Abstract
OBJECTIVE
To describe the prevalence of benzodiazepine use, sociodemographic and physical health factors associated with use, dosages taken, and directions for use among individuals aged 65 years and older.
DESIGN
Cross-sectional analysis of baseline data from the community-based, prospective observational Cardiovascular Health Study.
PATIENTS/PARTICIPANTS
Medicare eligibility lists from four U.S. communities were used to recruit a representative sample of 5,201 community-dwelling elderly, of which 5,181 participants met all study criteria.
MEASUREMENTS AND MAIN RESULTS
Among participants, 511 (9.9%) were taking at least one benzodiazepine, primarily anxiolytics (73%). Benzodiazepines were often prescribed to be taken pro re nata (PRN “as needed”), and 36.5% of prescriptions with instructions to be taken regularly were taken at a dose lower than prescribed. Reported over-the-counter (OTC) sleep aid medication use was 39.2% in benzodiazepine users and 3.3% in nonusers. In a multivariate logistic model, the significant independent correlates of benzodiazepine use were being white (odds ratio [OR] 1.9; 95% confidence interval [CI] 1.0, 3.4), female (OR 1.7; CI 1.4, 2.2), and living in Forsyth County, North Carolina, or Washington County, Maryland, compared with living in Sacramento County, California, or Allegheny County, Pennsylvania (OR 2.3; CI 1.4, 2.2); having coronary heart disease (OR 1.6; CI 1.2, 2.1), health status reported as poor or fair (OR 1.8; CI 1.4, 2.3), self-reported diagnosis of nervous or emotional disorder (OR 6.7; CI 5.1, 8.7), and reporting use of an OTC sleep aid medication (OR 18.7; CI 14.1, 24.7).
CONCLUSIONS
One in 10 participants reported taking a benzodiazepine, most frequently an anxiolytic, often at a lower dose than prescribed and usually PRN. The high prevalence of OTC sleep aid medication and benzodiazepine use may place the patient at increased risk of psychomotor impairment. Physicians should assess OTC sleep aid medication use when prescribing benzodiazepines.
Keywords: benzodiazepine; sleep aid medication, over-the-counter; Cardiovascular Health Study; elderly; anxiolytics
Benzodiazepines effectively treat anxiety and insomnia with minimal adverse effects in normal healthy individuals.1–6 However, in the elderly, the safety of benzodiazepines is less clear because of their impaired metabolic elimination and increased sensitivity.7–14 An estimated 10.9 million (6.2%) of U.S. adults purchased or obtained benzodiazepines in 1987, and of those aged 65 years and older (elderly), 11.3% to 13.7% reported taking a benzodiazepine in the previous year.15, 16 In 1988, individuals over 60 constituted approximately 20% of the U.S. population, yet received 49% of all prescriptions for benzodiazepines.17
Benzodiazepines are associated with a number of adverse effects including daytime sedation, ataxia, and slowed psychomotor performance.18–22 One of the more severe adverse effects of benzodiazepine use in the elderly is an increased risk of hip fracture.23–28 Concomitant use of benzodiazepines and other agents such as over-the-counter (OTC) sleep aid medication or alcohol may potentiate the adverse effects of benzodiazepines by increasing the risk of adverse outcomes.29 In addition, reviews of benzodiazepine prescribing practices for the elderly have shown that the dosages are not adjusted adequately for impaired metabolic elimination and increased sensitivity, potentially placing individuals at higher risk of adverse effects.17, 30
Some of the reported clinical correlates of benzodiazepine use in older subjects are the number of other medications taken, sleep disorders, and depression.31 In addition, benzodiazepine users are more likely to be white and college educated.31, 32 Important limitations of the previous studies in this area include nonvalidated patient-reported health status measures and medication use, data from a limited geographic distribution, no data on benzodiazepine dosages used, no data on the proportion of benzodiazepine prescriptions that were ordered to be taken daily versus pro re nata(PRN), and no data on OTC sleep aid medication use or alcohol use.31, 32
The Cardiovascular Health Study provided the opportunity to examine patterns of drug use and their correlates in a large, multisite, population-based study of community-dwelling older adults. One of its aims was to describe medication use in the community-dwelling elderly. The aims of the present study were to use the Cardiovascular Health Study data to describe benzodiazepine prevalence of use, dosages taken, directions for use, and patient reported use, and to explore the correlates of benzodiazepine use with various sociodemographic, psychosocial, and physical health factors.
METHODS
Setting
The Cardiovascular Health Study is a community-based, prospective, observational study of cardiovascular disease and stroke in community-dwelling adults aged 65 years and older, sponsored by the National Heart, Lung, and Blood Institute.
Subjects
Participants were sampled from Medicare eligibility lists in four U.S. communities: Washington County, Maryland; Allegheny County, Pennsylvania; Forsyth County, North Carolina; and Sacramento County, California. The Cardiovascular Health Study recruitment and design is described in detail elsewhere.32, 33 Among eligible respondents, 5,201 (57.6%) agreed to participate and were enrolled in the study between June 1989 and May 1990. Potential participants were excluded if they were institutionalized, wheelchair dependent in the home, undergoing treatment for cancer, or expected to move from the area in the next 3 years. Compared with nonenrollees, participants were less likely to self-report nervous or emotional disorders, high blood pressure, and stroke, and more likely to have quit smoking and to perceive their health status as very good or excellent.33
Measures
Medications.
Medication data were collected by medication inventories conducted by study interviewers in the participant's home. We used the method of an in-home medication inventory, which is thought to be the most reliable and valid approach to obtaining from subjects information about their use of medications.34 Participants were asked to provide the interviewers with the containers of all their current prescription medications. The operational definition of a current prescription medicine was one for which a prescription was written by a physician, and taken by the participant during the 2 weeks prior to the interview. During the in-home medication inventory, the interviewer simply transcribed from the prescription label the name of the drug, the strength, and the dosing instructions. After the transcription process was completed, the interviewer placed the medications in front of the participant and inquired how often each medication was taken on average during the previous 2 weeks.35 The measure of dose reported taken was created from the available data since the interviewers independently recorded the dose and instructions, and asked participants how frequently they took a medicine.36 For all scheduled benzodiazepine prescriptions, the dose reported taken was the calculated total daily dose taken from the patient response to the question, “How often the medication was taken on average during the previous 2 weeks?” Benzodiazepines prescribed to be taken PRN were excluded from analyses of the dose reported taken. The duration of therapy with specific medications was not assessed. Individuals were also asked if they used several nonprescription medications (e.g., OTC sleep aid medication) in the previous 2 weeks. Use of a benzodiazepine and an OTC sleep aid was defined as current if a patient reported their use within the previous 2 weeks.
Benzodiazepine Use.
Benzodiazepines were identified and classified on the basis of their FDA-approved indications as either a hypnotic (triazolam, temazepam, and flurazepam), or an anxiolytic (lorazepam, alprazolam, oxazepam, clonazepam, prazepam, chlordiazepoxide, clorazepate, and diazepam). Participants who were using benzodiazepines from different classes (i.e., they were using both a hypnotic and anxiolytic benzodiazepine) were assigned to the benzodiazepine class that corresponded to the benzodiazepine they were not taking PRN. In those cases in which the PRN status was the same for both drugs, the individual was classified as a combination user. For each of the 11 different benzodiazepines used by this cohort, a median scheduled dose was calculated using all prescriptions with instructions to use the benzodiazepine on a scheduled basis, excluding all PRN prescriptions. The median dose reported taken was also calculated for each of the 11 benzodiazepines using only the prescriptions with instructions to use the benzodiazepine on a scheduled basis, excluding all PRN prescriptions.
Covariates.
A physical examination and interview that included medical and personal history, functional status, and psychosocial questionnaires were administered at baseline. Patients did not undergo psychiatric evaluation. Sociodemographic data included age, gender, education, annual household income, marital status, and race. Psychosocial items included self-perceived global health-rating (excellent, very good, and good; fair and poor), measures of feelings about life as a whole (six categories ranging from 1 = delighted to 6 = terrible), satisfaction with the meaning and purpose of life (Likert scale, anchored by 1 = extremely satisfied and 10 = extremely dissatisfied), and social support (6-item composite scale ranging from 6 = least support to 24 = maximum support).37, 38 Physical functioning was assessed by activities of daily living (ADL) and instrumental activities of daily living (IADL) scores.39, 40 Behavioral data were collected on cigarette smoking and alcohol consumption measured in drinks per week. Sleep disorder information was collected from a medical history questionnaire.
Prevalent comorbid conditions included a history of coronary heart disease, cerebrovascular disease, congestive heart failure, intermittent claudication, hypertension, diabetes mellitus, pulmonary disease, and renal disease. Data were also collected on patient self-reported physician-diagnosed nervous or emotional disorders. Participants were asked: “Have you ever been told by a doctor that you currently have a nervous or emotional disorder?” Data were based exclusively on self-reports except for coronary heart disease, cerebrovascular disease, congestive heart failure, and intermittent claudication, which were validated by physical examination and review of the participant's hospital and physician records.
Statistical Analyses
All statistical analyses were performed using statistical analyses software (SAS version 6.04, SAS Institute, Cary, NC) on a personal computer. Descriptive statistics were used to compare benzodiazepine users to nonusers, nonusers to hypnotic benzodiazepine users, and nonusers to anxiolytic benzodiazepine users. For categorical comparisons, χ2 tests of independence were performed; for continuous measure comparisons, Student's t tests were performed. For the above analyses, data were also stratified by age and gender. Because there were multiple comparisons, a two-tailed p value <.01 was considered significant.
Multivariate analyses used logistic regression models to identify variables that were associated with benzodiazepine use. Variables associated with benzodiazepine use were selected by a logistic regression procedure for backward selection.41 Variables that did not reach a Wald χ2 significance level of .05 were removed from the model. The following variables were initially entered in the model: gender, age (65–69 years, 70–79 years, and 80+ years), race (white vs nonwhite) geographic residence (Forsyth County, NC, and Washington County, Md., vs Sacramento County, Calif., and Allegheny County, Pa.), marital status (married vs not married), education (high school graduate and beyond vs others), coronary heart disease, cerebrovascular disease, congestive heart failure, intermittent claudication, hypertension, pulmonary disease, kidney disease, self-reported diagnosis of nervous or emotional disorder, number of comorbid conditions, smoking, alcohol use, use of OTC sleep aid medication, number of medications (excluding benzodiazepine), self-perceived health (excellent, very good, and good vs fair and poor), and continuous measures of global health rating, feelings toward life, satisfaction with life, ADLs, and IADLs. In addition, for the model that identifies predictors of hypnotic users, PRN status was included as a covariate. Owing to the amount of missing data, income was not included in the above models.
RESULTS
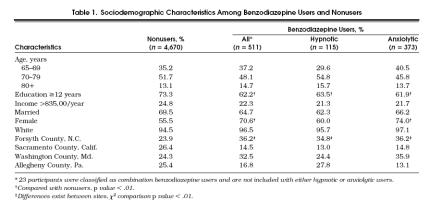
Of the 5,201 participants enrolled in the Cardiovascular Health Study, 20 were excluded from this study because of active cancer treatment (n= 14), or missing medication data (n= 6). Of the remaining 5,181 participants, 511 (9.9%) of the participants were taking at least one type of benzodiazepine. Among these, 115 (22.5%) were using hypnotic benzodiazepines, 373 (73%) were using anxiolytics and 23 (4.5%) were classified as combination benzodiazepine users (Table 1) Benzodiazepine users were less likely to have had at least 12 years of education and more likely to be women. Benzodiazepine use varied by geographic site, ranging from 5.7% to 14.2% of participants, p < .01. These differences in benzodiazepine use by geographic site also existed for anxiolytic and hypnotic benzodiazepine use. Anxiolytic benzodiazepine users were significantly more often women than men. Owing to the small number of participants classified as combination benzodiazepine users (n= 23), valid conclusions could not be made, and their results are not shown in the tables. Stratifying these analyses by age and gender did not change the results.
Tabel 1.
Sociodemographic Characteristics Among Benzodiazepine Users and Nonusers
Benzodiazepine Use
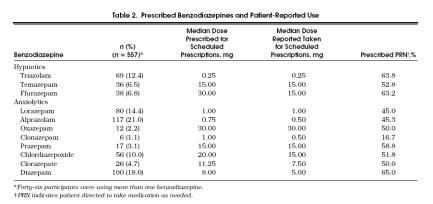
The most frequently prescribed benzodiazepines were alprazolam (21.0%), diazepam (18.0%), lorazepam (14.4%), and triazolam (12.4%) (Table 2); among the hypnotic and anxiolytic benzodiazepines, triazolam and alprazolam were the most frequently prescribed, respectively. Benzodiazepines were commonly prescribed to be taken as needed: for nearly all benzodiazepines, PRN status ranged from one half to nearly two thirds of the prescriptions. Of the 557 prescriptions for benzodiazepines, 257 (46.1%) were prescribed to be taken on a scheduled regimen. Of these 257 prescriptions, 94 (36.5%) were actually taken less frequently than prescribed, and 5 (1.9%) were taken more frequently than prescribed. Eleven different benzodiazepines were used among subjects in this cohort. Of the 11 different benzodiazepines used, prescriptions to be taken on a scheduled regimen were evaluated; 5 (45.5%) of the 11 benzodiazepines were reported to be taken at the median dose prescribed, and 6 (54.5%) were reported to be taken at a dose below the median dose prescribed.
Tabel 2.
Prescribed Benzodiazepines and Patient-Reported Use
Correlates of Benzodiazepine Use
Univariate Analyses.
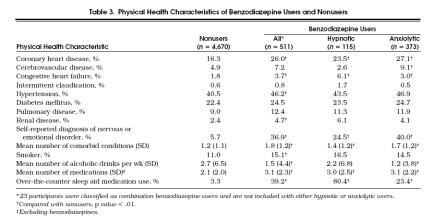
Benzodiazepine users had a significantly higher prevalence of coronary heart disease, congestive heart failure, renal disease, and self-reported diagnosis of nervous or emotional disorder than nonusers, p < .01 (Table 3 Cerebrovascular disease prevalence appeared to be higher in benzodiazepine users than in nonusers; however, analyses by anxiolytic and hypnotic benzodiazepine users showed a higher disease prevalence only among anxiolytic benzodiazepine users. The prevalence of smoking and hypertension was significantly higher in benzodiazepine users compared with nonusers, p < .01. The overall number of chronic comorbid conditions among nonusers was lower than in benzodiazepine users, p < .01. Excluding the benzodiazepine prescriptions, benzodiazepine users took more medications than nonusers (p < .01) and reported taking fewer drinks of alcohol per week (1.5 vs 2.7, p < .01) than nonusers. When analyzed by hypnotic and anxiolytic user groups, only the anxiolytic users used less alcohol than nonusers of benzodiazepine. Self-reported use of OTC sleep aid medication at some time in the previous 2 weeks was substantially higher among all benzodiazepine users (39.2%), hypnotic users (80.4%), and anxiolytic users (23.4%) compared with nonusers of benzodiazepine (3.3%), p < .01. When analyses were stratified by age and gender, differences in physical health and sociodemographic characteristics between benzodiazepine users and nonusers were few and relatively small, despite the large number of comparisons made. For this reason, age and gender differences are not reported here.
Table 3.
Physical Health Characteristics of Benzodiazepine Users and Nonusers
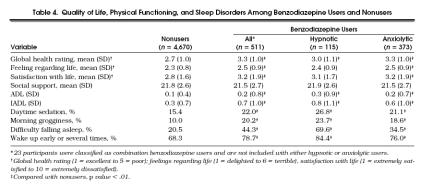
Compared with nonusers, all benzodiazepine users, hypnotic users, and anxiolytic users rated their overall health as worse, p < .01 (Table 4) All categories of benzodiazepine users reported more problems with ADLs and IADLs, more morning grogginess, difficulty falling asleep, and problems with waking up during the night than nonusers, p < .01. However, “feeling regarding life” and “satisfaction with life” scores did not differ between hypnotic users and nonusers.
Tabel 4.
Quality of Life, Physical Functioning, and Sleep Disorders Among Benzodiazepine Users and Nonusers
Multivariate Models.
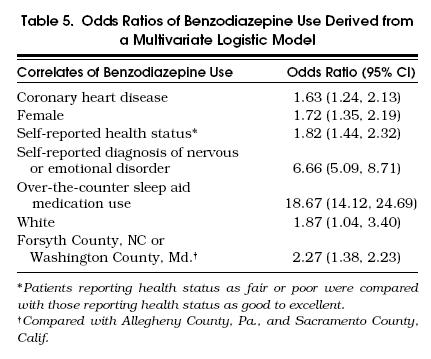
Results from multivariate analyses that compared benzodiazepine users to nonusers are presented in Table 5. Owing to missing data for covariates, 4,755 (91.8%) of the participants were used for these analyses. Benzodiazepine users were nearly 19 times more likely to report taking an OTC sleep aid medication than nonusers, and were approximately 7 times more likely to have self-reported a physician-diagnosed nervous or emotional disorder than nonusers. Benzodiazepine users were also more likely to be female and white; to be residing in Forsyth County, NC or Washington County, Md.; to report worse health; and to have a history of coronary heart disease.
Tabel 5.
Odds Ratios of Benzodiazepine Use Derived from a Multivariate Logistic Model
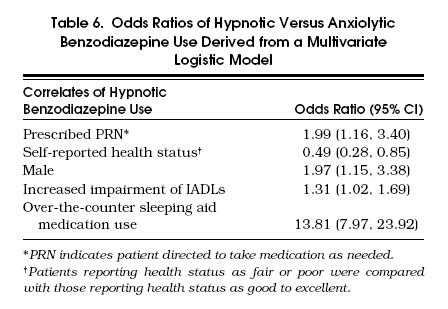
Results from multivariate analyses that compared anxiolytic users with hypnotic users are presented in Table 6. Results indicate that concomitant OTC sleep aid medication use was nearly 14-fold higher among hypnotic users than anxiolytic users. Hypnotic users were also more likely to be male, to be instructed to take the medication PRN, and to have greater impairments in their IADLs, and less likely to report their health as fair or poor.
Tabel 6.
Odds Ratios of Hypnotic Versus Anxiolytic Benzodiazepine Use Derived from a Multivariate Logistic Model
DISCUSSION
Nearly 10% of the Cardiovascular Health Study participants reported taking a benzodiazepine, most frequently an anxiolytic often prescribed to be taken PRN. Of those with prescription benzodiazepines to be taken on a scheduled basis, a substantial proportion reported taking less than the median prescribed dose (36.5%), and 6 of 11 benzodiazepines were reported to be taken at median doses lower than prescribed. When we evaluated the correlates of benzodiazepine use, we found two previously unreported variables correlated with benzodiazepine use: self-reported use of OTC sleep aid medication at some time in the previous 2 weeks (odds ratio [OR] 18.67; 95% confidence interval [CI] 14.12, 24.69) and coronary artery disease (OR 1.63; 95% CI 1.24, 2.13). The strong correlation between self-reported use of OTC sleep aid medication and benzodiazepine use is of particular concern because the active ingredient in all OTC sleep aid medications is an antihistamine, primarily diphenhydramine or doxylamine. The potential for adverse drug reactions with diphenhydramine and doxylamine are well known and especially troublesome in the elderly. These reactions are mediated through H1blockade and anticholingeric activity, resulting in central nervous system effects such as somnolence, diminished alertness, slowed reaction time, impairment of cognitive function, and dry mouth, blurred vision, urinary retention, and constipation.42, 43 Additive central nervous system effects of OTC sleep aid medications and benzodiazepines may place the patient at increased risk of psychomotor impairment. Especially troublesome is the likelihood that the physician prescribing the benzodiazepine is unaware of the patient's OTC sleep aid medication use, as patients rarely discuss OTC medication use with their physicians.44
There are several possible explanations for the reported use of both an OTC sleep aid medication and a benzodiazepine in the previous 2 weeks: e.g., the benzodiazepine was not effectively treating the sleep problem, and the individual was using both agents concomitantly; an OTC sleep aid was used prior to a new benzodiazepine prescription in the previous 2 weeks, or vice versa; or the individual was taking the benzodiazepine PRN and the OTC sleep aid medication PRN, potentially alternating the two. Among hypnotic benzodiazepine prescriptions, the latter explanation may be more likely because hypnotic benzodiazepine users were nearly two times more likely to have prescription directions as PRN than were anxiolytic benzodiazepine users.
The large portion of subjects reporting taking lower benzodiazepine doses than prescribed may be a result of side effects from accumulation of the benzodiazepine when taken on a regular schedule. Supporting this notion is our finding that benzodiazepines with long elimination half-lives (i.e., t1/2>24 h), flurazepam, clonazepam, chlordiazepoxide, clorazepate, and diazepam, all of which are likely to result in daytime sedation and drug accumulation in the elderly when taken on a scheduled basis, were taken at lower median doses than prescribed.7, 8 Prazepam was the only long-acting benzodiazepine reported taken at the median prescribed dose. Among the five short-acting benzodiazepines (i.e., t1/2≤ 24 h), triazolam, temazepam, lorazepam, oxazepam, and alprazolam, only alprazolam was taken at a median dose lower than prescribed. Thus, elderly participants on long-acting benzodiazepines may be noticing adverse effects including daytime sedation, ataxia, and slowed psychomotor performance and self-titrating their dose downward.9, 18–21
The high rate of PRN prescribing for benzodiazepine may suggest that physicians view the disorder for which the drug was prescribed as an intermittent problem. Among hypnotic benzodiazepines, another potential explanation for the high rate of PRN prescribing is the recommendation that when benzodiazepines are used to treat insomnia they should be taken no more often than 2 to 4 times a week to avoid dependence.45
The finding that coronary heart disease was independently correlated with benzodiazepine use is interesting because recent research indicates that a relationship between adverse cardiac events and psychological stress may be mediated by the occurrence of myocardial ischemia.46 Psychological stress is also associated with the use of anxiolytic benzodiazepines.32 Therefore, higher levels of stress may be associated with both benzodiazepine use and coronary heart disease.
The higher rates of all benzodiazepine use among women is consistent with epidemiologic data showing women have twice the prevalence of anxiety disorders.32, 47 With the higher prevalence of anxiety disorders in women, it is not surprising women were nearly three times more likely than men to be taking anxiolytic benzodiazepines. Men were twice as likely as women to take hypnotic benzodiazepines. Because the comparison was anxiolytic use to hypnotic use among women and men, the higher prevalence of hypnotic use found in men is likely the result of men using anxiolytics less, not higher hypnotics use.
Consistent with previous research of benzodiazepine use, a self-reported diagnosis of nervous or emotional disorder was strongly associated with benzodiazepine use, and participants who rated their health as fair or poor were more likely to use benzodiazepines.17, 32 However, these analyses did reveal that participants using anxiolytic benzodiazepines were significantly more likely to report their health as fair or poor than were hypnotic benzodiazepine users. The higher reported quality of life among hypnotic users may be the result of benzodiazepines providing greater relief in insomnia than in anxiety disorders.
Hypnotic benzodiazepine users were found to have greater impairments of their IADLs than anxiolytic benzodiazepine users. This could be due to the underlying sleep disorder or to adverse effects of the hypnotic benzodiazepine such as morning sedation. Because hypnotic benzodiazepines were more likely to be taken PRN, tolerance may not be developing to the sedative properties, or hypnotic benzodiazepines may be prescribed in excessive doses for these elderly participants.
Variation in benzodiazepine prescribing by geographic site was observed. Higher percentages of patients in the easternmost sites (Forsyth County, NC, and Washington County, Md.) reported taking benzodiazepines. It is unknown why this variation exists; however, unexplained variation in prescribing practices and use of medical resources by geographic location is not uncommon.7,47–49
Alcohol use was slightly lower in benzodiazepine users than in nonusers. This was potentially due to the warning patients generally received against alcohol use in combination with benzodiazepines from both the physician and the pharmacist or to underreporting of alcohol consumption among benzodiazepine users.
This study provides some of the best data currently available on benzodiazepine use. Despite this, it has several limitations. The cross-sectional nature of the study does not allow for differentiation of cause from effect. For example, although there was a higher incidence of nervous or emotional disorders among benzodiazepine users, it is unknown whether benzodiazepine use resulted in a higher incidence of nervous or emotional disorders or whether benzodiazepine use was a result of an emotional or nervous disorder. In addition, it is unknown whether lower alcohol use among benzodiazepine users was a result of less alcohol use due to a decline in patient-perceived need for alcohol or a result of a warning by a health care professional not to use alcohol with a benzodiazepine, or whether alcohol users were less likely to receive prescriptions for benzodiazepines. The sample was not fully representative of the population aged 65 years and older because of participant self-selection. Our sample participants were slightly healthier and had a better perception of their health than older persons in general.33 Therefore, generalization of our findings should be limited to our study population of noninstitutionalized elderly adults. The Cardiovascular Health Study did not examine the medical records to validate all participant-reported medical conditions. However. cardiovascular diseases were confirmed through medical record review. Reliance on participant self-report for OTC sleep aid medication use may have affected our estimates. We were unable to identify if benzodiazepine use and OTC sleep aid use was concurrent; however, patients did report use of both agents at some time in the previous 2 weeks. When assessing benzodiazepine therapy, the point in the course of benzodiazepine treatment was not determined. It is possible that patients received the benzodiazepine prescription at a time of high symptom severity prior to our study and since that time the severity of the condition lessened, potentially influencing benzodiazepine use patterns.
These analyses provide important information on the use of benzodiazepines in the elderly and their association with sociodemographic and health status factors. A substantial portion of elderly benzodiazepine users report taking a lower dose than prescribed and are often prescribed benzodiazepines to be taken PRN. In this study, OTC sleep aid medication and coronary heart disease were associated with benzodiazepine use. In addition, for the first time differences in sociodemographic and health status factors among hypnotic and anxiolytic users have been examined in the elderly. Our findings do not suggest clinicians should withhold benzodiazepines for the treatment of medical conditions for which benzodiazepines remain the drugs of choice. Consideration should be given to beginning therapy with a low dose of benzodiazepine and counseling the patient against OTC sleep aid use in conjunction with benzodiazepine therapy. Further research is required to explore the association between coronary heart disease and benzodiazepine use and to identify the potential negative outcomes of combination benzodiazepine and OTC sleep aid therapy. It is our suggestion that epidemiologic studies should evaluate hypnotic and anxiolytic benzodiazepine users separately.
Acknowledgments
Presented at the American College of Clinical Pharmacy, Spring Practice and Research Forum meeting, April 8, 1997.
Supported by contracts N01-HC-85079 through N01-HC-85086, from the National Heart, Lung, and Blood Institute, grants AG-09556 and AG13305 from the National Institute on Aging, and grants MH46015 and MH52247 from the National Institutes of Mental Health.
References
- 1.Gillin JC, Byerley WF. The diagnosis and management of insomnia. N Engl J Med. 1990;322:239–48. doi: 10.1056/NEJM199001253220406. [DOI] [PubMed] [Google Scholar]
- 2.Shader RI, Greenblatt DJ. Use of benzodiazepines in anxiety disorders. N Engl J Med. 1993;328:1398–1405. doi: 10.1056/NEJM199305133281907. [DOI] [PubMed] [Google Scholar]
- 3.Kales A, Bixler EO, Soldatos CR, Vela-Bueno A, Jacoby JA, Kales JD. Quazepam and temazepam: effect of short- and intermediate-term use and withdrawal. Clin Pharmacol Ther. 1986;39:345–52. doi: 10.1038/clpt.1986.51. [DOI] [PubMed] [Google Scholar]
- 4.Mendelson WB, Weingartner H, Greenblatt DJ, Garnett D, Willin JC. A clinical study of flurazepam. Sleep. 1982;5:350–60. doi: 10.1093/sleep/5.4.350. [DOI] [PubMed] [Google Scholar]
- 5.Cross-National Collaborative Panic Study Second Phase Investigators. Drug treatment of panic disorder, comparative efficacy of alprazolam, imipramine, and placebo. Br J Psychiatry. 1992;160:191–202. doi: 10.1192/bjp.160.2.191. [DOI] [PubMed] [Google Scholar]
- 6.Rickels K, Schweizer E, Csanalosi I, Case WG, Chung H. Long term treatment of anxiety and risk of withdrawal: prospective comparison of clorazepate and buspirone. Arch Gen Psychiatry. 1988;45:444–50. doi: 10.1001/archpsyc.1988.01800290060008. [DOI] [PubMed] [Google Scholar]
- 7.Greenblatt DJ, Harmatz JS, Shader RI. Clinical pharmacokinetics of anxiolytics and hypnotics in the elderly. Clin Pharmacokinet. 1991;21:262–73. doi: 10.2165/00003088-199121040-00003. [DOI] [PubMed] [Google Scholar]
- 8.Bertz RJ, Kroboth PD, Kroboth FJ, et al. Alprazolam in elderly and young men. Sensitivity and tolerance to psychomotor, sedative and memory effects. J Pharmacol Exp Ther. 1997;281:1317–29. [PubMed] [Google Scholar]
- 9.Greenblatt DJ, Harmatz JS, Shapiro L, Engelhardt N, Gouthro TA, Shader RI. Sensitivity to triazolam in the elderly. N Engl J Med. 1991;324:1691–8. doi: 10.1056/NEJM199106133242403. [DOI] [PubMed] [Google Scholar]
- 10.Smith RB, Divoll M, Gillespie WR, Greenblatt DJ. Effect of subject age and gender on the pharmacokinetics of oral triazolam and temazepam. J Clin Psychopharmacol. 1983;3:172–6. [PubMed] [Google Scholar]
- 11.Greenblatt DJ, Divoll M, Abernethy DR, Ochs HR, Shader RI. Benzodiazepine kinetics: implication for therapeutics and pharmacogeriatrics. Drug Metab Rev. 1983;14:251–92. doi: 10.3109/03602538308991391. [DOI] [PubMed] [Google Scholar]
- 12.Greenblatt DJ, Divoll M, Harmatz JS, McLaughlin DS, Shader RI. Kinetics and clinical effects of flurazepam in young and elderly noninsomniacs. Clin Pharmacol Ther. 1981;30:475–86. doi: 10.1038/clpt.1981.191. [DOI] [PubMed] [Google Scholar]
- 13.Greenblatt DJ, Allen MD, Shader RI. Toxicity of high-dose flurazepam in the elderly. Clin Pharmacol Ther. 1977;21:355–61. doi: 10.1002/cpt1977213355. [DOI] [PubMed] [Google Scholar]
- 14.Kruse WHH. Problems and pitfalls in the use of benzodiazepines in the elderly. Drug Safety. 1990;5:328–44. doi: 10.2165/00002018-199005050-00003. [DOI] [PubMed] [Google Scholar]
- 15.Olfson M, Pincus HA. Use of benzodiazepines in the community. Arch Intern Med. 1994;154:1235–40. [PubMed] [Google Scholar]
- 16.Stewart RB, Franklin EM, Moore MT, Hale WE. Changing patterns of psychotropic drug use in the elderly: a five-year update. DICP Ann Pharmacother. 1989;23:610–3. doi: 10.1177/1060028089023007-821. [DOI] [PubMed] [Google Scholar]
- 17.Wysowski DK, Baum C. Outpatient use of prescription sedative-hypnotic drugs in the United States,1980 through 1989. Arch Intern Med. 1991;151:1779–83. [PubMed] [Google Scholar]
- 18.Hindmarch I, Kerr JS, Sherwood N. The effects of alcohol and other drugs on psychomotor performance and cognitive function. Alcohol Alcoholism. 1991;26:71–9. [PubMed] [Google Scholar]
- 19.Heatherly DG, Nikaido AM, Bjornsson TD, Kitls C., Ellinwood EH., Jr Comparative pharmacokinetics and pharmacodynamics of lorazepam, alprazolam, and diazepam. Psychopharmacology (Berl) 1985;86:392–9. doi: 10.1007/BF00427897. [DOI] [PubMed] [Google Scholar]
- 20.Smith RB, Kroboth PD, Vanderlugt JT, Phillips JP, Juhl RP. Pharmacokinetics and pharmacodynamics of alprazolam after oral and IV administration. Psychopharmacology (Berl) 1984;84:452–6. doi: 10.1007/BF00431449. [DOI] [PubMed] [Google Scholar]
- 21.Linnoila M, Erwin CW, Brendle A, Simpson D. Psychomotor effects of diazepam in anxious patients and healthy volunteers. J Clin Psychopharmacol. 1983;3:88–96. [PubMed] [Google Scholar]
- 22.Hemmelgarn B, Suissa S, Huang A, Boivin JF, Pinard G. Benzodiazepine use and the risk of motor vehicle crash in elderly. JAMA. 1997;278:27–31. [PubMed] [Google Scholar]
- 23.Ray WA, Griffin MR, Downey W. Benzodiazepines of long and short elimination half-life and the risk of hip fracture. JAMA. 1989;262:3303–7. [PubMed] [Google Scholar]
- 24.Granek E, Baker SP, Abbey H, et al. Medications and diagnosis in relation to falls in a long-term care facility. J Am Geriatr Soc. 1987;35:503–11. doi: 10.1111/j.1532-5415.1987.tb01395.x. [DOI] [PubMed] [Google Scholar]
- 25.Rubenstein LZ, Josephson KR, Robbins AS. Falls in the nursing home. Ann Intern Med. 1994;121:442–51. doi: 10.7326/0003-4819-121-6-199409150-00009. [DOI] [PubMed] [Google Scholar]
- 26.Ray WA, Griffin MR, Downey W. Psychotropic drug use and the risk of hip fracture. N Engl J Med. 1987;316:363–9. doi: 10.1056/NEJM198702123160702. [DOI] [PubMed] [Google Scholar]
- 27.Cummings SR, Nevitt MC, Browner WS, et al. Risk factors for hip fracture in white women. N Engl J Med. 1995;332:767–73. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- 28.Herings RMC, Stricker BHC, de Boer A, Bakker A, Sturmans F. Benzodiazepines and the risk of falling leading to femur fractures: dosage more important than elimination half-life. Arch Intern Med. 1995;155:1801–7. [PubMed] [Google Scholar]
- 29.Woods JH, Katz JL, Winger G. Benzodiazepines: use, abuse, and consequences. Pharmacol Rev. 1992;44:151–347. [PubMed] [Google Scholar]
- 30.Nolan L, O'Malley K. Patients, prescribing, and benzodiazepines. Eur J Clin Pharmacol. 1988;35:225–9. doi: 10.1007/BF00558257. [DOI] [PubMed] [Google Scholar]
- 31.Mayer-Oakes SA, Kelman G, Beers MH, et al. Benzodiazepine use in older, community-dwelling southern Californians: prevalence and clinical observations. Ann Pharmacother. 1993;27:416–21. doi: 10.1177/106002809302700403. [DOI] [PubMed] [Google Scholar]
- 32.Swartz M, Landerman R, George LK, Melville ML, Blazer D, Smith K. Benzodiazepine anti-anxiety agents: prevalence and correlates of use in a southern community. Am J Public Health. 1991;81:592–6. doi: 10.2105/ajph.81.5.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tell GS, Fried LP, Hermanson B, Manolio TA, Newman AB, Borhani NO. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidemiol. 1993;3:358–66. doi: 10.1016/1047-2797(93)90062-9. [DOI] [PubMed] [Google Scholar]
- 34.Landry JA, Smyer MA, Tubman JG, Lago DJ, Roberts J, Simonson W. Validation of two methods of data collection of self-reported medicine use among the elderly. Gerontologist. 1988;28:672–6. doi: 10.1093/geront/28.5.672. [DOI] [PubMed] [Google Scholar]
- 35.Psaty BM, Lee M, Savage PJ, Rutan GH, German PS, Lyles M. Assessing the use of medications in the elderly: method and initial results in the Cardiovascular Health Study. J Clin Epidemiol. 1992;45:683–92. doi: 10.1016/0895-4356(92)90143-b. [DOI] [PubMed] [Google Scholar]
- 36.Schulz R, Mittelmark M, Kronmal R, et al. Predictors of perceived health status in elderly men and women. J Aging Health. 1994;6:419–47. doi: 10.1177/089826439400600401. [DOI] [PubMed] [Google Scholar]
- 37.Cohen S, Mermelstein R, Kamarack T, Hoberman HM. In: Social Support: Theory, Research, and Applications. Sarason IG, Sarason BR, editors. The Hague, Netherlands: Martinus Nijhoff; 1985. pp. 73–94. Measuring the functional components of social support. [Google Scholar]
- 38.Katz SC, Ford AB, Moskowitz RW, et al. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–9. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 39.Lawton MP, Brod EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–86. [PubMed] [Google Scholar]
- 40.Hosmer DW, Lemeshow S, editors. Applied Logistic Regression. New York, NY: Wiley and Sons; 1989. [Google Scholar]
- 41.Simons FE, Simons KJ. The pharmacology and use of H1-receptor-antagonist drugs. N Engl J Med. 1994;330:1663–70. doi: 10.1056/NEJM199406093302307. [DOI] [PubMed] [Google Scholar]
- 42.Simons FER. H1-receptor-antagonists: comparative tolerability and safety. Drug Safety. 1994;10:350–80. doi: 10.2165/00002018-199410050-00002. [DOI] [PubMed] [Google Scholar]
- 43.Lamy P. Over-the-counter medications: the drug interactions we overlook. J Am Geriatr Soc. 1982;30:S69. doi: 10.1111/j.1532-5415.1982.tb01359.x. [DOI] [PubMed] [Google Scholar]
- 44.Kupfer DJ, Reynolds CF. Management of insomnia. New Engl J Med. 1997;336:341–6. doi: 10.1056/NEJM199701303360506. [DOI] [PubMed] [Google Scholar]
- 45.Jiang W, Babyak M, Krantz DS, et al. Mental stress–induced myocardial ischemia and cardiac events. JAMA. 1996;275:1651–6. doi: 10.1001/jama.275.21.1651. [DOI] [PubMed] [Google Scholar]
- 46.Reich J. The epidemiology of anxiety. J Nerv Ment Dis. 1986;174:129–36. doi: 10.1097/00005053-198603000-00001. [DOI] [PubMed] [Google Scholar]
- 47.Chassin MR, Brook RH, Park RE, et al. Variations in the use of medical and surgical services by the medicare population. N Engl J Med. 1986;314:285–90. doi: 10.1056/NEJM198601303140505. [DOI] [PubMed] [Google Scholar]
- 48.Gilbert K, Gleason PP, Singer DE, et al. Variations in antimicrobial use and costs in more than 2000 patients with community-acquired pneumonia. Am J Med. 1998;104:17–27. doi: 10.1016/s0002-9343(97)00274-x. [DOI] [PubMed] [Google Scholar]
- 49.Mort EA. Clinical decision-making in the face of scientific uncertainty: hormone replacement therapy as an example. J Fam Pract. 1996;42:147–51. [PubMed] [Google Scholar]