 (HIV) epidemic, Pneumocystis carinii pneumonia (PCP) has been one of the most common and feared clinical complications.1,2,1–3Pneumocystis carinii pneumonia preventive therapy 4 and guidelines for the use of these agents 5 represent a major breakthrough in HIV clinical care because these drugs improve survival 6, 7 and save millions of dollars in health care costs yearly.8, 9 Yet even among persons with medical coverage for such care, use of PCP prophylactic drugs is deficient.10 Specific groups including women, illicit drug users, and persons of color often fail to receive prophylaxis.1,10–12 Little is known about the association of provider characteristics with delivery of PCP prophylaxis. Provider experience in AIDS care has been linked to improved HIV patient survival.3,4,13–15 This benefit may be mediated in part by improved HIV preventive care. Similar to preventive health care in general populations,16, 17 use of PCP prophylaxis may be greater in clinics featuring reminder systems and physician training or feedback. In this article, we examined two outcomes, delivery of PCP prophylaxis and primary PCP, and associations with study clinics' experience with AIDS care, specialty, and HIV-focused features.
METHODS
Clinic and Patient Data
Patient data were obtained from the New York State Medicaid HIV/AIDS Research Data Base and clinic data from a previously reported survey of clinics managing the care of patients with AIDS.18 The HIV/AIDS Data Base offered demographic data and longitudinally linked inpatient and outpatient claims for Medicaid enrollees with advanced HIV infection from 1984 through 1992. Criteria for entry into the database have been tested against AIDS registry data,19 and use patterns of AIDS-related diagnoses and HIV-specific treatments. Key clinical and demographic elements in the database have been rigorously evaluated.20
We surveyed 197 clinics identified from the HIV/AIDS Data Base as the usual source of care for one or more Medicaid enrollees initially diagnosed with AIDS in 1990.21 The usual source of care must have been visited at least twice and for more than 50% of a patient's ambulatory encounters.22 Sites that typically do not deliver longitudinal ambulatory care, such as emergency departments or surgical centers, were not considered in this process.
One-half hour telephone interviews, designed by five HIV expert clinicians, were conducted from December 1993 to April 1994 and completed by 179 (91% response rate) clinic medical directors or other administrators knowledgeable about clinic services and other features. Respondents were asked about clinic setting (i.e., hospital or free-standing), specialty, and the proportions of HIV-infected patients as well as the entire clinic enrolled on Medicaid. The director assessed the continuity of care for physicians and their HIV-infected patients on a Likert-type scale (1 = low to 10 = high). The clinic survey obtained the following information on HIV-focused features as well as their availability on-site or only in the institution: (1) a director of HIV/AIDS ambulatory services; (2) the use of clinicians outside of clinic staff to care for persons with PCP (comanagement); (3) the delivery of aerosolized pentamidine; (4) a HIV case manager who contacts HIV-infected patients at least every 3 months; (5) a protocol for initial HIV care evaluation; (6) multidisciplinary conferences on HIV care; and (7) enrollment in clinical trials. Of these HIV-related clinic attributes, only enrolling in clinical trials was not associated with the study outcomes (p>.40) and not considered further.
From pharmacy claims, we identified PCP prophylaxis medications (i.e., trimethoprim-sulfamethoxazole, aerosolized pentamidine, or dapsone),23 and antiretroviral drugs approved during our study years (i.e., zidovudine, didanosine, or zalcitabine). As in our previous work,10 PCP prophylaxis was defined as at least two claims for any prophylactic drug more than 30 but no more than 60 days apart. Claims preceding an episode of PCP by less than 1 month were excluded as more likely for treatment of active disease. If treatment had been initiated more than 3 weeks before other (non-PCP) inpatient stays, prophylaxis was considered continued in the hospital (inpatient medication data unavailable).
Medicaid files also offer gender, age, location of residence, and duration of Medicaid eligibility, but not racial-ethnic data. Clinical data on New York State Medicaid claims are coded by the International Classification of Diseases, Ninth Revision, Clinical Modification(ICD-9-CM), with up to five diagnoses per inpatient stay and two per outpatient visit. The date of first clinical AIDS diagnosis was specified according to the 1987 revision of the Centers for Disease Control and Prevention (CDC) AIDS Surveillance Case Definition that was operative in all the years of our study.21 A primary episode of PCP was identified from a coded inpatient diagnosis or, if recorded on an outpatient claim, when appearing on two claims at least 1 week apart. Because of drug interactions, intolerance, or toxicity, prophylaxis was expected to be less likely for persons with another chronic disease. Comorbid chronic disease during the year before AIDS diagnosis was identified from diagnoses for diabetes mellitus, asthma, emphysema, hypertension, coronary artery disease, peripheral vascular disease, alcoholic liver disease, rheumatoid arthritis, or renal insufficiency.10 A validated methodology identified illicit drug users from inpatient or outpatient coded diagnoses indicating dependence or abuse of cocaine, heroin, or other illicit substances (ICD-9-CM 304.0x, 304.2x, 304.5x, 304.7x, 305.5x, 305.6x, 304.9x), diagnostic-related groups (433–435), or from claims for drug treatment services such as methadone maintenance.20
Clinic specialty categories were constructed from survey responses: HIV/AIDS specialty (i.e., infectious disease or self-designated HIV/AIDS), hospital-based primary care (i.e., general internal medicine, family practice, general practice, or mixed general-subspecialty clinic), and free-standing primary care or other (i.e., community-based primary care clinics and obstetrics-gynecology or surgery clinics regardless of setting). Free-standing primary care and obstetrics-gynecology and surgery clinics were combined for analysis owing to similar associations with the outcome variables. Analyses of patients in free-standing primary care clinics alone produced similar results.
The AIDS experience of each study patient's clinic was determined by a three-step calculation. First, for each study year, we analyzed patterns of care for the study cohort on the New York State AIDS Data Base and determined the cumulative number of Medicaid enrollees with AIDS for whom each study clinic served as the usual source of care (in the year before and/or 3 months after the first AIDS-related condition). Second, for each study patient, we determined the cumulative number of AIDS Medicaid enrollees followed by the same clinic from 1986 through that patient's year of AIDS diagnosis. Third, to estimate the total AIDS experience, the cumulative number of Medicaid AIDS patients was divided by the clinic director's estimate of the proportion of the clinic's HIV population covered by Medicaid.
Study Population
We studied Medicaid enrollees whose first AIDS-related diagnosis was in 1990 through 1992, HIV infection was documented more than 2 months before AIDS diagnosis, and usual source of care was surveyed. Of 5,007 New York State Medicaid enrollees aged 13 to 60 years in the HIV/AIDS database, we excluded 1,632 with AIDS diagnosed before 1990, 453 with less than 6 months on Medicaid before AIDS diagnosis, 372 with their first HIV-related diagnosis or service less than 2 months before AIDS diagnosis, and 674 with 50% or less of their ambulatory visits with a surveyed clinic. The final study cohort totaled 1,876 persons treated by 125 (70%) of the surveyed clinics.
Analysis
Our two dependent variables were prescribed PCP prophylaxis within 6 months before AIDS diagnosis, and PCP as the initial AIDS diagnosis. We accounted for clustering of patients within clinics by estimating bivariate and multivariate logistic regression models using a general estimating equation (GEE) algorithm with the independence working correlation matrix.24 This analysis allows for the correlation of patients within clinics. As that correlation is not perfect, it is preferred to using the clinic as the unit of analysis. All p values and confidence intervals reported are robust to the correlation specification. We also examined the bivariate association of AIDS care experience with specific HIV services offered by the clinic and clinic specialty using the χ2 test. The bivariate association of the number of HIV services by clinic specialty and by AIDS experience was examined using the Kruskal-Wallis test.
Continuity of care and AIDS experience were grouped initially by quartile for analysis and, based on bivariate cluster-corrected results, grouped at the median in the multivariate models. Notably, analyses using the lowest and highest experience quartiles as cutpoints did not change the multivariable results. After analysis by decade, age was collapsed into two categories. The proportion of clinic patients on Medicaid was divided into 100% (the third tertile) and less than 100% because Medicaid-only clinics may be better equipped to deal with this population's social and health care issues.
For each outcome we examined two multivariate models adjusting for patient characteristics and differing clinic variables. The first model examined AIDS experience, clinic specialty, and the number of HIV-focused features. The second model included indicators for each HIV-related feature associated with the outcome in the bivariate analysis at p < .20. Analysis of first-degree interactions showed one between gender and illicit drug use so these two factors were categorized for the multivariate analysis into four combinations. We also observed an interaction between gender and year in the PCP prophylaxis model. Because of the complexity of interpreting the interactions between gender, drug use, and year, we report this gender-year interaction only descriptively. No significant (p < .05) interactions appeared between year and clinic characteristics. The GEE algorithm does not have a goodness-of-fit statistic so we obtained a Hosmer-Lemeshow statistic from ordinary logistic models.25 All models presented in tables have a p value from the Hosmer-Lemeshow statistic of .4 or greater.
RESULTS
Patient Characteristics
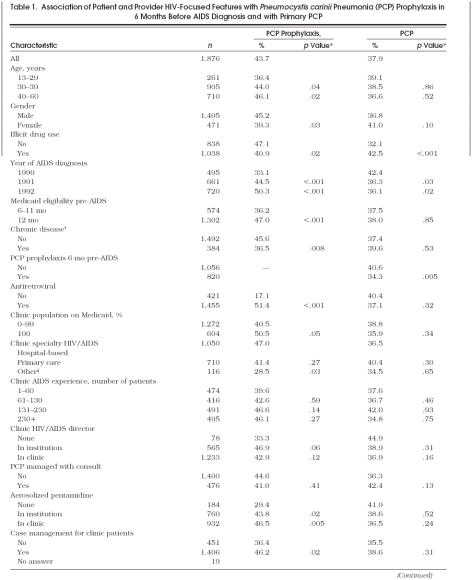
Of 1,876 individuals in our cohort, 44% had PCP prophylaxis and 38% had PCP as the initial AIDS-defining diagnosis. In bivariate analyses (Table 1), women, drug users, and persons with another chronic disease such as diabetes or hypertension were less likely to receive prophylaxis. Of these characteristics, only drug use was associated with a higher likelihood of PCP. Prophylaxis was more likely for older age groups and persons with complete Medicaid coverage in the year before AIDS diagnosis. The proportion of the cohort on prophylaxis increased over time, but the decline in the proportion with PCP was less dramatic and leveled off in 1992. Antiretroviral therapy was associated with increased proportions on PCP prophylaxis while PCP prophylaxis itself was strongly associated with a lower rate of PCP.
Table 1.
Association of Patient and Provider HIV-Focused Features with Pneumocystis carinii Pneumonia (PCP) Prophylaxis in 6 Months Before AIDS Diagnosis and with Primary PCP
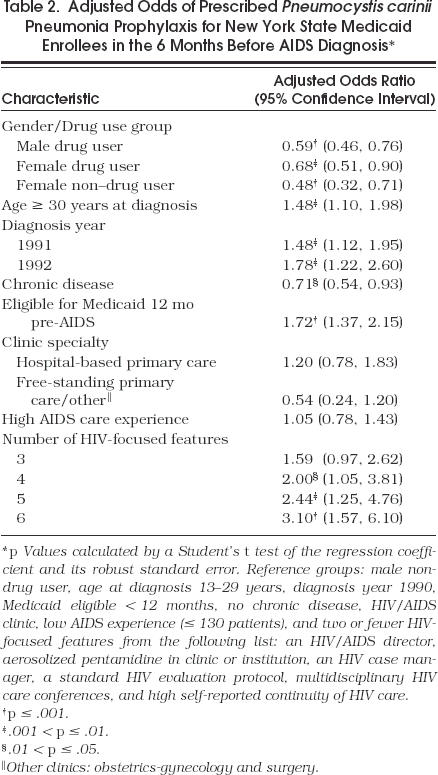
After adjustment, persons aged at least 30 years or with longer Medicaid eligibility were still more likely to be on prophylaxis, while those with another chronic disease were 30% less likely (Table 2 The multivariate analyses showed a significant interaction for gender by drug use and for gender by year of diagnosis (in the PCP prophylaxis model only). The adjusted odds of prescribed PCP prophylaxis for drug users of both genders and for female non–drug users were 30% to 50% lower than male non–drug users. To explore the year effect, we examined bivariate associations of four gender–drug use categories by year of diagnosis. In 1990, proportions on prophylaxis were 20% for female drug users, 32% for male drug users, 26% for female non–drug users, and 44% for male non–drug users. By 1992, the differences across these four groups had decreased substantially: 55% for female drug users, 46% for male drug users, 46% for female non–drug users, and 54% for male non–drug users.
Table 2.
Adjusted Odds of Prescribed Pneumocystis carinii Pneumonia Prophylaxis for New York State Medicaid Enrollees in the 6 Months Before AIDS Diagnosis*
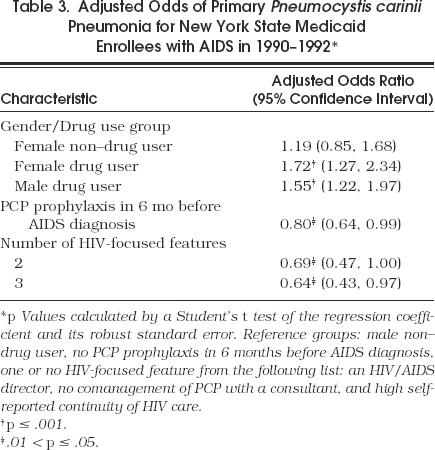
In the multivariate model with PCP as the dependent variable, PCP prophylaxis before AIDS diagnosis was associated with a 20% reduction in the adjusted odds of PCP. Only drug users had increased adjusted odds of developing PCP compared with male non–drug users (Table 3)
Table 3.
Adjusted Odds of Primary Pneumocystis carinii Pneumonia for New York State Medicaid Enrollees with AIDS in 1990–1992*
Clinic Characteristics
HIV/AIDS specialty clinics managed 56% of the cohort. Of patients in free-standing primary care or other clinics, most (n= 112) were in primary care clinics with only a few (n= 4) in surgical or obstetrics-gynecology clinics. Patients in free-standing primary care or other clinics were less likely to have PCP prophylaxis prescribed than those in HIV/AIDS clinics. The cumulative AIDS experience of clinics ranged from 1 to 1,000 patients with a median of 131. The proportion of patients on prophylaxis increased with experience, but this was not significant, nor was experience related to the proportion with PCP (Table 1). Neither clinic specialty nor AIDS experience had an adjusted association with prophylaxis (Table 2).
Although many clinic HIV-related features had higher proportions of patients on prophylaxis, significant associations appeared for only three: aerosolized pentamidine on-site, case management, and multidisciplinary conferences on HIV care (Table 1). Among patients on prophylaxis, aerosolized pentamidine was used more commonly by those in clinics offering the drug on-site than by those in clinics that did not (46% vs 24%, p= .002). Director-rated greater continuity of care was the only clinic feature associated with a lower rate of PCP.
The number of clinic HIV-focused features varied significantly by specialty with an average of 5.2 services for HIV/AIDS clinics compared with 3.7 and 3.6 features (p < .001), respectively, for free-standing primary care or other clinics and hospital-based primary care clinics. The number of features also differed between highest and lowest AIDS experience quartiles (4.3 vs 4.8, respectively, p < .001).
We observed a striking association between the total number of clinic HIV-focused features and patient receipt of PCP prophylaxis (Table 2). All the features listed on Table 1 were considered except for the use of consultants in managing patients with PCP as this characteristic was poorly associated with prophylaxis. Patients in sites with two or fewer such features (comprising 18% of the study cohort) served as the reference group. As the number of HIV features rose, the adjusted odds of prophylaxis increased monotonically with patients in clinics with six HIV-related features (31% of the cohort) having threefold higher adjusted odds of prophylaxis.
In another model that examined specific HIV-related features rather than the number of features (not shown), only availability of aerosolized pentamidine in the clinic or institution had a significant association with this outcome (adjusted odds ratio [AOR] 1.64; 95% confidence interval [CI] 1.02, 2.65). Adjusted odds ratios in this model were increased but did not achieve significance for case management (AOR 1.30; 95% CI 0.97, 1.76) and multidisciplinary conferences (AOR 1.55; 95% CI 0.97, 2.50).
Because more-knowledgeable HIV-infected patients might have prompted their provider to prescribe prophylaxis, we estimated the model in Table 2 for only drug users, who may represent a less-informed group. Compared with drug users in sites with no more than two HIV features, the AOR of drug users in clinics offering six such features was 4.44 (95% CI 1.90, 10.37). Thus, we observed an even stronger association of the number of HIV-focused features with receipt of prophylaxis by drug users.
Multivariate analysis did not show a significant association for clinic specialty, AIDS experience, or any of the separate HIV-focused features with a patient's risk of PCP as the initial AIDS diagnosis. However, compared with patients in sites with only one or none of three features (i.e., HIV/AIDS director, not needing consultants to manage PCP care, and self-reported continuity of HIV care), patient in sites with two of three features had a lower risk of PCP (Table 3).
DISCUSSION
Pneumocystis carinii pneumonia prophylaxis has become a standard quality-of-care measure for persons with advanced HIV disease. The number of HIV-related features offered by clinics serving as the usual source of care for our study cohort was a stronger predictor than either clinic AIDS care experience or specialty of PCP prophylaxis and PCP as the initial AIDS diagnosis. Several of these HIV-focused features resemble interventions used by multifaceted cancer prevention programs.26 Receipt of various cancer prevention services has shown greater improvement when both providers and patients receive reminders and educational materials.27 An initial HIV-evaluation protocol may serve the same purpose as chart-based cancer prevention reminders.7,8,9,27–30 Widely observed deficiencies in HIV care knowledge 31, 32 are likely to be improved by appointing a director of HIV care, holding multidisciplinary HIV conferences, and having clinic providers prepared to manage PCP without consultants.
Physician–HIV patient continuity of care was also positively associated with PCP prophylaxis. In general populations, increased continuity of care improves patient clinical outcomes and satisfaction.33 Increased continuity of care helps the physician keep abreast of the patient's changing clinical status and allows initiation of prophylactic therapy in a timely fashion. In our study, continuity of care was assessed only by clinic directors' ratings, but half rated continuity as low or moderate, indicating an effort to be self-critical.
Offering aerosolized pentamidine on-site was related to greater PCP prophylaxis rates. This service probably improves PCP prevention by promoting patient convenience and access to therapy, but it resulted in greater use of this less effective form of prophylaxis. However, our study largely preceded evidence that alternatives such as trimethoprim-sulfamethoxazole are more effective.34, 35
Case management was associated with greater odds of PCP prophylaxis and, in other chronically ill populations, improved delivery of preventive health care.36 Case managers work to improve access to care and other services and may also help patients comply with HIV-related care including medication use. Unfortunately, we did not know the case manager's location or the content of his or her interactions with patients. A survey of 175 AIDS case managers found that those based in hospitals had more clinical training and experience with drug-using populations than those in the community.37 Further research should study case managers' preparation and types of counseling and assistance.
We acknowledge that we are only able to examine associations. Therefore, the clinic characteristics in our analysis may be markers for clinics with other unmeasured features that may actually be responsible for the observed benefits. In addition, we cannot evaluate specific physician characteristics associated with PCP quality of care.
Only 44% of our cohort received prophylaxis, slightly higher than the 40% rate observed for patients with CD4 counts less than 200/μL when they first visited a clinical trial center in a similar time frame as our study.12 Reassuringly, the odds of prophylaxis in our cohort increased significantly over time so that current rates are likely to be higher. As reported by others,38 the rate of PCP has declined less impressively in the 1990s.
Drug users of both genders were more likely to develop PCP than male non–drug users. Fortunately, we observed a marked trend toward greater prophylaxis use by female drug users by the last year of our study. However, female non–drug users and male drug users still lagged behind in their use of prophylaxis in our last study year. Older age was a significant predictor of greater prophylaxis use but was not associated with PCP. We could not examine the association of race-ethnicity with these two outcomes, but other researchers have reported that African Americans have lower adjusted odds of PCP prophylaxis than whites.11 Access to care and prescribing habits of providers have been postulated as contributing to this finding.11 We also could not assess patient compliance and toxicity. Drug toxicity, either real or theoretical, may explain the lower use of prophylaxis by persons with comorbidities such as hypertension or diabetes.
Several other limitations of this study should be acknowledged. We focused on clinic and not on private group or individual practitioner care. Although clinics are the most common source of longitudinal ambulatory care for persons with AIDS in the United States,22, 39 the quality of HIV care in private offices is also important to investigate. Other research suggests that private practitioners may adopt standard elements of HIV care more slowly.40 We studied only patients with a usual source of care, but those without a usual source of care are even less likely to receive PCP prophylaxis.11 In addition, we did not examine patients in managed care arrangements.
Our experience measure is based on a count of Medicaid-enrolled persons with AIDS followed in study clinics adjusted for the proportion of all persons with AIDS in the clinic who are covered by Medicaid. As the median proportion reported by study clinic directors was 85%, AIDS patients in study clinics were predominantly enrolled in Medicaid. In other analyses, we observed longer survival for Medicaid-enrolled women with AIDS treated in clinics with greater experience, measured using a similar approach from claims and interview data.41 The lack of an association of experience with PCP prevention is unexpected but may be due to the possibility that patients in highly experienced clinics may not develop AIDS-defining conditions (needed to be included in our cohort) due to appropriate prophylaxis or antiretroviral therapy.
In support of the completeness of our identification of primary PCP cases, however, PCP was the initial AIDS diagnosis for 40% of reported New York State AIDS cases versus 38% of our study population (Jones J, New York State Department of Health, personal communication). Theoretically, some in our study cohort might not have needed prophylaxis, but this group is most likely small because CD4 T-lymphocyte counts are generally well below 200/μL when AIDS-related conditions occur,42 and other indications for prophylaxis such as oral candidiasis often precede an AIDS diagnosis.5 Our cohort had been diagnosed with HIV infection more than 2 months before their first AIDS diagnosis, giving their providers sufficient time to start prophylaxis. The generalizability of our data to other HIV populations or demographic groups is not known. However, New York State is an epicenter of the HIV epidemic in the United States,43 and Medicaid is the most common payer for AIDS care nationally.
In recent years, the New York State Department of Health has taken a proactive stance in regard to HIV care and mandated that many of these HIV-focused features be provided by clinics that receive enhanced payments under fee-for-service arrangements for HIV-infected Medicaid enrollees.44 Efforts are under way to move New York State Medicaid enrollees with HIV infection into managed care settings that offer the special expertise and HIV-focused features needed to ensure quality care. Our data support such efforts to concentrate HIV care in settings that have the support and expertise needed to manage this complex population.
Acknowledgments
The authors thank the following experts who helped in the development of the survey instrument: David Rose, MD, Kathryn Anastos, MD, Gary Burke, MD, Nilsa Gutierrez, MD, and Jay Dobkin, MD. We also thank the clinic directors and others who completed our survey.
References
- 1.Phair J, Munoz A, Detels R, et al. The risk of Pneumocystis carinii pneumonia among men infected with human immunodeficiency virus type 1. N Engl J Med. 1990;322:161–5. doi: 10.1056/NEJM199001183220304. [DOI] [PubMed] [Google Scholar]
- 2.Turner B J, Markson LE, McKee L, Houchens R, Fanning T. The AIDS-defining diagnosis and subsequent complications: a survival-based severity index. J Acquir Immune Defic Syndr. 1991;4:1059–71. [PubMed] [Google Scholar]
- 3.Sha BE, Benson CA, Pottage CJ, Urbanski PA, Daugherty SR, Kessler HA. HIV infection in women: an observational study of clinical characteristics, disease progression, and survival for a cohort of women in Chicago. J Acquir Immune Defic Syndr. 1995;8:486–95. [PubMed] [Google Scholar]
- 4.Simonds RJ, Hughes WT, Feinberg J, Navin TR. Pneumocystis carinii pneumonia in persons infected with human immunodeficiency virus. Clin Infect Dis. 1995;21:S44–8. doi: 10.1093/clinids/21.supplement_1.s44. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Recommendations for prophylaxis against Pneumocystis carinii pneumonia for adults and adolescents infected with human immunodeficiency virus. MMWR. 1992;41:1–11. [PubMed] [Google Scholar]
- 6.Mallal SA, Martinez P, French M, James IR, Dawkins RL. Severity and outcome of Pneumocytis carinii pneumonia (PCP) in patients of known and unknown HIV status. J Acquir Immune Defic Syndr. 1994;7:148–53. [PubMed] [Google Scholar]
- 7.Saah AJ, Hoover DR, Peng Y, et al. Predictors for failure of Pneumocystis carinii pneumonia prophylaxis. JAMA. 1995;273:1197–1202. [PubMed] [Google Scholar]
- 8.Freedberg KA, Tosteson ANA, Cohen CJ, Cotton DJ. Primary prophylaxis for Pneumocystis carinii pneumonia in HIV-infected people with CD4 counts below 200/mm3: a cost-effectiveness analysis. J Acquir Immune Defic Syndr. 1991;4:521–31. [PubMed] [Google Scholar]
- 9.Gallant JE, McAvinue SM, Moore RD, Bartlett JG, Stanton DL, Chaisson RE. The impact of prophylaxis on outcome and resource utilization in Pneumocystis carinii pneumonia. Chest. 1995;107:1018–23. doi: 10.1378/chest.107.4.1018. [DOI] [PubMed] [Google Scholar]
- 10.Turner BJ, Markson LE, McKee LJ, Houchens R, Fanning T. Health care delivery, zidovudine use, and survival of women and men with AIDS. J Acquir Immune Defic Syndr. 1994;7:1250–62. [PubMed] [Google Scholar]
- 11.Moore RD, Stanton D, Gopalan R, Chaisson RE. Racial differences in the use of drug therapy for HIV disease in an urban community. N Engl J Med. 1994;330:763–8. doi: 10.1056/NEJM199403173301107. [DOI] [PubMed] [Google Scholar]
- 12.Glassroth J, Jordan M, Wallace JM, et al. Use of preventive interventions by persons infected with type-1 human immunodeficiency virus (HIV-1) Am J Prev Med. 1994;10:259–66. [PubMed] [Google Scholar]
- 13.Kitahata MM, Koepsell TD, Deyo RA, Maxwell CL, Dodge WT, Wagner EH. Physicians' experience with the acquired immunodeficiency syndrome as a factor in patients' survival. N Engl J Med. 1996;334:701–6. doi: 10.1056/NEJM199603143341106. [DOI] [PubMed] [Google Scholar]
- 14.Ball JK, Turner B J. Hospital Studies Program Research Note 15. Rockville, Md: Agency for Health Care Policy and Research; 1991. AIDS in U.S. hospitals in 1986–87: a national perspective. DHHS publication (PH)91-0015. August. [Google Scholar]
- 15.Bennett CL, Garfinkle JB, Greenfield S, et al. The relation between hospital experience and in-hospital mortality for patients with AIDS-related PCP. JAMA. 1989;261:2975–9. [PubMed] [Google Scholar]
- 16.McPhee SJ, Detmer WM. Office-based interventions to improve delivery of cancer prevention services by primary care physicians. Cancer. 1993;72:1100–12. doi: 10.1002/1097-0142(19930801)72:3+<1100::aid-cncr2820721327>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 17.Wender RC. Cancer screening and prevention in primary care: obstacles for physicians. Cancer. 1993;72:1093–9. doi: 10.1002/1097-0142(19930801)72:3+<1093::aid-cncr2820721326>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 18.Markson LE, Turner BJ, Cocroft J, Houchens R, Fanning TR. Clinic services for persons with AIDS: experience in a high prevalence state. J Gen Intern Med. 1997;12:141–9. doi: 10.1007/s11606-006-5021-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keyes M, Andrews R, Mason M. A methodology for building an AIDS research file using Medicaid claims and administrative data bases. J Acquir Immune Defic Syndr. 1991;4:1015–24. [PubMed] [Google Scholar]
- 20.Fanning TR, Turner BJ, Cosler LE, et al. Quality of Medicaid data for HIV/AIDS research: examination of a statewide database. AIDS Publ Pol J. 1995;10:39–47. [Google Scholar]
- 21.Centers for Disease Control and Prevention. Classification system for human immunodeficiency virus (HIV) infection in children under 13 years of age. MMWR. 1987;36:25–35. [PubMed] [Google Scholar]
- 22.Turner BJ, McKee L, Fanning T, Markson LE. AIDS specialist versus generalist ambulatory care for advanced HIV infection and impact on hospital use. Med Care. 1994;32:902–16. doi: 10.1097/00005650-199409000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan JE, Masur H, Holmes KK, et al. USPHS/IDDSA guidelines for the prevention of opportunistic infections in persons infected with human immunodeficiency virus type 1. Clin Infect Dis. 1995;21:S12–31. doi: 10.1093/clinids/21.supplement_1.s12. [DOI] [PubMed] [Google Scholar]
- 24.Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:12–22. [Google Scholar]
- 25.Hosmer DW, Lemeshow S. Applied Logistic Regression. New York, NY: John Wiley and Sons; 1989. [Google Scholar]
- 26.Rimer BK, Ross E, Balshem A, Engstrom PF. The effect of a comprehensive breast screening program on self-reported mammography use by primary care physicians and women in a health maintenance program. J Am Board Fam Pract. 1993;6:443–51. [PubMed] [Google Scholar]
- 27.Turner BJ, Day SC, Borenstein B. A controlled trial to improve delivery of preventive care: physician or patient reminder? J Gen Intern Med. 1989;4:403–9. doi: 10.1007/BF02599691. [DOI] [PubMed] [Google Scholar]
- 28.Cheney C, Famsdell JW. Effect of medical records' checklists on implementation of periodic health measures. Am J Med. 1987;83:129–36. doi: 10.1016/0002-9343(87)90507-9. [DOI] [PubMed] [Google Scholar]
- 29.Tierney WM, Hui SL, McDonald CJ. Delayed feedback of physician performance versus immediate reminders to perform preventive care: effect on physician compliance. Med Care. 1986;24:659–66. doi: 10.1097/00005650-198608000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Hahn DL. Systematic cholesterol screening during acute care visits. J Am Board Fam Pract. 1993;6:529–36. [PubMed] [Google Scholar]
- 31.Paauw DS, Wenrich MD, Curtis JR, Carline JD, Ramsey PG. Ability of primary care physicians to recognize physical findings associated with HIV infection. JAMA. 1995;274:1380–2. [PubMed] [Google Scholar]
- 32.Curtis JR, Paauw DS, Wenrich MD, Carline JD, Ramsey PG. Ability of primary care physicians to diagnose and manage Pneumocystis carinii pneumonia. J Gen Intern Med. 1995;10:395–9. doi: 10.1007/BF02599841. [DOI] [PubMed] [Google Scholar]
- 33.Smith CS. The impact of an ambulatory firm system on quality and continuity of care. Med Care. 1995;33:221–6. doi: 10.1097/00005650-199503000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Schneider MME, Hoepelman AIM, Eeftinck Schattenkerk JK, et al. A controlled trial of aerosolized pentamidine or trimethodprim-sulfamethoxazole as primary prophylaxis against Pneumocystis carinii pneumonia in patients with human immunodeficiency virus infection. N Eng J Med. 1992;327:1836–41. doi: 10.1056/NEJM199212243272603. [DOI] [PubMed] [Google Scholar]
- 35.Bozzette SA, Finkelstein DM, Spector SA, Frame P, Powderly WG. A randomized trial of three antipneumocystis agents in patients with advanced human immunodeficiency virus infection. NIAID AIDS Clinical Trials Group. N Engl J Med. 1995;332:693–9. doi: 10.1056/NEJM199503163321101. [DOI] [PubMed] [Google Scholar]
- 36.Erkel EA. The impact of case management in preventive services. J Nurs Adm. 1993;23:27–32. [PubMed] [Google Scholar]
- 37.Piette J, Fleishman JA, Mor V, Dill A. A comparison of hospital and community case management programs for persons with AIDS. Med Care. 1990;28:746–55. doi: 10.1097/00005650-199008000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Moore RD, Chaisson RE. Natural history of opportunistic disease in an HIV-infected urban clinical cohort. Ann Intern Med. 1996;124:633–42. doi: 10.7326/0003-4819-124-7-199604010-00003. [DOI] [PubMed] [Google Scholar]
- 39.Crystal S. Health-care barriers and utilization patterns among intravenous-drug users with HIV disease. AIDS Pub Pol J. 1992;7:187–98. [Google Scholar]
- 40.Markson LE, Cosler LE, Turner BJ. Implications of generalists' slow adoption of zidovudine in clinical practice. Arch Intern Med. 1994;154:1497–1504. [PubMed] [Google Scholar]
- 41.Laine C, Markson LE, McKee LJ, Hauck WW, Fanning TR, Turner BJ. The relationship of clinic experience with advanced HIV and survival of women with AIDS. AIDS; 19th Annual National Meeting of the Society of General Internal Medicine; May 1996; Washington, DC. In press. Presented at the. [DOI] [PubMed] [Google Scholar]
- 42.Turner BJ, Hecht R, Ismail R. CD4+T-lymphocyte measures in the treatment of individuals infected with human immunodeficiency virus type 1. Arch Intern Med. 1994;154:1561–73. [PubMed] [Google Scholar]
- 43.Centers for Disease Control and Prevention. HIV/AIDS Surveillance Report. Atlanta, Ga: CDC; December 1995. [Google Scholar]
- 44.Health Facilities Series: H-86, D&TC-45, HMO-39 89-99. Albany, NY: New York State Department of Health; March 7 1991. New Medicaid Reimbursement Rates for HIV Primary Care Visits. [Google Scholar]