Abstract
OBJECTIVE
To determine the association between asymptomatic carotid bruits and the development of subsequent stroke in older adults with isolated systolic hypertension.
DESIGN
Retrospective cohort study.
SETTING
The Systolic Hypertension in the Elderly Program (SHEP), a 5-year randomized trial testing the efficacy of treating systolic hypertension in noninstitutionalized persons aged 60 years or older. From the original 4,736 SHEP participants, we identified a cohort of 4,442 persons who had no prior history of stroke, transient ischemic attack, or myocardial infarction at randomization.
MEASUREMENTS AND MAIN RESULTS
The end point for this ancillary study was the development of a stroke. The average follow-up was 4.2 years. Carotid bruits were found in 284 (6.4%) of the participants at baseline. Strokes developed in 21 (7.4%) of those with carotid bruits and in 210 (5.0%) of those without carotid bruits. The unadjusted risk of stroke among persons with carotid bruits was 1.53 (95% confidence interval [CI] 0.98, 2.40). Adjusting for age, gender, race, blood pressure, smoking, lipid levels, self-reported aspirin use, and treatment group assignment, the relative risk of stroke among persons with asymptomatic carotid bruits was 1.29 (95% CI 0.80, 2.06). Among SHEP enrollees aged 60 to 69 years, there was a trend (p=.08) toward increased risk (relative risk [RR] 2.05; 95% CI 0.92, 4.68) of subsequent stroke in persons with, compared to those without, carotid bruits. However, among enrollees aged 70 years or over, there was no relation between carotid bruit and subsequent stroke (RR 0.98; 95% CI 0.55, 1.76). In no other subgroup of SHEP enrollees did the presence of carotid bruit independently predict stroke.
CONCLUSIONS
Although we cannot rule out a small increased risk of stroke associated with bruits in asymptomatic SHEP enrollees aged 60 to 69 years, the utility of carotid bruits as a marker for increased risk of stroke among asymptomatic elderly with isolated systolic hypertension aged 70 years or older is limited.
Keywords: carotid bruits, asymptomatic, stroke, elderly, Systolic Hypertension in the Elderly Program (SHEP)
Stroke is an important cause of morbidity, mortality, loss of independence, and institutionalization. Age is a major risk factor for stroke, with rates doubling in each decade after 55 independent of other known risk factors. 1 Hypertension, cigarette smoking, diabetes, and atrial fibrillation are also major risk factors for stroke. 2 Although the development of symptomatic carotid disease (e.g., transient ischemic attacks [TIAs]) identifies persons at high risk of subsequent strokes, most strokes occur without previous symptoms. 3 Thus, the identification and management of asymptomatic elderly at high risk of subsequent stroke is an important public health issue. In asymptomatic persons with atrial fibrillation, the utilization of oral anticoagulants reduces the risk of stroke. 4 However, the prevention of stroke for asymptomatic persons without atrial fibrillation depends largely on reducing known risk factors and, in appropriate candidates, identifying persons with surgically remediable high-grade carotid artery stenosis. 2–4
Auscultation for carotid bruits is noninvasive and inexpensive, but its utility in detecting asymptomatic older adults at increased risk of stroke has been incompletely studied. 5 Early studies found an increased risk of carotid bruits among persons suffering stroke compared with control subjects 6, 7 ; however, these studies were nonblinded and potentially subject to observer bias. Population-based prospective studies found that persons with carotid bruits were at higher risk of subsequent stroke, 8–10 but age-specific risks were not reported. In contrast, a study of nursing home residents aged 75 years or older found no increased risk of stroke among persons with asymptomatic carotid bruits. However, sample size was small, and the generalizability of this finding to less frail elderly is not known. 11 Given the age-related prevalence of carotid bruits, and the paucity of data on the utility of neck auscultation to predict stroke in older adults, we conducted a retrospective cohort study of Systolic Hypertension in the Elderly Program (SHEP) participants to determine the risk of stroke associated with asymptomatic carotid bruits.
METHODS
Study Population and Events
The Systolic Hypertension and the Elderly Program was a randomized, placebo-controlled, multicenter trial designed to test whether drug treatment of isolated systolic hypertension in older adults reduces the risk of fatal or nonfatal stroke. A total of 4,736 persons were enrolled in SHEP from a total of 447,921 screenees and followed for an average of 4.5 years. Men and women aged 60 years or older were screened at two baseline visits and considered eligible if the average of four blood pressure readings at the first and second baseline visit consisted of a systolic blood pressure of at least 160 and less than 220 mm Hg and a diastolic blood pressure of less than 90 mm Hg. Potential enrollees were excluded from the study if there was a history of stroke with residual paresis; a history of TIA and carotid bruit or two TIAs in the same distribution; atrial fibrillation or flutter; insulin or warfarin use; history of coronary artery bypass surgery or myocardial infarction within 6 month of screening; or dementia or residence in a nursing home (fewer than 5% of SHEP enrollees reported limitation in activities of daily living 12). Other exclusion criteria have been previously described. 12, 13
Although there was no specific training on neck auscultation, the presence and location of carotid bruits was recorded as an explicit part of the physical examination prior to study entry in all SHEP enrollees. The end point adjudication committee reviewed medical records and radiographic studies for persons who developed symptoms consistent with stroke or TIA. Cerebrovascular events were classified by event type and location.
Analytic Strategy
Study follow-up began at the randomization date and ended with the date of a fatal or nonfatal stroke. Because we wanted to study the risk of stroke in asymptomatic persons, time after a TIA was censored. Of 131 events censored in this manner, only 10% of strokes occurred within 4 months of the TIA. Death unrelated to stroke, loss to follow-up, and the end of the SHEP study were other censoring events.
Univariate statistical analysis was performed using χ 2 t statistics where appropriate. Univariate and multivariate relative risks were estimated using Cox proportional hazards models. 14 The proportional hazards assumption that the risk of stroke over time was parallel in persons with and those without carotid bruits, was not rejected (p= .45). Variables included in the multivariate models were age, gender, race, systolic and diastolic blood pressure, pulse pressure, total cholesterol, high-density lipoprotein (HDL), the presence of electrocardiographic (ECG) abnormalities, self-reported smoking and aspirin use at randomization, history of diabetes, and assignment to active treatment or placebo. To create risk strata, we identified six independent (p < .05) risk factors for stroke in the SHEP cohort: age 70 years or over, current smoking, history of diabetes, systolic blood pressure above median (170 mm Hg) at randomization, the presence of at least one ECG abnormality at randomization, and HDL < 43 mg/dL (lowest quartile). Persons with two or fewer risk factors were classified as the “low-risk” group, others were considered the “high-risk” group. All power calculations were based on a two-sided α = 0.05 and β = 0.2 using the software by Dupont and Plummer, 15 and other procedures were performed utilizing SAS software (version 6.11). All reported p values are two-tailed.
RESULTS
Characteristics of Study Cohort
Of the 4,736 study participants, we excluded 294 subjects felt by study clinicians to have any prior history of stroke, TIA, or myocardial infarction at randomization. Thus, our cohort consisted of 4,442 SHEP enrollees. The mean (± SD) age was 71.5 ± 6.72 years, 57.8% were women, 78.9% were white, and 50.2% were assigned to active treatment. The mean (± SD) systolic and diastolic blood pressures at baseline were 170.3 ± 9.38 and 76.5 ± 9.70 mm Hg, respectively. The mean serum cholesterol (± SD) and HDL levels at baseline were 236 ± 44 and 54 ± 15 mg/dL, respectively. Twelve percent were current smokers, 10.1% had a history of diabetes, and 60% had at least one abnormality on resting ECG.
Distribution of Risk Factors for Cerebrovascular Disease Among Persons With or Without Carotid Bruits
Of the 4,442 SHEP enrollees in the cohort, bruits were found in 284 (6.4%, range in centers 1.8% –12.5%), of which 124 (44%) were bilateral. Carotid bruits were found more commonly (p < .05) among older and nonwhite subjects. Persons with bruits were more likely to smoke, had higher systolic blood pressure, lower diastolic blood pressure, wider pulse pressure, higher cholesterol levels, and more frequent abnormal ECG findings than persons without carotid bruits. Potential stroke risk factors including male gender, diabetes, and HDL levels were equally distributed among persons with and those without carotid bruits (Table 1) Persons with carotid bruits were more likely than persons without carotid bruits to use aspirin (22% vs 16%, p= .02) and be randomized to placebo (58% vs 50%, p= .006).
Table 1.
Distribution of Potential Risk Factors for Cerebrovascular Disease Among Persons With and Without Asymptomatic Carotid Bruits*

Relation of Bruit to Development of Stroke
The cohort was followed for 18,488 person-years, during which 231 strokes occurred yielding an event rate of 1.24 (95% CI 1.08, 1.41) per 100 person-years. Persons with carotid bruits were followed for 1,129 person-years, during which 21 strokes occurred for an event rate of 1.86 (95% CI 1.07, 2.64) per 100 person-years. Persons without bruits contributed 17,359 person-years of observation, during which 210 strokes occurred for an event rate of rate of 1.21 (95% CI 1.04, 1.37) per 100 person-years.
Among persons with unilateral carotid bruits, the rate of stroke was 2.21 (95% CI 1.06, 3.36), whereas the rate among persons with bilateral carotid bruits was 1.40 (95% CI 0.37, 2.44) per 100 person-years. In 7 of 14 persons with unilateral carotid bruits who developed stroke, the distribution of stroke was known; of these, 4 were ipsilateral and 3 were contralateral to the bruit.
The unadjusted relative risk (RR) of stroke among persons with carotid bruits was 1.53 (95% CI 0.98, 2.40). Controlling only for treatment group assignment, the RR of stroke among persons with, compared to those without, carotid bruits was 1.47 (95% CI 0.94, 2.31). This fell to 1.29 (95% CI 0.80, 2.06) when other potential risk factors, described above, were included in the model.
Among the 2,169 persons (49%) in the low-risk group, 68 (3.1%) developed subsequent stroke. Of the remaining 2,273 enrollees (51%) in the high-risk group, 163 (7.2%) developed a completed stroke. Among persons at low risk, the univariate risk of stroke associated with asymptomatic carotid bruits was 1.38 (95% CI 0.50, 3.80), whereas among high-risk persons the univariate RR of stroke was 1.36 (95% CI 0.82, 2.25).
Among SHEP enrollees aged 60 to 69 years, there was a trend (p= .08) toward increased risk (RR 2.05; 95% CI 0.92, 4.68) of subsequent stroke in persons with, compared to those without, carotid bruits. However, among enrollees aged 70 years or over, there was no relation between carotid bruit and subsequent stroke (RR 0.98; 95% CI 0.55, 1.76). In the other subgroups, the RR of stroke varied between 1.1 and 1.8, and the lower bound of the 95% CI was 0.8 or less (Fig. 1).
Figure 1.
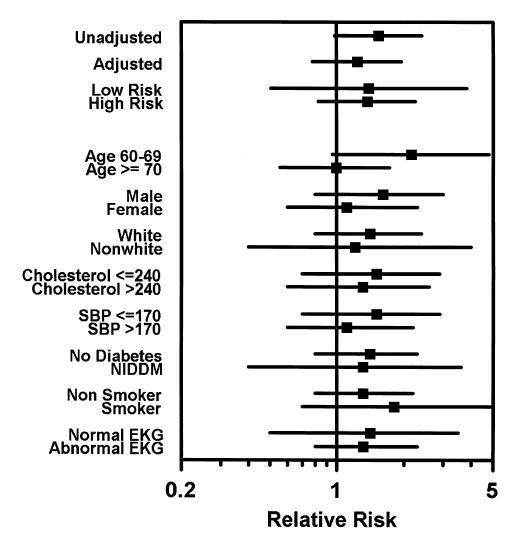
The relative risk (boxes) and 95% confidence intervals (lines) of completed stroke among SHEP study subjects with, compared to those without, asymptomatic carotid bruits at randomization date. SBP signifies systolic blood pressure in mm Hg.
DISCUSSION
In this cohort of high-functioning elderly with isolated systolic hypertension, more than 6% of enrollees were found to have asymptomatic carotid bruits at randomization, and of these, nearly half had bilateral carotid bruits. Persons with carotid bruits were more likely to have other risk factors for atherosclerosis. Although persons with carotid bruit were 50% more likely to develop a completed stroke, this relation was confounded by more important stroke risk factors. As in previous studies, 8, 9 events were as likely to occur contralateral to the bruit, when the location of the stroke was known. Although in nearly every subgroup persons with bruits were more likely than those without bruits to develop a completed stroke, in none of these categories was the strength of the association statistically significant. These results, combined with recent findings that SHEP enrollees with carotid bruits were at increased risk of myocardial infarction, 16 confirm the accepted notion that carotid bruits are a sign of generalized atherosclerosis, rather than a useful marker of clinically significant carotid artery disease.
Although there was a trend toward increased risk of stroke associated with asymptomatic carotid bruits among enrollees aged 60 to 69 years, there was no association of carotid bruits with subsequent strokes in persons aged 70 years or older. In no other subgroup was the presence of carotid bruit associated with subsequent stroke. It is not clear why the cerebrovascular prognostic significance associated with carotid bruits decreases in the very old. Older adults may have a higher prevalence of completely occluded carotid arteries, which are thought not to produce bruits. Thus, if strokes are more likely to occur in persons with complete obstruction, the relation between bruit and subsequent stroke may deteriorate in the elderly. The prevalence of cardiac disease increases with age, and radiated murmurs or venous hums may decrease the positive predictive value of carotid bruits for carotid stenosis. However, this was not observed by Davies and Humphrey, who found the positive predictive value of carotid bruits for carotid stenosis increased from 48% among persons less than 55 years to 75% in persons 55 years or older. 17 This raises the possibility that the relation between degree of carotid stenosis and subsequent stroke lessens with advancing age. Although untested, it is biologically plausible given evidence that other risk factors for atherosclerosis, such as hypertension, 18 or hypercholesterolemia, 19, 20 do not perform as well in the prediction of cardiovascular disease in the elderly.
The large numbers of persons aged 70 years and older in SHEP may explain the lack of association of carotid bruits with subsequent stroke in this cohort compared with previous population-based studies. 8–10 Our findings are consistent with those of Van Ruiswyk and colleagues, 11 who found that carotid bruits did not predict stroke (RR 1.1; 95% CI 0.45, 2.7) in a cohort of 241 frail nursing home residents aged 75 years or older. However, in addition to providing well-defined cohort and adjudicated end points, our study subjects more closely resemble patients who would be candidates for office screening—they were at increased risk of stroke, but otherwise were in relatively good health, and would have the most to lose from a catastrophic event. Also, our large sample size could detect an RR of 1.9, compared with 3.1, of stroke among persons with bruits, and could permit the evaluation of subgroups.
Two potential limitations to this study should be discussed. First, participants in SHEP were highly selected as the study enrolled only 1% of persons screened. Thus, the generalizability of findings to other elderly or hyperpertensive populations may be limited. However, many of the traditional risk factors for coronary heart disease, such as cholesterol, smoking, and history of diabetes, predict these events in SHEP, 16 suggesting some generalizability of our findings beyond this study population. Second, details about bruit sound quality were not included in the SHEP study. Although some cross-sectional studies have shown that “localized” bruits predict underlying carotid stenosis better than “diffuse” bruits, 21, 22 the risk of stroke does not vary between persons with diffuse or localized bruits. 10 Others argue that persons with bruits that extend through diastole are at increased stroke risk, 21 but interobserver agreement regarding bruit duration, pitch, and intensity is low. 22
Auscultatory findings are subjective in nature, and the accuracy of auscultation for carotid bruits in SHEP could be lower than in other settings. In SHEP, auscultation for bruit was part of a general physical examination, and multiple physicians at each site collected data. Because bruit was not the primary variable of interest, there was no systematic training of observers. The wide range of bruits detected in the 16 study centers supports this possibility. Also, in one study center, SHEP examiners identified bruits in 8 (7.3%) of 109 participants; whereas a single observer, as a part of an ancillary study, detected 19 bruits in 99 (19.2%) of the members of the same cohort. 23 However, in SHEP the overall prevalence and location of bruits is similar to that in other studies, 9, 10 suggesting that, however imprecise, our findings probably apply to the performance of neck auscultation in a typical clinical setting.
Various authorities have published recommendations for and against neck auscultation for carotid bruits. The Canadian Task Force on the Periodic Health Examination recommended against screening for bruits in asymptomatic persons, whereas the American Academy of Family Physicians recommends auscultation for carotid bruits in people aged 40 years and older with risk factors for cerebrovascular or cardiovascular disease, or those with a history of cardiovascular disease. 24 The United States Preventive Services Task Force offers a “C” recommendation: there is insufficient evidence to recommend for or against screening for asymptomatic carotid disease utilizing physical examination. 24
Despite the lack of proven efficacy and despite expert opinions, 25, 26 detecting a carotid bruit creates concern among clinicians. Clinician overestimates of the prognostic significance of carotid bruits, especially in the very old, can result in pursuit of expensive and high-risk management strategies. For example, nearly a quarter of neurologists surveyed would initiate an evaluation for carotid endarterectomy when presented a vignette describing an 81-year-old woman with nonspecific symptoms and a loud carotid bruit. 27 Also, in a recent “Clinical Crossroads,” auscultation of a carotid bruit in an asymptomatic 79-year-old man was the cue to obtain an ultrasound examination in search of carotid stenosis. 28 Our data show that carotid bruits in asymptomatic elderly with isolated systolic hypertension—even those with multiple other risk factors—do not identify persons at increased risk of subsequent stroke. Although we cannot rule out a small increased risk of stroke associated with bruits in asymptomatic SHEP enrollees aged 60 to 69 years, the utility of carotid bruits as a marker for increased stroke risk among asymptomatic elderly with isolated systolic hypertension aged 70 years or older is limited. Further studies are needed to develop more promising approaches for the prevention of cerebrovascular disease in the elderly.
References
- 1.Adams HP, Gordon DL. Epidemiology of and stroke-preventive strategies for atherothromboembolic brain infarction in the elderly. Clin Geriatr Med. 1991;7:401–16. [PubMed] [Google Scholar]
- 2.Bronner LL, Kanter DS, Manson JE. Primary prevention of stroke. N Engl J Med. 1995;333:1392–400. doi: 10.1056/NEJM199511233332106. [DOI] [PubMed] [Google Scholar]
- 3.Lefkowitz D. Asymptomatic carotid artery disease in the elderly. Diagnosis and management strategies. Clin Geriatr Med. 1991;7:417–28. [PubMed] [Google Scholar]
- 4.Matchar DB, McCrory DC, Barnett HJ, Feussner JR. Medical treatment for stroke prevention. Ann Intern Med. 1994;121:41–53. doi: 10.7326/0003-4819-121-1-199407010-00008. [DOI] [PubMed] [Google Scholar]
- 5.Sauve JS, Laupacis A, Ostbye T, Feagan B, Sackett DL. Does this patient have a clinically important carotid bruit? JAMA. 1993;270:2843–5. doi: 10.1001/jama.1993.03510230081040. [DOI] [PubMed] [Google Scholar]
- 6.Imataka K, Seki A, Takahashi N, Fujii J. Carotid bruits and their clinical significance. Jpn Heart J. 1984;25:725–31. doi: 10.1536/ihj.25.725. [DOI] [PubMed] [Google Scholar]
- 7.Rennie L, Ejrup E, McDowell F. Arterial bruits in cerebrovascular disease. Neurology. 1964;14:751–6. doi: 10.1212/wnl.14.8_part_1.751. [DOI] [PubMed] [Google Scholar]
- 8.Wolf PA, Kannel WB, Sorlie P, McNamara P. Asymptomatic carotid bruit and risk of stroke. The Framingham study. JAMA. 1981;245:1442–5. [PubMed] [Google Scholar]
- 9.Heyman A, Wilkinson WE, Heyden S, et al. Risk of stroke in asymptomatic persons with cervical arterial bruits: a population study in Evans County, Georgia. N Engl J Med. 1980;302:838–41. doi: 10.1056/NEJM198004103021504. [DOI] [PubMed] [Google Scholar]
- 10.Wiebers DO, Whisnant JP, Sandok BA, O'Fallon WM. Prospective comparison of a cohort with asymptomatic carotid bruit and a population-based cohort without carotid bruit. Stroke. 1990;21:984–8. doi: 10.1161/01.str.21.7.984. [DOI] [PubMed] [Google Scholar]
- 11.Van Ruiswyk J, Noble H, Sigmann P. The natural history of carotid bruits in elderly persons. Ann Intern Med. 1990;112:340–3. doi: 10.7326/0003-4819-112-5-340. [DOI] [PubMed] [Google Scholar]
- 12.Anonymous Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertensionFinal results of the Systolic Hypertension in the Elderly Program (SHEP) SHEP Cooperative Research Group. JAMA. 1991;265:3255–64. [PubMed] [Google Scholar]
- 13.Bearden D, Allman R, McDonald R, Miller S, Pressel S, Petrovitch H. Age, race, and gender variation in the utilization of coronary artery bypass surgery and angioplasty in SHEP. SHEP Cooperative Research Group. Systolic Hypertension in the Elderly Program. J Am Geriatr Soc. 1994;42:1143–9. doi: 10.1111/j.1532-5415.1994.tb06979.x. [DOI] [PubMed] [Google Scholar]
- 14.Cox DR. Regression models and life-tables. J R Stat Soc [B] 1972;34:187–220. [Google Scholar]
- 15.Dupont WD, Plummer DP. Power and sampling calculations: a review and computer program. Control Clin Trials. 1990;11:116–28. doi: 10.1016/0197-2456(90)90005-m. [DOI] [PubMed] [Google Scholar]
- 16.Frost PH, Davis BR, Burlando AJ, et al. Coronary heart disease risk factors in men and women aged 60 years and older: findings from the Systolic Hypertension in the Elderly Program. Circulation. 1996;94:26–34. doi: 10.1161/01.cir.94.1.26. [DOI] [PubMed] [Google Scholar]
- 17.Davies KN, Humphrey PR. Do carotid bruits predict disease of the internal carotid arteries? Postgrad Med J. 1994;70:433–5. doi: 10.1136/pgmj.70.824.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curb JD, Abbott RD, MacLean CJ, et al. Age-related changes in stroke risk in men with hypertension and normal blood pressure. Stroke. 1996;27:819–24. doi: 10.1161/01.str.27.5.819. [DOI] [PubMed] [Google Scholar]
- 19.Prospective Studies Collaboration Cholesterol, diastolic blood pressure, and stroke: 13,000 strokes in 450,000 people in 45 prospective cohorts. Prospective studies collaboration. Lancet. 1995;346:1647–53. [PubMed] [Google Scholar]
- 20.Benfante R, Reed D. Is elevated scrum cholesterol level a risk factor for coronary heart disease in the elderly? JAMA. 1990;263:393–6. [PubMed] [Google Scholar]
- 21.Lubic LG. Detection of carotid stenosis by physical examination. JAMA. 1994;271:1908. . Letter. [PubMed] [Google Scholar]
- 22.Chambers BR, Norris JW. Clinical significance of asymptomatic neck bruits. Neurology. 1985;35:742–5. doi: 10.1212/wnl.35.5.742. [DOI] [PubMed] [Google Scholar]
- 23.Sutton KC, Dai WS, Kuller LH. Asymptomatic carotid artery bruits in a population of elderly adults with isolated systolic hypertension. Stroke. 1985;16:781–4. doi: 10.1161/01.str.16.5.781. [DOI] [PubMed] [Google Scholar]
- 24.Screening for asymptomatic carotid artery stenosis DiGuiseppi C, Atkins D, Woolf SH. Guide to Clinical Preventive Services. 2nd ed. Alexandria, Va: International Medical Publishing; 1996. U.S. Preventive Services Task Force. 53–61. In. [Google Scholar]
- 25.Khaira HS, Crowson MC. Carotid bruit: does it matter? Br J Hosp Med. 1995;53:426–7. Editorial. [PubMed] [Google Scholar]
- 26.Kuller LH, Sutton KC. Carotid artery bruit: is it safe and effective to auscultate the neck? Stroke. 1984;15:944–7. doi: 10.1161/01.str.15.6.944. [DOI] [PubMed] [Google Scholar]
- 27.Anderson DC. How Twin Cities neurologists treat ischemic stroke. Policies and trends. Arch Neurol. 1993;50:1098–103. doi: 10.1001/archneur.1993.00540100083023. [DOI] [PubMed] [Google Scholar]
- 28.Caplan LR. A 79-year-old musician with asymptomatic carotid artery disease. JAMA. 1995;274:1383–9. [PubMed] [Google Scholar]


