Abstract
We describe a central venous catheter-related (Port-A-Cath; Smiths Industries Medical Systems [SIMS] Deltec, Inc., St. Paul, Minn.) infection caused by Rhodotorula glutinis in a 51-year-old man with nasopharyngeal carcinoma. He was treated with fluconazole for 8 weeks and had the catheter removed. Two isolates of R. glutinis recovered from blood specimens (one obtained via peripheral veins and one via the catheter) before administration of fluconazole and one recovered from the removed catheter 17 days after initiation of fluconazole therapy exhibited high-level resistance to fluconazole (MICs, >256 μg/ml). These three isolates were found to belong to a single clone on the basis of identical antibiotypes determined by the E test (PDM Epsilometer; AB Biodisk, Solna, Sweden) and biotypes determined by API ID32 C (bioMerieux, Marcy I'Etoile, France) and their identical random amplified polymorphic DNA patterns.
Species of the genus Rhodotorula are commensals in the natural environment and in humans (1, 2, 4; M. Cowan and J. Allen, Letter, J. Hosp. Infect. 19:293, 1991). Infections due to Rhodotorula species have rarely been reported (1, 2, 5, 8-10, 12, 13). The majority of these infections are associated with the use of indwelling venous catheters in patients with underlying malignancy (1, 2, 5, 10, 12, 13). Several Rhodotorula species have been described as human pathogens, among which R. rubra is the most common species (4, 8, 12). Although there have been reports demonstrating three cases of culture-proven R. glutinis fungemia in patients with intravenous catheters (12, 13), none of these have described the use of molecular typing methods to demonstrate the genetic identity of multiple isolates of the organism from peripheral blood or from the catheter.
Case report.
A 51-year-old man was found to have nasopharyngeal carcinoma in May 1990 and received radiotherapy and intensive chemotherapy from June to August 1990. He was healthy for the following 7 years. Unfortunately, multiple bone metastases were noticed in January 1998. He received local radiotherapy for bone lesions and eight courses of chemotherapy with various regimens from July 1998 to July 1999. A Port-A-Cath catheter (Smiths Industries Medical Systems [SIMS] Deltec, Inc., St. Paul, Minn.) was implanted in June 1999. From June 1999 to October 2000, he was admitted to the hospital three times due to facial cellulitis (caused by group G Streptococcus on one occasion and of unknown etiology on two occasions) and was treated successfully with antibiotics. The catheter was flushed each time during his monthly visits to the oncology clinic.
On 18 November 2000, 1 day after the catheter was flushed, he developed a fever and visited the emergency department. There were no obvious inflammatory signs over the catheter site. His white blood count was 4,200/mm3 with 78% neutrophils. The fever disappeared spontaneously within 1 day. Culture of one blood specimen aspirated via the catheter during the febrile period was inoculated into one BACTEC Myco/F Lytic bottle (Becton Dickinson, Sparks, Md.), which yielded a red yeast (Rhodotorula species) (isolate A). Fever recurred 5 days later and again disappeared spontaneously. Blood cultures collected from peripheral veins and from the catheter at the emergency department also yielded red yeasts 5 days after incubation (the isolates were not preserved). He returned to the outpatient clinic on 29 November 2000 and remained afebrile. Fluconazole (200 mg per day) was administered orally beginning on 30 November. Unfortunately, fever up to 38.3°C occurred on 3 December and he was admitted to the hospital.
After admission, Port-A-Cath-related fungemia was suspected. Removal of the vascular catheter and treatment with amphotericin B were recommended, but the patient insisted on keeping the catheter and remaining on fluconazole therapy because of fear of the informed side effects of amphotericin B. Intravenous fluconazole (400 mg per day) was given immediately after blood cultures were performed. His fever subsided 2 days later. Five days after the patient's fever had subsided, cultures from the blood also yielded a red yeast, which was identified as R. glutinis (isolate B) (see below). Two blood cultures performed on the 7th and 12th days after initiation of intravenous fluconazole did not disclose the presence of the red yeast. The catheter was subsequently removed on the 17th day of hospitalization, and cultures of blood drawn from peripheral veins and the catheter and of blood-like material between the disk and metal portions of the catheter were also done. A Gram stain of the blood-like material disclosed many yeast-like organisms, which were later found to be red yeasts (isolate C). The culture of blood drawn from the catheter and peripheral veins did not yield the organism. The patient remained afebrile after initiation of fluconazole therapy, and the drug treatment was continued for about 4 weeks. He was discharged on 29 December 2000, and oral fluconazole (600 mg per day) was administrated for an additional 4 weeks. Two sets of blood cultures were collected 1 month after discontinuation of fluconazole therapy and were negative for the organism. At the 3-month follow-up, there was no evidence of recurrence.
Microbiology.
All isolates (A, B, and C) were identified as R. glutinis by use of conventional methods (orange colonies on Sabouraud dextrose agar at 25°C and oval budding cells without pseudohyphae on cornmeal-Tween 80 agar [Difco Laboratories, Detroit, Mich.]) and API ID32 C (code number 5475750313; probability of species identification, 99.7%; typicality index, 99.6%) (bioMerieux, Marcy I'Etoile, France). For comparison, two isolates of R. glutinis (one blood isolate [D] and one isolate from pus [E] recovered from two patients seen at the National Taiwan University Hospital in 1999 and 2000, respectively) were also included in the study as control strains. The MICs for these isolates, determined by the E test (PDM Epsilometer; AB Biodisk, Solna, Sweden), were measured on RPMI 1640-2% glucose agar medium supplemented with morpholinepropanesulfonic acid (MOPS) as previously described (6). The MICs were read after 2 days of incubation at 37°C. Measurement of the MICs of fluconazole for these isolates was also performed using the methods recommended by the National Committee for Clinical Laboratory Standards (11). Random amplified polymorphic DNA (RAPD) patterns of these isolates were determined by arbitrarily primed PCR as described previously (6). The three oligonucleotide primers used, M13 (5′-GAGGGTGGCGGTTCT-3′), OPH-15 (5′-AATGGCGCAG-3′), and OPH-19 (5′-CTGACCAGCC-3′) were chosen from 20 primers in a kit (OPH-1 to OPH-20) purchased from Operon Technologies, Inc. (Alameda, Calif.).
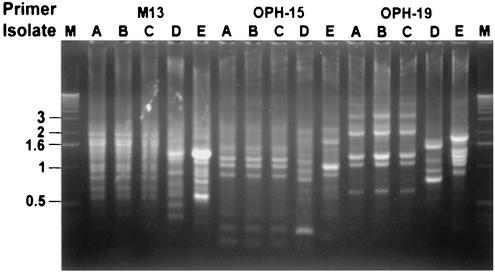
Isolates A, B, and C had identical antibiotypes: amphotericin B MICs of 0.125 μg/ml, fluconazole MICs of >256 μg/ml (the MICs determined by the E test and the NCCLS method were identical), ketoconazole MICs of 0.25 μg/ml, itraconazole MICs of 8 μg/ml, and flucytosine MICs of 0.06 μg/ml. However, for isolates D and E, the itraconazole MICs were 0.5 and 1 μg/ml, respectively, while the MICs of the other four agents were within one to two twofold dilutions of those for isolates A, B, and C. Isolates A, B, and C had identical RAPD patterns (they shared every band), and these patterns were different from those of the three control isolates (Fig. 1).
FIG. 1.
RAPD patterns of the five isolates of R. glutinis obtained with three primers: M13, OPH-15, and OPH-19. Lanes M, molecular size markers (1-kb ladder; Gibco BRL, Gaithersburg, Md.); lanes A to E, patterns for isolates A to E, respectively. See the text for the designation of these isolates.
Discussion.
Our findings are unique for three reasons. First, although catheter-related R. glutinis infection has been previously described, our report using phenotypic and genotypic typing methods clearly demonstrates that R. glutinis caused catheter-related infection (isolates recovered from peripheral blood and the inner part of the catheter had identical phenotypes and genotypes). The recurrent nature of infection was due to the persistence of this organism in the catheter for a period of at least 1 month. Second, strains of R. glutinis are intrinsically highly resistant to fluconazole, and amphotericin B is the drug of choice for treating infections caused by this organism. Fluconazole at a dosage of 400 mg per day administered intravenously seemed to improve our patient's clinical condition and remove the yeasts from his bloodstream. Obviously, after 17 days of treatment, this agent failed to clear this organism from the inside of the catheter. The reason why the organism was cleared from the bloodstream but not from the inner part of the catheter (the concentrations of fluconazole running through the catheter would be theoretically highest) before the removal of the catheter is not fully understood. The probable immunocompetent status of the patient and the high fungal burden within the inner part of the catheter might contribute in part to this phenomenon. Moreover, biofilm formation by organisms such as Candida albicans has been demonstrated to contribute to the persistence of the organism and drug resistance (3). Determining whether this is also true of R. glutinis requires further investigation. Finally, our study clearly demonstrated that RAPD patterns generated by arbitrarily primed PCR were acceptable as a typing method for a variety of fungi, including R. glutinis isolates (6, 7).
Previous susceptibility studies of Rhodotorula species have shown that all tested isolates were susceptible to amphotericin B (MICs ranged from 0.125 to 1.6 μg/ml), ketoconazole (MICs of ≤0.25 μg/ml), itraconazole (MICs ranged from 0.25 to 12.8 μg/ml), and flucytosine (MICs of ≤0.25 μg/ml) and resistant to fluconazole (MICs of ≥32 μg/ml) (4). Among the 13 R. glutinis isolates tested by Galan-Sanchez et al. (4), 92.3% were highly resistant to fluconazole (MICs of ≥256 μg/ml). The susceptibility results for our isolates, i.e., high-level resistance to fluconazole and high MICs of itraconazole, were consistent with these previous findings (4). Fluconazole is not recommended as the drug of choice for the treatment of infections due to Rhodotorula species (2, 4, 9, 13). In one previous report, fluconazole failed to clear R. glutinis within a central venous catheter, which led to recurrent sepsis 5 days after the initiation of fluconazole treatment, necessitating subsequent amphotericin B treatment in a nonneutropenic patient with acute leukemia (13). The initial satisfactory improvement of our patient's clinical condition was the reason why we continued fluconazole therapy for him.
Although strains of Rhodotorula species have been shown to cause a variety of severe infections in humans, these organisms have frequently been reported as contaminants in culture plates or medical equipment because of their ubiquitous existence in the natural environment and have been known to cause nosocomial pseudoepidemics (1, 2, 4, 7, 14; Cowan and Allen, letter). In our patient, R. glutinis might have entered into the bloodstream via repeated flushing with contaminated normal saline or via an inadequately disinfected skin surface and then further multiplied and persisted within the catheter.
In conclusion, we report a microbiologically documented central venous catheter-related infection caused by R. glutinis in a nonneutropenic cancer patient. Although the isolate was highly resistant to fluconazole in vitro, this agent (high dose) was able to clear the organism from the bloodstream and the patient received fluconazole treatment for 8 weeks and had the catheter removed. Amphotericin B remains the most active agent against this organism and should be used as the treatment of choice.
REFERENCES
- 1.Alliot, C., B. Desablens, R. Garidi, and S. Tabuteau. 2000. Opportunistic infection with Rhodotorula in cancer patients treated by chemotherapy: two case reports. Clin. Oncol. 12:115-117. [DOI] [PubMed] [Google Scholar]
- 2.Braun, D. K., and C. A. Kauffman. 1992. Rhodotorula fungemia: a life-threatening complication of indwelling central venous catheters. Mycoses 35:305-308. [DOI] [PubMed] [Google Scholar]
- 3.Chandra, J., D. M. Kuhn, P. K. Mukherjee, L. L. Hoyer, T. McCormick, and M. A. Ghannoum. 2001. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J. Bacteriol. 183:5385-5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galan-Sanchez, F., P. Garcia-Martos, C. Rodriguez-Ramos, P. Marin-Casanova, and J. Mira-Gutierrez. 1999. Microbiological characteristics and susceptibility patterns of strains of Rhodotorula isolated from clinical specimens. Mycopathologia 145:109-112. [DOI] [PubMed] [Google Scholar]
- 5.Goldani, L. Z., D. E. Craven, and A. M. Sugar. 1995. Central venous catheter infection with Rhodotorula minuta in a patient with AIDS taking suppressive doses of fluconazole. J. Med. Vet. Mycol. 33:267-270. [DOI] [PubMed] [Google Scholar]
- 6.Hsueh, P. R., L. J. Teng, C. C. Hung, J. H. Hsu, P. C. Yang, S. W. Ho, and K. T. Luh. 2000. Molecular evidence for strain dissemination of Penicillium marneffei: an emerging pathogen in Taiwan. J. Infect. Dis. 181:1706-1712. [DOI] [PubMed] [Google Scholar]
- 7.Hsueh, P. R., L. J. Teng, J. H. Hsu, Y. S. Liaw, Y. C. Chen, Y. S. Pan, H. J. Pan, P. C. Yang, S. W Ho, and K. T. Luh. 2001. A Nosocomial pseudoepidemic caused by Exophiala jeanselmei among patients following sonography-guided aspiration of thoracic lesions. J. Formosan Med. Assoc. 100:613-619. [PubMed] [Google Scholar]
- 8.Kiehn, T. E., E. Gorey, A. E. Brown, F. F. Edwards, and D. Armstrong. 1992. Sepsis due to Rhodotorula related to use of indwelling central venous catheters. Clin. Infect. Dis. 14:841-846. [DOI] [PubMed] [Google Scholar]
- 9.Kiraz, N., Z. Gulbas, and Y. Akgun. 2000. Rhodotorula rubra fungemia due to use of indwelling venous catheters. Mycoses 43:209-210. [DOI] [PubMed] [Google Scholar]
- 10.Krcmery, V., I. Krupova, and D. W. Denning. 1999. Invasive yeast infections other than Candida species in acute leukaemia. J. Hosp. Infect. 41:181-194. [DOI] [PubMed] [Google Scholar]
- 11.National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 12.Pien, F. D., R. L. Thompson, D. Deye, and G. D. Roberts. 1980. Rhodotorula septicemia: two cases and review of the literature. Mayo Clin. Proc. 55:258-260. [PubMed] [Google Scholar]
- 13.Sheu, M. J., C. C. Wang, C. C. Wang, W. J. Shi, and M. L. Chu. 1994. Rhodotorula septicemia: report of a case. J. Formosan Med. Assoc. 93:645-647. [PubMed] [Google Scholar]
- 14.Whitlock, M. W. L., R. A. Dietrich, E. H. Steimke, and M. F. Tenholder. 1992. Rhodotorula rubra contamination in fiberoptic bronchoscopy. Chest 102:1516-1519. [DOI] [PubMed] [Google Scholar]