Abstract
OBJECTIVE
To determine, in a representative sample of patients drawn from a variety of hospitals, the degree of adherence to consensus recommendations for anticoagulation among patients with deep vein thrombosis or pulmonary embolism.
DESIGN
Cross-sectional review of a population-based random sample.
SETTING
Twenty-one randomly selected Pennsylvania hospitals.
PATIENTS
Of 357 randomly selected Medicare beneficiaries discharged from study hospitals with a diagnosis of deep venous thrombosis or pulmonary embolism during 1992, 43 charts were not reviewed for administrative reasons, 31 were miscoded or not treated with intravenous administration of heparin, and 13 were excluded for other reasons, leaving 270 in the final sample.
MEASUREMENTS AND MAIN RESULTS
Overall, 179 patients (66%, 95% confidence interval [CI] 59%, 72%) received therapeutic anticoagulation (two consecutive partial thromboplastin times more than 1.5 times control) within 24 hours of starting heparin. Platelet counts were checked at least once during the first week of heparin therapy in 66% (95% CI 58%, 74%). At least 5 days of heparin therapy was given to 84% (95% CI 79%, 87%). Among 266 (99%) of the patients receiving warfarin, 193 (72%; 95% CI 63%, 80%) received heparin until the prothrombin time ratio or International Normalized Ratio was therapeutic. Patients who were started on warfarin therapy within 2 days of heparin had decreased length of stay (geometric mean 8.2 vs 9.7 days, p = .003). Compliance varied among hospitals.
CONCLUSIONS
In a wide variety of hospitals, we found fair, but variable, compliance with consensus recommendations for anticoagulation of patients with venous thromboembolic disease. Simple interventions to improve compliance with these recommendations might improve quality of care and reduce costs.
Keywords: venous thromboembolism, warfarin, heparin, monitoring, quality of care
Deep vein thrombosis (DVT) is a common disease in elderly Americans.1, 2 The most dreaded complication, pulmonary embolism, has a high mortality rate in this population, and most deaths occur before the pulmonary embolism is treated.2 The standard approach to treatment is acute anticoagulation with carefully administered and monitored intravenous (IV) heparin, followed by a period of oral anticoagulation with warfarin.3
Since 1985, the National Heart Lung and Blood Institute (NHLBI) and/or the American College of Chest Physicians (ACCP) have convened four consensus conferences on anticoagulation therapy, which have provided guidance for anticoagulant use in this setting.3–6 At these conferences, experts in anticoagulation therapy generate consensus recommendations for therapy, based on a thorough review of the literature using strict rules of evidence.7 These conferences have suggested that early achievement of therapeutic levels of anticoagulation might decrease recurrences; routine monitoring of platelet counts is indicated because of heparin-induced thrombocytopenia; heparin should be administered for at least 5 days in patients with DVT or pulmonary embolism, and until therapeutic anticoagulation with warfarin has been achieved and maintained for 2 days; and warfarin therapy can be instituted concurrently with heparin without increased risk of bleeding.3
Investigators have examined the ability of clinicians to achieve the first of these goals, adequate anticoagulation within 24 hours of the initiation of heparin, in a variety of settings.8–12 In general, the results have been disappointing, with the proportion of patients achieving this goal ranging from 22%11 to 60%,10 depending on the study. Exceptions have been studies of hospitals where heparin dosing nomograms have been used to guide therapy and the proportion of patients achieving adequate anticoagulation within 24 hours of the initiation of heparin has been significantly higher.8, 10, 13–16
Each of these studies, however, reflects practice in one or only a few hospitals. Further, none has examined other important features of heparin dosing, such as the frequency with which platelet counts are monitored, or the extent to which early initiation of warfarin therapy has been adopted in the community.
Because of these limitations, and as part of a plan to examine and improve the quality of care for Medicare beneficiaries in Pennsylvania, we conducted a study of anticoagulation for patients with DVT and pulmonary embolism in 21 randomly selected Pennsylvania hospitals. We compared practices observed in this study with those recommended in the 1989 ACCP-sponsored consensus conference. We emphasize, with the 1995 conference cochairs,17 that these ”recommendations are not rigid but are offered as guidelines for consideration by physicians who should adapt them to individual patient circumstances.” Our use of the word ”compliance” with these recommendations is not meant to imply that noncompliance represents suboptimal practice.
METHODS
Patients
We first identified all patients admitted to Pennsylvania hospitals with DVT or pulmonary embolism in the principal diagnosis position during the period January 1 to December 31, 1992. For each patient, the first such admission was selected. We used the following codes for DVT: 451.11, femoral vein phlebitis and thrombophlebitis; 451.19, thrombophlebitis and phlebitis of other deep vessels of the leg, not elsewhere classified (NEC); 451.2, thrombophlebitis and phlebitis of leg, unspecified; 451.81, iliac vein thrombophlebitis and phlebitis; 453.8, venous thrombosis, NEC; 453.9, venous thrombosis, of unspecified site. We also used code 415.1, pulmonary embolism and infarction. We excluded patients with a diagnosis code for vena cava filter placement, those who were discharged dead, and those who were transferred to another acute care facility. We then used a computerized random number generator to randomly select a set of 21 study hospitals, after stratifying by size and geographic location (eastern vs western Pennsylvania, urban vs rural). Finally, we randomly selected up to 18 qualifying discharges from each facility, with a target of 15 eligible discharges from each facility. The limited number of hospitals in the study and the limited number of patients per hospital were dictated by the cost of the interventions that we planned as part of the overall study.
Data Collection
A trained data abstractor reviewed each chart to confirm that the attending physician treated the patient for DVT or pulmonary embolism and that a vena cava filter had not been placed. We did not require laboratory confirmation of DVT or pulmonary embolism (e.g., venogram or ventilation perfusion scan results), as our focus was on therapy, given that the attending physician believed DVT or pulmonary embolism was present (regardless of whether the diagnosis was correct). We excluded patients for whom the attending physician discontinued therapy in the first 3 days because of a decision not to treat the DVT or pulmonary embolism (usually because of popliteal vein thrombosis). We also excluded those who were initially treated with thrombolytics or therapeutic doses of subcutaneous heparin because we were concerned about the dosing and monitoring of IV heparin therapy. Finally, we excluded patients who had been taking warfarin prior to admission, because the dosing of heparin in such patients might be atypical.
The abstractors extracted data regarding initial heparin dose, duration of heparin therapy, timing and results of the first six partial thromboplastin time (PTT) tests obtained during the 72 hours after starting heparin therapy and timing of up to seven platelet counts obtained after starting heparin therapy. In addition, they determined the date of the initial dose of warfarin, and the prothrombin time (PT) ratio most closely preceding the time the heparin was discontinued.
Analysis
For each patient, we determined whether five process end points were achieved. First was the time to the initial therapeutic PTT. Patients were considered to have achieved a therapeutic PTT at the time of the first of two consecutive PTTs that were more than 1.5 times control, excluding any PTTs obtained within 3 hours of the initial heparin bolus. When a hospital used a range of normal rather than a single control value, we considered the midpoint of the normal range to be the control value. Recent research makes it clear that the traditional use of 1.5 times control as the lower limit of the therapeutic range is frequently inaccurate because the therapeutic range actually varies considerably among lots of reagent and among laboratories.18 It was not feasible to perform such studies on the reagents being used in each facility, so the traditional range was used. The second end point was whether at least one platelet count was obtained during the first 3 and the first 7 days of therapy. Third was whether the patient received at least 5 days (120 hours) of IV heparin therapy. We did not require that the PTT be therapeutic throughout this time. Fourth was the time from the onset of heparin therapy to warfarin initiation. Fifth was whether heparin therapy was continued until the patient was therapeutically anticoagulated with warfarin, as measured by a PT ratio greater than or equal to 1.3, or an International Normalized Ratio (INR) greater than or equal to 2.0. This PT ratio implies an International Sensitivity Index (ISI) of approximately 2.6, typical of reagents used in North America.19 However, it is possible that the specific values varied somewhat among hospitals. The 1989 ACCP consensus recommendations suggest use of a PT ratio of 1.3 when INR is not available.5
In addition to presenting descriptive statistics, we examined whether age, gender, and hospital influenced whether or not patients achieved these end points. We used the generalized estimating equation (GEE) method to calculate population estimates of rates, proportions, and means, and the 95% confidence intervals (CIs) for these estimates. This method adjusts for the clustering of observations (patients) within the sampled hospitals.20 Our analysis of time to therapeutic PTT used survival analysis techniques because we only collected data on PTT for the first 72 hours, and some patients were censored prior to achieving a therapeutic PTT. Early warfarin administration had been associated with shorter length of stay in previous studies,21 so we compared length of stay in patients who began warfarin within 2 days of starting heparin with that in patients who started warfarin later. Since length of stay was not normally distributed, we used a log transformation. The GEE was implemented in the SAS statistical package (SAS Institute, Cary, NC). Time to an event was analyzed by the Coxph program in Splus version 3.3 (StatSci, Seattle, Wash.), which adjusts for the effect of clustering of patients within hospitals.
RESULTS
Data Analysis
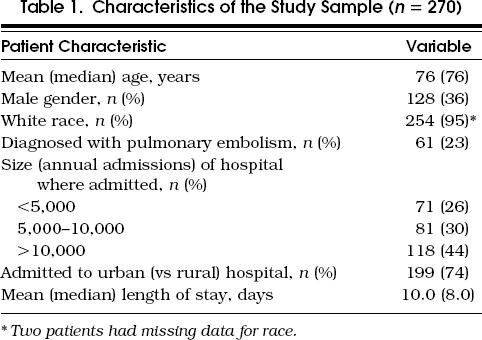
The sampling process produced 21 hospitals across the state. Six of these were rural. Of the 15 urban hospitals, 6 were small (defined as fewer than 10,000 Medicare discharges annually). Of the 357 patient charts requested from these hospitals, we received 313 usable charts. We excluded 13 because the diagnosis of DVT or pulmonary embolism had been miscoded, 18 because the patient was not treated with IV heparin, and 12 for other reasons (vena caval filter, death, transfer, on warfarin at admission). All but one hospital, which was rural, contributed at least 11 patients— the maximum contributed by a hospital was 16. The characteristics of the study population are shown in Table 1
Table 1.
Characteristics of the Study Sample (n= 270)
The majority of patients (172, 64%) were given a 5,000-U bolus of heparin to start therapy, along with an initial infusion rate of 1,000 U/h (146 patients, 54%). The distribution of times to therapeutic anticoagulation is shown in Figure 1. The median time to the first check of PTT after starting the infusion was 6.25 hours. Following a subtherapeutic PTT result, the geometric mean time until a repeated PTT was drawn was 10.2 hours (95% CI 8.9, 11.7 hours). Within 24 hours of the start of heparin therapy, 66% (95% CI 59%, 72%) of patients had achieved therapeutic anticoagulation. The duration of heparin therapy was 120 hours or more (5 days) in 226 (84%; 95% CI 79%, 87%). The distribution of heparin therapy duration is shown in Figure 2.
Figure 1.
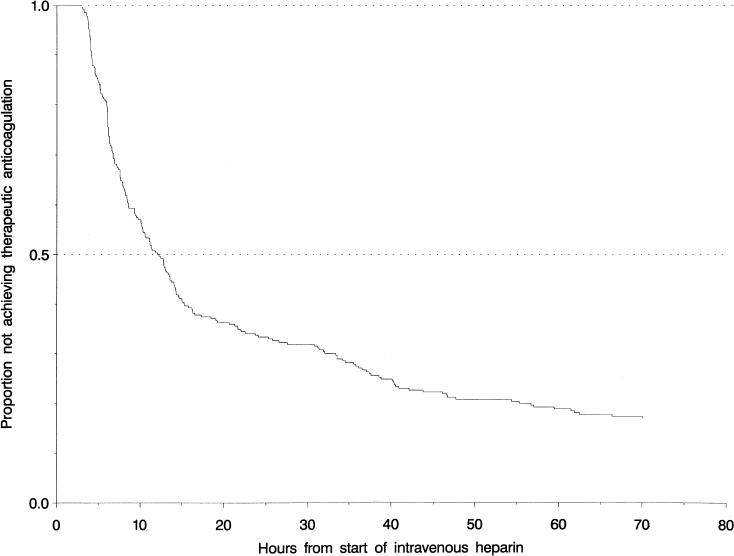
Time (in hours) from start of heparin to therapeutic partial thromboplastin time among patients treated for venous thromboembolic disease.
Figure 2.
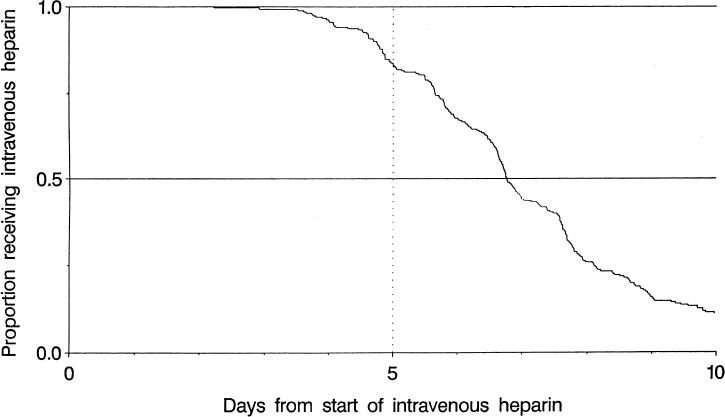
Duration of intravenous heparin therapy among patients treated for venous thromboembolic disease.
Platelets were monitored at least once during the first 7 days of treatment in 177 patients (66%; 95% CI 58%, 74%) and during the 3 days after starting heparin in 148 (55%; 95% CI 46%, 62%). Virtually all patients (266, 99%) were discharged on warfarin. Among these patients the heparin was continued until the PT was therapeutic in 193 (72%; 95% CI 63%, 80%). Only 155 (59%; 95% CI 51%, 67%) of the patients discharged on warfarin began warfarin within 2 days of heparin. The proportion of patients whose management met each of the recommendations we examined is summarized in Table 2
Table 2.
Proportion of Patients Meeting Consensus Guidelines
The hospital to which the patient was admitted was a significant predictor of time to a therapeutic PTT (median time for a hospital ranged from 6.5 to 40 hours, p= .009), as well as duration of heparin therapy (median duration for a hospital ranged from 5.8 to 8.7 days, p < .001).
Age and gender did not differ between those who achieved and those who did not achieve any of the end points. Similarly, although there was a tendency for women to achieve a therapeutic PTT faster than men, the difference was of borderline statistical significance (p= .067).
Patients who began warfarin within 2 days of initiation of heparin therapy had a significantly shorter median length of stay than those in whom warfarin was started later (geometric mean 8.2 vs 9.7 days, p= .003). In addition to time of warfarin initiation, the hospital where a patient was admitted also predicted length of stay.
We incidentally noted that 11 study hospitals were still reporting PT ratios rather than INRs when the study ended in December 1992. Follow-up in 1995 revealed that all had since adopted use of the INR.
DISCUSSION
In this study of heparin dosing practices in a large representative population of elderly Medicare beneficiaries with DVT or pulmonary embolism, we found that approximately two thirds achieved a therapeutic PTT during the first 24 hours of therapy. This is somewhat better compliance with this guideline than was found in studies of practice in hospitals in Tennessee,12 North Carolina,8 Utah,10 Canada,9 and England.11 This result is remarkable given that most other studies classified patients who had only a transiently therapeutic PTT during the first 24 hours as therapeutic, while we required that consecutive PTTs be within the therapeutic range in order to avoid giving credit for patient who had had a PTT that was therapeutic following a heparin bolus. When we recalculated our measured compliance using this criterion (but excluding therapeutic PTTs obtained within 4 hours of starting treatment), it rose to 72%.
The reason for the superior performance we found is unclear. It may be that the older age of patients in the present study allows the traditional starting dose of a 5,000-U bolus and 1,000 U/h to put more patients into the therapeutic range, even though there is evidence from a randomized controlled trial that a heparin dosing nomogram using a more aggressive starting dose is more effective than a nomogram using these traditional starting doses.13 Alternatively, evidence in the literature supporting more aggressive heparin dosing may have been translated into improved practice by the physicians practicing in the study hospitals.
The proportion of patients achieving a therapeutic PTT within 24 hours is less, however, than the 77% to 99% proportion reported by a number of hospitals following the introduction of dosing nomograms.8, 10, 13–16 One other study showed that use of a nomogram allowed achievement of this goal in 66% of patients, but this was a marked improvement over the 38% of patients who reached the goal prior to the nomogram.9 None of the hospitals in the study was routinely using a dosing nomogram by the end of 1992; therefore, such protocols likely represent one way to improve heparin dosing practices. Despite the absence of dosing nomograms in any of the hospitals, there was significant variation among hospitals in the proportion of patients who achieved a therapeutic PTT within 24 hours. In visits to present these data at 10 of the hospitals, no clear explanations for this variation emerged.
This is the first study to demonstrate significant differences between clinical practice and a number of other consensus recommendations including routine monitoring of platelet counts, duration of heparin therapy, and early initiation of warfarin in this population. Early warfarin administration might also represent an opportunity for substantial cost savings. Our finding that early warfarin administration was associated with a 1.5-day decrease in median length of stay is supported by randomized trial data demonstrating that this approach is both safe and cost-effective.14, 21 Our data suggest that 3,240 (42%) of the 7,714 Pennsylvania Medicare beneficiaries (unpublished analysis of Medicare claims by KePRO) admitted with DVT during 1992 did not start warfarin therapy within 2 days of starting heparin, therefore, improved compliance with early warfarin initiation could reduce total hospital days for this group by nearly 5,000 days. Similar savings might be expected in other parts of the country.
There are a number of potential reasons for the suboptimal compliance noted in our study. First, the attending physicians might not agree with the consensus recommendations. Indeed, the primary study end point, achievement of a therapeutic PTT within 24 hours, is not supported by any randomized controlled trial, but rather by retrospective comparisons of patients who did and did not achieve this end point.3, 22 Moreover, some researchers have questioned the validity of the assertion that not achieving therapeutic anticoagulation early in the course of treatment leads to unacceptably high recurrence rates.23 This is supported by our observation that, for patients in our study, the rate of readmission for DVT or pulmonary embolism during the 90 days following the index admission was just 2.5%, and was similar in patients who did and patient who did not achieve therapeutic anticoagulation within 24 hours of starting heparin (data not shown). However, in presentations of the data to physicians practicing at 10 of the 21 study hospitals, none disagreed with the consensus recommendations that we used as benchmarks.
Second, the nature of the study population may have made the recommendations inappropriate for the individual patients. However, we restricted our study to those patients in whom intravenous heparin was the initial therapy and excluded those who had placement of a vena cava filter, were transferred, or died during the hospitalization. Further, we note that virtually all of the study population was discharged on warfarin. Each of these factors suggests that the practices seen in our study reflect the results of straightforward treatment of elderly patients with intravenous heparin for DVT and pulmonary embolism.
Third, inadequate monitoring of PTTs and platelet counts might reflect physicians' responses to perceived fiscal constraints. However, the best compliance was with the guideline that 5 days of heparin therapy be provided—perhaps the most expensive of the end points to achieve. Further, the early use of warfarin, which most evidence suggests would lead to cost savings, was not widespread. The wide spectrum of end points examined makes it clear that our results reflect more than a problem with a single aspect of care (e.g., poor turnaround time in the clinical laboratory).
Finally, one might question whether it is reasonable to expect 100% compliance with these recommendations, given the wide diversity of clinical situations faced by individual clinicians and patients. Moreover, dosing of unfractionated heparin is notoriously difficult. We agree that 100% compliance is unlikely to be achieved, but point out that three of five recommendations (for early warfarin use, for therapeutic PTT within 24 hours, and for routine checks of platelet counts) were met by fewer than two thirds of the patients in the sample. Moreover, evidence in the literature suggests that the degree of compliance can be increased with relatively simple interventions. Further, each of the hospitals with which we discussed the study results believed that compliance could be improved with relatively simple interventions, such as physician education or institution of a nomogram. The results of these interventions are currently being evaluated.
In conclusion, we have shown that the delivery of heparin therapy for DVT and pulmonary embolism to Pennsylvania Medicare beneficiaries often fails to achieve a number of process objectives for such therapy set forth in ACCP consensus recommendations published in 1989.5 Unlike previous studies, our study reflects clinical practice with a random sample of patients who were drawn from a diverse sample of hospitals, and thus are likely to reflect representative anticoagulation practices in older patients throughout the state. We found that compliance with at least one of these recommendations, achieving early therapeutic levels of anticoagulation, exceeded that which was reported in the literature.
Acknowledgments
The analyses on which this publication is based were performed under contract 500-96-P708, entitled ”Utilization and Quality Peer Review Organization for the Commonwealth of Pennsylvania,” sponsored by the Health Care Financing Administration, Department of Health and Human Services. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. The authors assume full responsibility for the accuracy and completeness of the ideas presented. This article is a direct result of the Health Care Quality Improvement Program initiated by the Health Care Financing Administration, which has encouraged identification of quality improvement projects derived from analysis of patterns of care, and therefore required no special funding on the part of this contractor. Ideas and contributions to the authors concerning experience in engaging with issues presented are welcomed.
References
- 1.Anderson FA, Wheeler HB. Physician practices in the management of venous thromboembolism: a community-wide survey. J Vasc Surg. 1992;16:707–14. doi: 10.1067/mva.1992.41080. [DOI] [PubMed] [Google Scholar]
- 2.Goldhaber SZ, Morpurgo M. Diagnosis, treatment and prevention of pulmonary embolism. JAMA. 1992;268:1727–33. [PubMed] [Google Scholar]
- 3.Hyers TM, Hull RD, Weg JG. Antithrombotic therapy for venous thromboembolic disease. Chest. 1995;108(suppl):S335–51. doi: 10.1378/chest.108.4_supplement.335s. [DOI] [PubMed] [Google Scholar]
- 4.Hyers TM, Hull RD, Weg JG. Antithrombotic therapy for venous thromboembolic disease. Chest. 1986;89(suppl):S26–35. doi: 10.1378/chest.89.2_supplement.26s. [DOI] [PubMed] [Google Scholar]
- 5.Hyers TM, Hull RD, Weg JG. Antithrombotic therapy for venous thromboembolic disease. Chest. 1989;95(suppl):S37–51. doi: 10.1378/chest.95.2_supplement.37s. [DOI] [PubMed] [Google Scholar]
- 6.Hyers TM, Hull RD, Weg JG. Antithrombotic therapy for venous thromboembolic disease. Chest. 1992;102(suppl):S408–25. doi: 10.1378/chest.102.4_supplement.408s. [DOI] [PubMed] [Google Scholar]
- 7.Cook DJ, Guyatt GH, Laupacis A, Sackett DL, Goldberg RJ. Clinical recommendations using levels of evidence for antithrombotic agents. Chest. 1995;108(suppl):S227–30. doi: 10.1378/chest.108.4_supplement.227s. [DOI] [PubMed] [Google Scholar]
- 8.Gunnarsson PS, Sawyer WT, Montague D, Williams ML, Dupuis RE, Caiola SM. Appropriate use of heparin: empiric vs nomogram-based dosing. Arch Intern Med. 1995;155:526–32. [PubMed] [Google Scholar]
- 9.Cruickshank MK, Levine MN, Hirsh J, Roberts R, Siguenza M. A standard heparin nomogram for the management of heparin therapy. Arch Intern Med. 1991;151:333–7. [PubMed] [Google Scholar]
- 10.Eliott GC, Hiltunen SJ, Suchyta M, et al. Physician-guided treatment compared to a heparin protocol for deep vein thrombosis. Arch Intern Med. 1994;154:999–1004. [PubMed] [Google Scholar]
- 11.Fennerty AG, Thomas P, Backhouse G, Bentley P, Campbell IA. Audit control of heparin treatment. BMJ. 1985;290:27–8. doi: 10.1136/bmj.290.6461.27-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wheeler AP, Jaquiss RDB, Newman JH. Physician practices in the treatment of pulmonary embolism and deep venous thrombosis. Arch Intern Med. 1988;148:1321–5. [PubMed] [Google Scholar]
- 13.Raschke RA, Reilly BM, Guidry JR, Fontana JR, Srinivas S. The weight-based heparin dosing nomogram compared with a ”standard care” nomogram: a randomized controlled trial. Ann Intern Med. 1993;119:874–81. doi: 10.7326/0003-4819-119-9-199311010-00002. [DOI] [PubMed] [Google Scholar]
- 14.Hull RD, Raskob GE, Rosenbloom D, et al. Optimal therapeutic level of heparin therapy in patients with venous thrombosis. Arch Intern Med. 1992;152:1589–95. [PubMed] [Google Scholar]
- 15.Hollingsworth JA, Rowe BH, Brisebois FJ, Thompson PR, Fabris LM. The successful application of a heparin nomogram in a community hospital. Arch Intern Med. 1995;155:2095–2100. [PubMed] [Google Scholar]
- 16.Raschke RA, Gollihare B, Peirce JC. The effectiveness of implementing the weight-based heparin nomogram as a practice guideline. Arch Intern Med. 1996;156:1645–9. [PubMed] [Google Scholar]
- 17.Dalen JE, Hirsh J. Introduction to the Fourth ACCP Consensus Conference on Antithrombotic Therapy. Chest. 1995;108(suppl):S225–6. [Google Scholar]
- 18.Hirsh J, Raschke R, Warkentin TE, Dalen JE, Deykin D, Poller L. Heparin: mechanism of action, pharmacokinetics, dosing considerations, monitoring, efficacy and safety. Chest. 1995;108(suppl):S258–75. doi: 10.1378/chest.108.4_supplement.258s. [DOI] [PubMed] [Google Scholar]
- 19.Hirsh J, Dalen JE, Deykin D, Poller L, Bussey H. Oral anticoagulants: mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest. 1995;108(suppl):S231–46. doi: 10.1378/chest.108.4_supplement.231s. [DOI] [PubMed] [Google Scholar]
- 20.Diggle PJ, Liang KY, Zeger SL. Analysis of Longitudinal Data. Oxford, UK: Clarendon Press; 1994. pp. 146–68. [Google Scholar]
- 21.Gallus A, Tillett J, Jackaman J, Mills W, Wycherley A. Safety and efficacy of warfarin started early after submassive venous thrombosis or pulmonary embolism. Lancet. 1986;2:1293–6. doi: 10.1016/s0140-6736(86)91431-5. [DOI] [PubMed] [Google Scholar]
- 22.Hull RD, Raskob GE, Hirsh J, et al. Continuous intravenous heparin compared with intermittent subcutaneous heparin in the initial treatment of proximal-vein thrombosis. N Engl J Med. 1986;315:1109–14. doi: 10.1056/NEJM198610303151801. [DOI] [PubMed] [Google Scholar]
- 23.Anand S, Ginsberg JS, Kearon C, Gent M, Hirsh J. The relation between the activated partial thromboplastin time response and recurrence in patients with venous thrombosis treated with continuous intravenous heparin. Arch Intern Med. 1996;156:1677–81. [PubMed] [Google Scholar]



