Abstract
OBJECTIVE
To assess the extent to which an age-associated reduction in mammography use can be explained by declining self-reported health status.
DESIGN
We analyzed data from the 1992 National Health Interview Survey (NHIS) and Cancer Control Supplement. Logistic regression analysis was used to evaluate the association between age, health status (self-reported health and limitations in major activity), and other variables potentially related to mammography use within the past 1 year (recent mammography).
PARTICIPANTS
Of 12,035 NHIS respondents we restricted our analysis to the 1,772 women aged 50 years or older who reported one or more lifetime mammograms. We excluded women without a mammogram (n = 937) because we were interested in factors related to recent use versus past use of mammography.
MEASUREMENTS AND MAIN RESULTS
The percentage of women with a recent mammogram declined with increasing age, and the age association was independent of other factors including health status (adjusted odds ratio [OR] comparing women aged 75 years or older with those aged 50 to 64 years was 0.54; 95% confidence interval [CI] 0.41, 0.70). This age effect persisted in an analysis restricted to women reporting good or better health (adjusted OR was 0.60, 95% CI 0.44, 0.80).
CONCLUSION
The observed decline in recent mammography use with advancing age was not explained by variation in health status. Because healthy elderly women may live long enough to realize the potential benefit of screening mammography, factors responsible for its reduced use should be identified. Doing so will allow for the selective promotion of screening mammography among those older women most likely to benefit.
Keywords: mammography, age factors, aging, breast cancer, health status
Despite the increased risk of breast cancer occurrence and mortality that accompanies aging, and the demonstrated effectiveness of screening mammography as a method to reduce mortality related to breast cancer, use of screening mammography declines with advancing age.1–5 Several factors may contribute to this pattern, including the absence of studies demonstrating the effectiveness of screening mammography among women aged 75 years or older, lower levels of interest in or knowledge about breast cancer control practices among older women, or competing health care needs and priorities.6 Poor health status could also reduce patient or physician enthusiasm for a procedure such as mammography because the anticipated benefit of screening may not be realized for many years following the procedure. An age-associated decline in health status could thus provide another potential explanation for the reduced use of mammography among older women.
In order to understand better the relations among age, health status, and mammography use, we examined data from the 1992 National Health Interview Survey (NHIS) and Cancer Control Supplement.7 An earlier analysis of the 1987 NHIS data demonstrated that mammography use did decline with age and that this effect was unexplained by poor health status.1 As contrasted with earlier studies,1, 2 our focus was on mammography use within the 12 months prior to interview because we postulated that current measures of health would manifest their greatest impact on current rather than historical mammography use. Furthermore, to reduce the likelihood of a potential age-related cohort effect, we limited the analysis to women who had ever had a mammogram. By excluding women who had never had a mammogram, we examined the relation of current health status to contemporary mammography use among women who had previously demonstrated their interest and acceptance of the procedure.
METHODS
Data for this analysis were obtained from the public use tapes of the 1992 NHIS, including the Cancer Control Supplements. The NHIS collects health and demographic information from a sample of the civilian, noninstitutionalized U.S. population who are aged 18 years or older. All adult family members in a surveyed household were asked to participate in the interview. Proxy respondents were accepted for family members who were incapable of responding for themselves, or who were not at home during the interview. Information that was collected included the incidence of acute health conditions, the prevalence of chronic health conditions, restrictions or limitations in daily activity, self-assessed health status, and utilization of health services. For the Cancer Control Supplements, one respondent from a surveyed family was randomly selected to also complete one of two cancer surveys (cancer epidemiology or cancer control). The cancer supplements were available for 12,035 respondents. Of these, we initially restricted our evaluation to the 2,709 women aged 50 years or older. Because we were interested in comparing factors related to recent mammography versus past mammography, women who never had a mammogram (n = 937) were also excluded from the analysis.
Mammography was categorized into two levels: reported mammogram within the 12 months that preceded the interview versus last mammogram more than 12 months previously. Age was categorized into three age groups: 50–64 years, 65–74 years, and 75 years or older. Self-reported health status was collapsed into three categories: good or better, fair, and poor. Limitations in activity (long-term reduction in major activities of daily living resulting from chronic disease or impairment) were dichotomized as one or more limitations in major activities, and no limitations. Other variables included as potential confounders were education, race, insurance status, the belief that family history of breast cancer is a risk factor for breast cancer, smoking status, and usual source of health care. There were 41 persons with missing data in one or more of these variables. These records were excluded from our statistical analysis; thus, our final models were conducted on data from 1,730 women. Certain variables that were of interest to us, particularly poverty status and last visit to the gynecologist, were missing on too many records to include them in our analysis.
We used unconditional logistic regression to evaluate the extent to which age and self-reported health status were related to having undergone mammography in the past year (recent mammography), relative to women with a reported history of mammography, but not within 12 months before the interview date. Women for whom there was a positive history of mammography but the time of mammography was unknown were assumed to have not had a mammogram within 12 months and were included in the reference group. We calculated crude odds ratios (ORs) for age and for self-reported health status, and then evaluated the influence of adjusting for each of the potential confounding variables mentioned above on age and self-reported health. To evaluate potential interaction effects, the relative likelihood of having undergone recent mammography in relation to age (crude and adjusted odds) was estimated within each category of self-reported health.
RESULTS
Characteristics of Study Sample Women and Their Relation to Recent Mammography Use
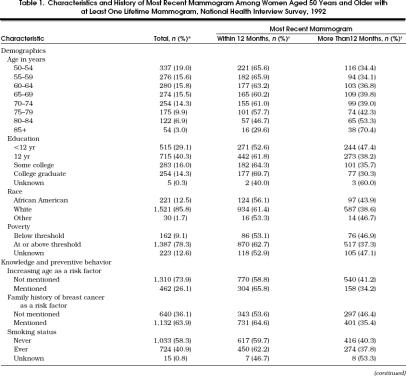
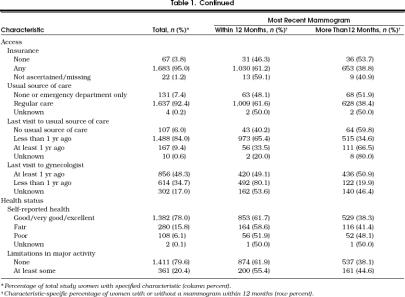
There were 2,709 women aged 50 years or older who participated in the 1992 NHIS. The characteristics of the 1,772 women (65.4%) who reported at least one previous mammogram are presented in Table 1. Approximately three quarters of the study women reported their health as good or better, and a similar proportion reported no limitations in any major activity.
Table 1.
Characteristics and History of Most Recent Mammogram Among Women Aged 50 Years and Older with at Least One Lifetime Mammogram, National Health Interview Survey, 1992
As shown in Table 1, the percentage of women with a recent mammogram declined with advancing age, and this decline was most pronounced among the groups of women aged 75 or older. Among women aged 50 to 59 years, approximately 66% reported a mammogram within the preceding 12 months as compared with approximately 60% of those aged 65 to 74 years and 30% of those aged 85 years or older. Recent mammography was also more frequent among women with a higher educational level, those who identified family history as a breast cancer risk factor, those who were insured, those who had a usual source of health care, those with better self-reported health, and those with no reported limitation of major activities.
Relations Between Age, Reported Health Status, and Recent Mammography Use
Figure 1 displays the percentage of women who had a mammogram within the preceding 12 months stratified by age (50–64, 65–74, 75+) and the two measures of reported health status. The lower panel of Figure 1 presents results for the two levels of limitation of major activities (none or any), and the top panel presents the same information for the three levels of self-reported health (poor, fair, and good or better).Figure 1 illustrates lower percentages of recent mammography use as age increases and as self-reported health status declines. The combination of oldest age and most impaired health status is associated with the lowest observed rates of mammography use. For example, among women aged 50 to 64 years, those with good or better health had a mammography rate (65%) comparable to that observed for women reporting poor health (62%). However, among women aged 75 years or older, the percentage with a recent mammogram among those reporting good health was 53% but declined to 28% among women reporting poor health (upper panel, Fig. 1). The pattern is similar for limitations of activity (lower panel, Fig. 1).
Figure 1.
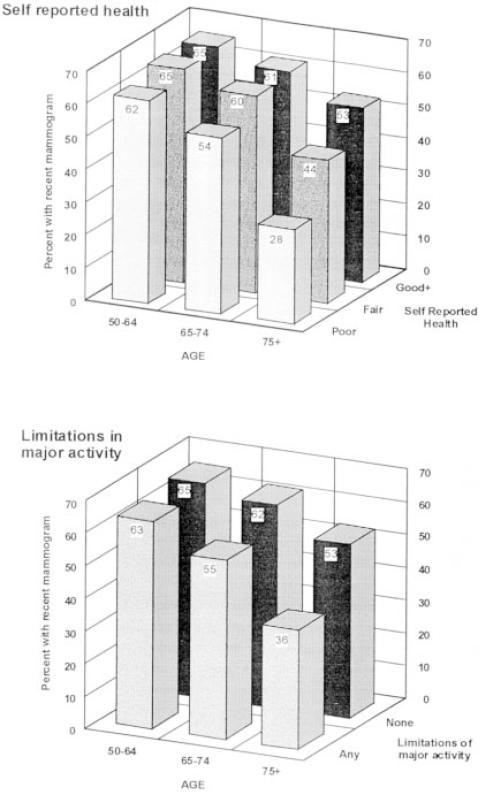
Relation of age and health status to completion of mammography within the preceding 12 months (top panel, for level of self-reported health; lower panel, for limitations in major activity).
Relation of Age to Recent Mammography Use, Controlling for Reported Health Status and Other Patient Characteristics
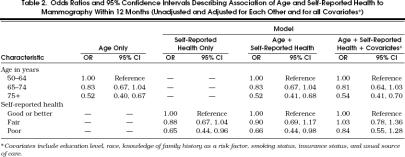
Table 2 presents the results of logistic regression analyses that examined the relation of age and reported health status to recent mammography (unadjusted, adjusted for each other, and adjusted for all covariates). The odds of a recent mammogram declined with advancing age and this association was unaffected after adjustment for health status and the remaining covariates. The odds of a recent mammogram were approximately 20% lower among women aged 65 to 74 years and nearly 50% lower among women aged 75 years or older as compared with women aged 50 to 64 years (adjusted OR for women 75+ = 0.54; 95% confidence interval [CI] 0.41, 0.70). There was no evidence of a significant interaction between age and health status in the logistic regression analysis (p= .46).
Table 2.
Odds Ratios and 95% Confidence Intervals Describing Association of Age and Self-Reported Health to Mammography Within 12 Months (Unadjusted and Adjusted for Each Other and for all Covariates*)
The unadjusted odds of recent mammography also declined with reduced levels of self-reported health. This effect persisted after adjustment for age, but was attenuated and no longer statistically significant after adjustment for the remaining covariates (Table 2). Analyses using self-reported functional limitations rather than self-reported health as the indicator of health status provided essentially similar results.
In order to examine further the relation of age to recent mammography use independent of age-associated changes in health status, we performed another analysis restricted to the subgroup of women reporting good or better health. Among this subgroup of healthy women the adjusted odds of a recent mammogram for those aged 65 to 74 years relative to those aged 50 to 64 years was 0.84 (95% CI 0.65, 1.09), and 0.60 (95% CI 0.44, 0.80) for those aged 75 years and older. Similar patterns were noted in analyses restricted to women reporting fair health or poor health (data not presented).
DISCUSSION
Among our study sample, approximately 60% of women aged 50 years or older reported having had a screening mammogram within the 12 months preceding their NHIS interview. As have others, we noted that the use of mammography declined with advancing age.1, 2, 4 This effect was most substantial for women over the age of 75, among whom the odds of a recent mammography were 50% lower than among women aged 50 to 64 years. Although we identified many factors that were independently associated with lower rates of mammography utilization, including demographic, socioeconomic, and health-care-related characteristics, none explained or modified the age-mammography association. We also evaluated whether or not an age-associated decline in health status might “explain” the observed age-related pattern, but noted that mammography use declined with age among women in each stratum of self-reported health. Even for those reporting good or better health, the odds of a recent mammogram were 40% lower among women aged 75 years or older as compared with those aged 50 to 64 years. Thus, poor health, as indicated by self-reported health status, and the other patient characteristics we have examined do not fully explain the substantial decline in recent mammography use among this sample of women.
From a decision-making perspective, reduce mammography use among older women may reflect the priorities and attitudes of the women themselves or of their physicians (who most often initiate the mammography referral process). Older women report reduced enthusiasm for screening mammography, and the recommendation of a health care professional is commonly cited as a primary determinant of mammography use.8–10 Physician surveys reported reduced rates of mammography referral for women over the age of 75 to 80.11–13 The primary explanation offered by surveyed physicians was related to the uncertain benefit of screening mammography among elderly women in general, and those with serious health impairments in particular.11, 12 This reported pattern is consistent with our observations, although we have no data concerning actual physician referral practices among the study women. Consideration of both mammography effectiveness and patient life expectancy are relevant to understanding these reported and observed patterns of mammography use.
If screening mammography is to reduce mortality from breast cancer, two assumptions must be met—first, the procedure itself must be effective, and second, a woman must be expected to live long enough to experience the benefit. Available evidence neither supports nor refutes the potential benefit of mammography among women aged 75 years or older,14 although the increased incidence of breast cancer, the operating characteristics of screening mammography, and other theoretical considerations suggest that the procedure could be beneficial among healthy elders.6, 15, 16 Nevertheless, the absence of definitive evidence of effectiveness and the absence of organizational endorsement of screening mammography for women over the age of 75 could contribute to the observed paucity of screening mammography use among elderly women.
Anticipated life expectancy is a relevant consideration because the benefit from screening mammography appears only after an interval of perhaps 7 to 9 years.17 Women with a lesser life expectancy might not be expected to benefit from screening mammography. Anticipated life expectancy for the average 75-year-old women is approximately 12 years (and most likely longer for those in good health).18 Thus, age considerations alone would not justify a decision to forgo screening mammography for a healthy 75-year-old woman. Conversely, life expectancy at age 75 may be reduced to only 4 years if serious comorbidity is present and earlier diagnosis of breast cancer may not confer the same advantage for women with severe comorbidity as it does for healthier women.1, 19 Thus, reduced use of mammography among elderly women reporting poor health could reflect a “rational” clinical or personal decision about health care priorities. Data concerning life expectancy considerations and mammography decision making by patients or physicians is not, however, available in the NHIS. Further research in this area is necessary if we are to better understand these factors as determinants of mammography use among older women.
Several limitations of this study must be acknowledged. Although based on a nationally representative sample, our estimates are not weighted in accord with the complex sampling design of the NHIS. Thus, although our estimates of mammography proportions and associated relations are internally valid, they cannot be extrapolated to the population from which the sample was drawn. Furthermore, we limited our analysis to women who reported ever having completed mammography, and thus, our estimates of mammography use exceed those reported by others.2 Had we included all women in our analysis, our estimate of having had a mammogram within 12 months would have been approximately 40% (rather than 60%), a figure consistent with the frequency of mammography use reported in previous studies.2 We used this restriction to focus on the current impact of health status on recent mammography utilization among women who might reasonably be expected to be referred for and accept the procedure. In particular, we wanted to reduce the potential impact of a cohort effect among elderly women who were unlikely to engage in mammography for long-established reasons such as disinterest unrelated to current health status. This concern appears well founded. We noted that only 42 (6%) of 666 women aged 75 years or older reported that their first mammography had been obtained in the preceding 2 years.
We used mammography within the preceding 12 months as our primary outcome measure, rather than within the past 3 years as used in some other analyses of the NHIS data,1, 2 or 2 years (the interval recommended by some authorities).14 We reasoned that reported current health status (as measured in the NHIS) would most likely influence recent rather than more remote behavior, and therefore emphasized the most recent opportunity to have completed mammography. Finally, as necessitated by the content of the NHIS, our measure of health status was self-reported rather than clinically measured, and only self-reported mammography history was available. However, self-reported health has been previously validated as a correlate of measured health status and a predictor of mortality,20 and given the consistency of our findings across all levels of reported health status (and reported functional limitations), it is unlikely that an alternative measure of health status would substantively alter our conclusions. Although we recognize that self-reported mammography use is subject to error, an age-associated decline in mammography use has been consistently observed in a variety of data sources.1, 2, 4 Furthermore, we have no reason to suspect that older women in good health (as in our subgroup analysis) are more likely than younger women to underreport recent mammography, and thus we do not believe that systematic misclassification bias explains the age-associated decline in mammography uses we have observed.
Our data were collected in the 1992 NHIS, and thus not all women (or their physicians) may have been fully aware that screening mammography had recently become a covered Medicare benefit (as of January 1, 1991). Although the removal of a financial barrier to mammography might be expected to reduce the age-associated decline in its use, initial observations have been less encouraging.21–23 Further investigation will be required to determine if the pattern of mammography use we have observed will continue to characterize mammography use among older women in the future.
In summary, we have demonstrated that recent use of mammography declined with advancing age in a group of older women and that this effect was not attributable to declines in reported health status or to other measured characteristics. Ill health did appear to be of particular relevance to mammography use among the oldest women in this study. This may be appropriate given anticipations of life expectancy and benefit. However, mammography use declined with age even among the healthiest older women. Recommendations concerning mammography use among women older than age 70 emphasize individualized decision making and consideration of anticipated life expectancy.6, 14, 24 Because chronologic age and health status together predict life expectancy, reliance on age alone as a guideline for mammography-related decision making is inadequate. If the potential benefits of mammography are to be enjoyed by those women who might be expected to live long enough to benefit from the procedure, targeted interventions will be required to promote procedure use among them.
References
- 1.Mor V, Pacala JT, Rakowski W. Mammography for older women: who uses, who benefits? J Gerontol. 1992;47:43–9. [PubMed] [Google Scholar]
- 2.Anderson LM, May DS. Has the use of cervical, breast, and colorectal cancer screening increased in the United States? Am J Public Health. 1995;85:840–2. doi: 10.2105/ajph.85.6.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costanza ME. The extent of breast cancer screening in older women. Cancer. 1994;74:2046–50. doi: 10.1002/1097-0142(19941001)74:7+<2046::aid-cncr2820741710>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 4.Use of mammography services by women aged ≥65 years enrolled in Medicare—United States 1991–1993. MMWR. 1995;44:777–81. [PubMed] [Google Scholar]
- 5.Burns RB, McCarthy EP, Freund KM, et al. Variability in mammography use among older women. J Am Geriatr Soc. 1996;44:922–6. doi: 10.1111/j.1532-5415.1996.tb01861.x. [DOI] [PubMed] [Google Scholar]
- 6.Costanza ME. Issues in breast cancer screening in older women. Cancer. 1994;74:2009–15. doi: 10.1002/1097-0142(19941001)74:7+<2009::aid-cncr2820741704>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 7.Benson V, Marano MA. Current estimates from the National Health Interview Survey, 1992. National Center for Health Statistics. Vital Health Stat 10. 1994:189. [PubMed] [Google Scholar]
- 8.NCI Breast Cancer Screening Consortium Screening mammography: a missed clinical opportunity?Results of the NCI Breast Cancer Screening Consortium and National Health Interview Survey Studies. JAMA. 1990;264:54–8. [PubMed] [Google Scholar]
- 9.Champion V. Relationship of age to mammography compliance. Cancer. 1994;74:329–35. doi: 10.1002/cncr.2820741318. [DOI] [PubMed] [Google Scholar]
- 10.Rimer BK, Ross E, Cristinzio CS, King E. Older women's participation in breast screening. J Gerontol. 1992;47:85–91. [PubMed] [Google Scholar]
- 11.Roetzheim RG, Fox SA, Leake B. Physician-reported determinants of screening mammography in older women: the impact of physician and practice characteristics. J Am Geriatr Soc. 1995;43:1398–402. doi: 10.1111/j.1532-5415.1995.tb06621.x. [DOI] [PubMed] [Google Scholar]
- 12.Marwill SL, Freund KM, Barry PP. Patient factors associated with breast cancer screening among women. J Am Geriatr Soc. 1996;44:1210–4. doi: 10.1111/j.1532-5415.1996.tb01371.x. [DOI] [PubMed] [Google Scholar]
- 13.Grady KE, Lemkau JP, McVay JM, et al. Clinical decision-making and mammography referral. Prev Med. 1996;25:327–38. doi: 10.1006/pmed.1996.0063. [DOI] [PubMed] [Google Scholar]
- 14.US Preventive Services Task Force . Guide to Clinical Preventive Services. 2nd ed. Baltimore, Md: Williams & Wilkins; 1996. p. 73. [Google Scholar]
- 15.Mandelblatt JS, Wheat ME, Monane M, Moshief RD, Hollenberg JP, Tang J. Breast cancer screening for elderly women with and without comorbid conditions. A decision analysis model. Ann Intern Med. 1992;116:722–30. doi: 10.7326/0003-4819-116-9-722. [DOI] [PubMed] [Google Scholar]
- 16.Kerlikowske K, Grady D, Barclay J, Sickles EA, Ernster V. Likelihood ratios for modern screening mammography: risk of breast cancer based on age and mammographic interpretation. JAMA. 1996;276:39–43. doi: 10.1001/jama.276.1.39. [DOI] [PubMed] [Google Scholar]
- 17.Kerlikowske K, Grady D, Rubin SM, Sandrock C, Ernster VL. Efficacy of screening mammography: a meta-analysis. JAMA. 1995;273:149–54. [PubMed] [Google Scholar]
- 18.National Center for Health Statistics . Health, United States, 1994. Hyattsville, Md: Public Health Service; 1995. pp. 1–307. [Google Scholar]
- 19.Satariano WA, Ragland DR. The effect of comorbidity on 3-year survival of women with primary breast cancer. Ann Intern Med. 1994;120:104–10. doi: 10.7326/0003-4819-120-2-199401150-00002. [DOI] [PubMed] [Google Scholar]
- 20.Idler EL, Benyamini Y. Self-rated health and mortality: a review of twenty-seven community studies. J Health Soc Beh. 1997;38:21–37. [PubMed] [Google Scholar]
- 21.Burg MA, Lane DS. Mammography referrals for elderly women: is Medicare reimbursement likely to make a difference? Health Serv Res. 1992;27:505–16. [PMC free article] [PubMed] [Google Scholar]
- 22.Kiefe CI, McKay SV, Halevy A, Brody BA. Is cost a barrier to screening mammography for low-income women receiving Medicare benefits? A randomized trial. Arch Intern Med. 1994;154:1217–24. [PubMed] [Google Scholar]
- 23.Blustein J. Medicare coverage, supplemental insurance, and the use of mammography by older women. N Engl J Med. 1995;332:1138–43. doi: 10.1056/NEJM199504273321706. [DOI] [PubMed] [Google Scholar]
- 24.Sener SF. Breast cancer in older women: screening and selection of locoregional therapy. Semin Surg Oncol. 1996;12:328–31. doi: 10.1002/(SICI)1098-2388(199609/10)12:5<328::AID-SSU7>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]