Abstract
OBJECTIVE
To determine the sensitivity and specificity of mean corpuscular volume, transferrin saturation, total iron-binding capacity, and ferritin level in determining iron deficiency in a population of anemic veterans with a wide variety of general medical diagnoses.
DESIGN
Retrospective chart review.
SETTING
Hospitals of the Department of Veterans Affairs in Madison and Milwaukee, Wisconsin.
PARTICIPANTS
One hundred one anemic veterans with any medical condition who underwent bone marrow aspiration and serum iron studies.
MEASUREMENTS AND MAIN RESULTS
Using the presence or absence of bone marrow hemosiderin as the reference standard, the sensitivity and specificity of the following serum iron indicators were calculated: mean corpuscular volume, transferrin saturation, total iron-binding capacity, and ferritin level. Of these patients, 41 (40.6%) were categorized as iron deficient, with no stainable bone marrow hemosiderin. A serum ferritin level ≤100 μg/L provided the best sensitivity (64.9%) and specificity (96.1%) for evaluating iron stores in this patient population. When performed within 24 hours of bone marrow examination, a serum ferritin level ≤100 μg/L was 100% accurate in separating iron-deficient from iron-sufficient patients. None of the other serum iron indicators alone or in combination performed better than ferritin level alone.
CONCLUSIONS
In a population of anemic veterans with a wide variety of concomitant medical problems, a serum ferritin level ≤100 μg/L was optimal for determining iron deficiency. This is higher than the ferritin level of ≤50 μg/L cited in standard textbooks as evidence of iron deficiency in patients with inflammation, infection, or malignancy.
Keywords: iron deficiency, anemia, ferritin, total iron-binding capacity, transferrin saturation
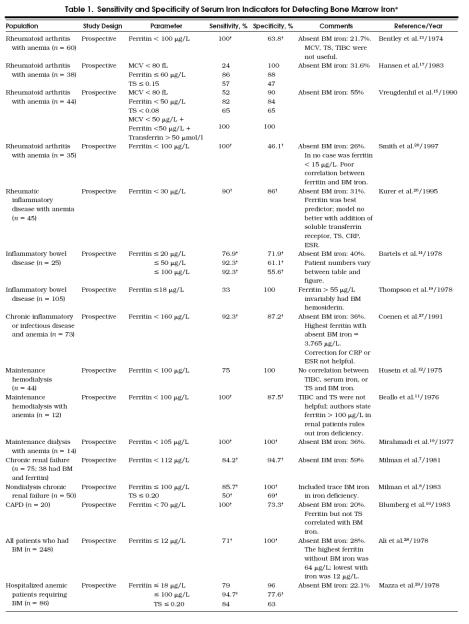
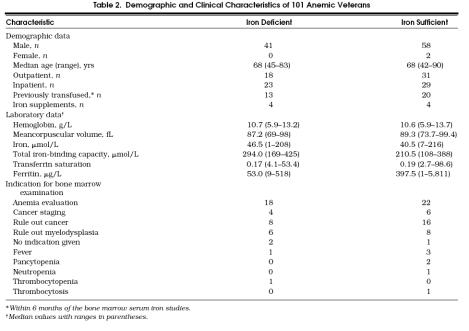
Anemia of chronic disease classically refers to anemia in patients with adequate iron stores occurring in conjunction with chronic inflammation, infection, and malignancy,1–3 or chronic renal failure.4–12 Numerous studies have evaluated the efficacy of serum iron indicators as gauges of iron stores in anemic patients with these chronic diseases.4–26 A number of these studies have correlated serum iron measures with bone marrow hemosiderin (Table 1)27––33 What appears to be less clear is the efficacy of serum iron indicators for anemic patients with medical problems other than infection, inflammation, malignancy, or chronic renal failure. We wondered if the findings of earlier research evaluating the performance of serum iron parameters in the anemia of chronic disease applied to patients with a wider spectrum of concomitant medical conditions. Such a question is relevant as the serum iron indicators are routinely ordered in the evaluation of anemia at substantial cost.
Table 1.
Sensitivity and Specificity of Serum Iron Indicators for Detecting Bone Marrow Iron*
The intention of this retrospective chart review was to determine the sensitivity and specificity of routinely ordered serum indicators of iron status, mean corpuscular volume (MCV), transferrin saturation, total iron-binding capacity (TIBC), and ferritin level, in a population of anemic veterans with any acute or chronic concomitant medical problem. Bone marrow hemosiderin on an aspirated smear was designated as the reference standard for determining iron deficiency. Despite its semiquantitative and subjective classification of bone marrow iron, the bone marrow aspirate remains the best estimate of the presence or absence of iron deficiency available to the practicing physician.4, 33
METHODS
Participants
A computer-generated search of hospital records determined that 943 bone marrow examinations were performed at the Department of Veterans Affairs (VA) Medical Centers in Madison and Milwaukee, Wisconsin, from January 1989 to June 1994. Patients were eligible for further study only if they had serum iron indicators (MCV, transferrin saturation, TIBC, and ferritin level) measured within 30 days of a bone marrow examination, microcytic or normocytic (MCV ≤ 100 fL) anemia (hemoglobin ≤ 140 g/L [14 g/dL]), and a concomitant acute or chronic medical problem. A total of 842 patients were excluded from further study owing to macrocytosis (n= 29), lack of iron studies (n= 574), remote iron studies drawn more than 30 days from the time of bone marrow examination (n= 89), an absence of or inadequate aspirate specimen (n= 35), insufficient documentation (n= 9), absence of anemia (n= 19), and chart unavailability (n= 87). This left 101 patients for further evaluation.
Diagnoses
Medical records of the remaining 101 patients were reviewed. Any diagnosis documented in the patient record was recorded as a chronic medical problem including hypertension, chronic obstructive pulmonary disease, diabetes, cancer, vascular disease, arthritis, renal failure, and autoimmune disorders. An acute medical problem was usually an infection or an exacerbation of a chronic condition. Most subjects had more than two chronic medical problems, and the most active problem at the time of the bone marrow examination and serum iron studies was ascertained from the medical record. Pertinent laboratory data (e.g., liver enzymes, renal function, erythrocyte sedimentation rate) were also recorded when available.
Biochemical Studies
Hematologic and serum biochemical analyses were performed by automated methods in the clinical laboratories at the two hospitals.
Bone Marrow Examinations
The marrow aspirates were prepared and examined in the clinical laboratories at the two hospitals. All were obtained from the iliac crest, placed in ethylenediaminetetraacetic acid (EDTA), concentrated, smeared on glass slides, and coverslipped. Stains for morphology were prepared in Wright-Giemsa, and iron was stained with Prussian blue with positive controls on each slide. Bone marrow iron was examined under oil immersion to look for ringed sideroblasts. Iron was quantified using a grade of 0 to 6. Iron deficiency was defined as the total absence of bone marrow hemosiderin. Any amount of stainable iron resulted in the placement of a patient in the iron-sufficient category. Because of the widely held opinion that a serum ferritin level>100 μg/L excludes the diagnosis of iron deficiency, we personally reexamined 4 of the 13 bone marrow specimens interpreted initially as iron deficient from patients with ferritin levels>100 μg/L with a hematologist blinded to the ferritin level and other clinical information. All four specimens were confirmed to have absent iron.
Statistical Analysis
Once the patients were classified as iron deficient or sufficient, sensitivities, specificities, and predictive values for the various serum iron indicators were calculated.34 Receiver-operating characteristic curves were constructed to aid in choosing the optimal cutoff point for each test in the assessment of iron status.34
The data were compared for the iron-deficient and iron-sufficient groups using the Mann-Whitney U Test. Statistical significance was assigned at p≤ .01 to adjust for multiple comparisons.
RESULTS
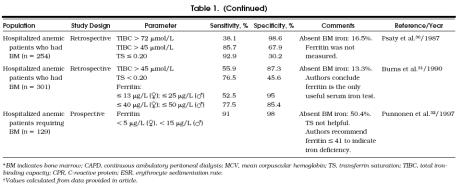
Of the 101 patients, 41 (40.6%) were iron deficient and 60 (59.4%) were iron sufficient. Other demographic and clinical information is presented in Table 2. The only significant differences found among the data for the iron-deficient and iron-sufficient groups were TIBC (p≤ .001) and ferritin level (p≤ .001). Forty-five patients had diseases typically associated with the anemia of chronic disease. Eight of these 45 and 5 other patients had renal failure (serum creatinine level>153 μmol/L [2.0 mg/dL]). The other 51 had a spectrum of medical illnesses including chronic obstructive pulmonary disease, heart disease, hypertension, and diabetes.
Table 2.
Demographic and Clinical Characteristics of 101 Anemic Veterans
Of all the serum iron indicators studied, serum ferritin level proved to best reflect iron status (Fig. 1). Using a ferritin value ≤100 μg/L maximized its sensitivity (64.9%) and specificity (96.1%) for the detection of iron deficiency in this population of patients and gave a positive predictive value of 92% and a negative predictive value of 79%. Exclusion of patients with infection, inflammation, and malignancy (n= 45) did not substantially alter the sensitivity or specificity (73.1% and 100%, respectively). The five patients with renal failure who were iron deficient had ferritin levels ranging from 29 to 487 μg/L. Although standard references,1, 2, 35 even within the same text,35 vary widely on ferritin levels said to indicate iron deficiency, serum ferritin levels ≤12 μg/L and ≤20 μg/L are frequently cited.35 In our patients, these levels had sensitivities of 8.1% and 18.9% and specificities of 98% and 98%, respectively. Positive and negative predictive values for levels ≤20 μg/L were 87% and 62%, respectively, and for levels ≤12 μg/L, 75% and 60%, respectively.
Figure 1.
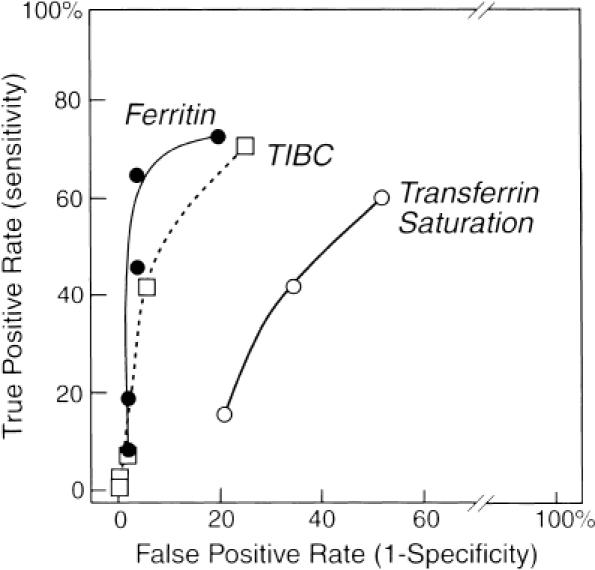
Receiver -operating characteristic curves for serum iron indicators using the presence or absence of bone marrow hemosiderin as the reference standard for iron sufficiency or deficiency, respectively. The points along the curves from left to right represent calculations for ferritin level ≤12, ≤20, ≤50, ≤100, and ≤150 μg/L; total iron-binding capacity ≥81, ≥72, ≥63, ≥54, and ≥45 μmol/L; and transferrin saturation value of 0.10, 0.1, 5, and 0.20.
None of the other serum iron indicators performed as well as ferritin.Figure 2 shows that the TIBC and transferrin saturation displayed considerable overlap between the iron-deficient and iron-sufficient groups. A transferrin saturation ≤0.20 had a sensitivity of 60.5% and specificity of 48.1%. Total iron-binding capacity ≥45 μmol/L had a sensitivity of 71% and a specificity of 75%. Mean corpuscular volume ≤80 fL had a sensitivity of 7.1% and a specificity of 88.3%. Combining a ferritin level ≤100 μg/L with TIBC ≥45 μmol/L did not improve the performance of ferritin level alone, yielding a sensitivity of 55.9% and specificity of 100%. Similarly, no improvement occurred by combining a ferritin value ≤100 μg/L with TIBC ≥45 μmol/L and transferrin saturation of 0.20, yielding a sensitivity of 31.4% and a specificity of 100%.
Figure 2.
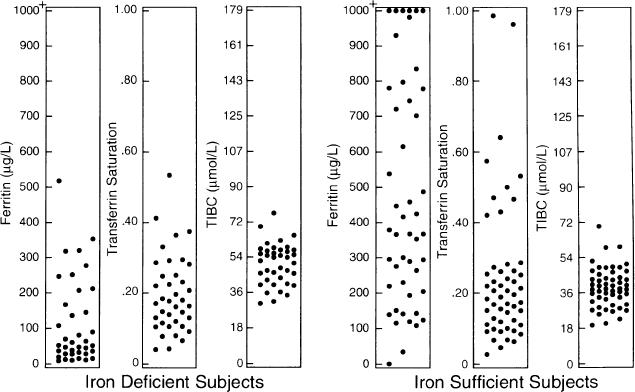
Serum iron indicators in iron-deficient and iron-sufficient patients demonstrating considerable overlap between the two groups.
If only those patients (n= 26) who had serum ferritin levels determined within 1 day of their bone marrow examinations were used in the calculation of sensitivity and specificity, both improved to 100% for ferritin level ≤100 μg/L. Transferrin saturation, TIBC, MCV, ferritin level ≤12 μg/L or ferritin level ≤20 μg/L did not consistently improve in performance for this group although TIBC ≥45 μmol/L improved to a sensitivity of 81.8% and specificity of 84.6%.
DISCUSSION
In this retrospective study of anemic veterans with concomitant medical illnesses who had serum iron indicators measured and bone marrow examinations within 30 days, we found that a ferritin level ≤100 μg/L outperformed MCV, transferrin saturation, and TIBC, either alone or in combination, in correctly diagnosing iron deficiency. However, because of its relatively low sensitivity, using a cutoff ferritin level ≤100 μg/L to indicate the presence of iron deficiency would still have missed more than one third of the patients lacking bone marrow iron stores. The prevalence of iron deficiency (40.6%) in our patients is consistent with that found by bone marrow iron examination in anemic patients with reumatoid arthritis (55%),15 patients with other chronic inflammatory or infectious diseases (36%),27 and hospitalized patients requiring a bone marrow examination (50.4%).32 Assuming this prevalence is similar for veterans with concomitant medical illness in other medical centers, the positive and negative predictive values of 92% and 79%, respectively, for a ferritin value ≤100 μg/L should be reasonable estimates for the clinician.
A serum ferritin level ≤50 μg/L is cited as evidence for iron deficiency in anemic patients with coexisting chronic disease states such as inflammation, infection, and malignancy.1, 3 Our study suggests that this cutoff may be too low in anemic patients seen at VA medical centers. Serum ferritin levels>100 μg/L,35 and>150 μg/L,2 are stated in medical textbooks to reassure clinicians that iron deficiency is not present. This was not the case in the present study. For example, the highest ferritin level found in the iron-deficient group was 518 μg/L. Conversely, one patient in the iron-sufficient group had a ferritin level of 1 μg/L.
Seven previous studies examined the efficacy of various serum iron indicators in diagnosing iron deficiency in patients with medical problems not limited to the usual medical conditions associated with the anemia of chronic disease.28–32, 36, 37 Six of these included assessment of bone marrow iron.28–32, 36 Five of these studies were in hospitalized patients, and one appears to have included both inpatients and outpatients.28 Sensitivity and specificity of serum ferritin level was provided or could be calculated from the data provided in five of these studies.28–31, 36 Both Mazza et al.29 and Ali et al.28 found the sensitivity and specificity of serum ferritin for detecting absent bone marrow iron to be similar to those found in our study, but with much lower cutoffs for ferritin level (18 μg/L and 12 μg/L, respectively). We were able to calculate a sensitivity of 94.7% and a specificity of 77.6% for serum ferritin level ≤100 μg/L using the data points provided in the study by Mazza et al.29 One possible explanation for greater sensitivity and lower specificity in this study compared with our results is that 11 (58%) of 19 iron-deficient subjects evaluated by Mazza et al. were classified as having uncomplicated iron deficiency (i.e., simple blood loss) without other diseases. Our study also included both inpatients and outpatients, and our subjects were almost entirely male. Witte et al. attempted to adjust the serum ferritin level cutoff for iron deficiency according to the erythrocyte sedimentation rate in 43 hospitalized patients,36 but subsequent studies found this prediction model invalid.26, 27
Transferrin saturation ≤20% was more sensitive (76.5%–92.9%) for iron deficiency in three studies that investigated this parameter in unselected hospitalized patients,29––31 compared with our study, which found a transferrin saturation of ≤20% to be only 60.5% sensitive. This may again be attributed to the proportionately fewer patients with uncomplicated iron-deficiency anemia in our study. The specificity of the transferrin saturation was poor in all studies. Transferrin saturation has also been shown to correlate poorly with bone marrow iron stores in patients with anemia and the more traditional chronic diseases.10, 11, 13, 26, 32
Our finding that both the sensitivity and specificity of a ferritin level ≤100 μg/L improved to 100% if only patients (n= 26) with serum ferritin obtained within 1 day of their bone marrow examinations were included suggests that the diagnostic accuracy of serum ferritin level for iron deficiency is actually better than the results reflected from evaluation of ferritin level and bone marrow examination within 30 days. Although this same-day correlation of serum ferritin level with bone marrow iron value strengthens the clinicians' confidence in using a serum ferritin value ≤100 μg/L as evidence of iron deficiency, sensitivity remained low for ferritin levels ≤12 μg/L and ≤20 μg/L, the more commonly used cutoff levels recommended as indicating iron deficiency. Furthermore, evaluation of the anemic patient typically proceeds in a stepwise fashion such that the correlation of serum ferritin level and bone marrow iron value within 30 days of each other may more closely approximate the time frame of these tests in actual medical practice.
Caution is urged in extrapolating the results of this study in mostly male veterans to other patient populations. We also remind the reader that our sample was from the population of anemic patients who received bone marrow examinations. We have no information on those who were anemic and did not have bone marrow iron stores evaluated. Nevertheless, our findings suggest that, at least in this patient population, serum iron indicators other than ferritin level do not discriminate accurately between the presence and absence of bone marrow iron. Their performance is similarly poor in populations of patients with inflammatory rheumatic disease,26 hematologic malignancies,18 or chronic renal failure.12 We suggest that there is abundant evidence from studies in patients with inflammation, infection, maligancy, or chronic renal failure and growing evidence from studies in patients with unselected acute and chronic illnesses that transferrin saturation and TIBC can be eliminated from the evaluation of microcytic or normocytic anemia.
In summary, our review of serum iron indicators and bone marrow iron stores in 101 anemic veteran patients suggests that in this limited population a serum ferritin value ≤100 μg/L has a high specificity for diagnosing the presence of iron deficiency in patients with a wide variety of chronic and acute medical problems. If the ferritin level determination and bone marrow examination are performed within 24 hours of each other, the sensitivity of a serum ferritin level ≤100 μg/L for iron deficiency is also high. Our results suggest that the recommended serum ferritin threshold of ≤50 μg/L for diagnosing iron deficiency in anemic patients with coexisting inflammation, infection, or malignancy may be too low in anemic patients with a wider variety of acute and chronic illnesses receiving care at the nation's 172 VA medical facilities and that raising the threshold to ≤100 μg/L may be preferable. Despite the routine practice of ordering transferrin saturation and TIBC tests to evaluate anemic patients, we found in this setting that these measures offered nothing beyond the serum ferritin level in discriminating iron-deficient from iron-sufficient patients. Furthermore, when there is any clinical ambiguity, the clinician may still need to examine bone marrow iron stores to diagnose iron deficiency.
Acknowledgments
This work was supported by the Madison Geriatric Research Education and Clinical Center (GRECC) and the University of Wisconsin Department of Medicine.
The authors acknowledge Kathryn Klechner for assistance with illustrations, Archie MacKinney, MD, for his advice and assistance with bone marrow aspirate reviews, and Ellen Roecker, PhD, for assistance with statistical analysis.
References
- 1.Bunn HF. Anemia associated with chronic disorders. In: Isselbacher KJ, Martin JB, Braunwald E, Kauci AS, Wilson JD, Kasper DL, editors. Harrison's Principles of Internal Medicine. 13th ed. New York, NY: McGraw-Hill; 1994. pp. 1732–4. In. eds. [Google Scholar]
- 2.Erslev AJ. Anemia of chronic disease. In: Beutler E, Lichtman MA, Caller BS, Kipps TS, editors. Williams Hematology. 5th ed. New York, NY: McGraw-Hill; 1995. pp. 518–24. In: eds. [Google Scholar]
- 3.Sears DA. Anemia of chronic disease. Med Clin North Am. 1992;76:567–79. doi: 10.1016/s0025-7125(16)30340-6. [DOI] [PubMed] [Google Scholar]
- 4.Kraus JR, Stolc V. Serum ferritin and bone marrow iron stores. Am J Clin Pathol. 1979;72:817–20. doi: 10.1093/ajcp/72.5.817. [DOI] [PubMed] [Google Scholar]
- 5.Bell JD, Kincaid WR, Morgan RG, et al. Serum ferritin assay and bone-marrow iron stores in patients on maintenance hemodialysis. Kidney Int. 1980;17:237–41. doi: 10.1038/ki.1980.27. [DOI] [PubMed] [Google Scholar]
- 6.Milman N, Christensen TE, Pedersen NS, Visfeldt J. Peritoneal dialysis and hemodialysis patients with chronic renal failure. Acta Med Scand. 1980;207:201–5. doi: 10.1111/j.0954-6820.1980.tb09706.x. [DOI] [PubMed] [Google Scholar]
- 7.Milman N, Christensen TE, Visfelt J. Diagnostic efficacy of various laboratory tests in the assessment of bone marrow iron stores in patients with chronic uraemia. Scand J Haematol. 1981;26:257–64. doi: 10.1111/j.1600-0609.1981.tb01657.x. [DOI] [PubMed] [Google Scholar]
- 8.Milman N, Bangsboll S, Pedersen NS, Visfeldt J. Serum ferritin in non-dialysis patients with chronic renal failure: relation to bone marrow iron stores. Scand J Haematol. 1983;30:337–44. doi: 10.1111/j.1600-0609.1983.tb01502.x. [DOI] [PubMed] [Google Scholar]
- 9.Mirahmadi KS, Paul WL, Winer RL, et al. Serum ferritin level: determinant of iron requirement in hemodialysis patients. JAMA. 1977;238:601–3. doi: 10.1001/jama.238.7.601. [DOI] [PubMed] [Google Scholar]
- 10.Blumberg AB, Marti HRM, Graber CG. Serum ferritin and bone marrow iron in patients undergoing continuous ambulatory peritoneal dialysis. JAMA. 1983;250:3317–9. [PubMed] [Google Scholar]
- 11.Beallo R, Dallman PR, Schoenfeld PY, Humphreys MH. Serum ferritin and iron deficiency in patients on chronic hemodialysis. Trans Am Soc Artif Intern Organs. 1976;22:73–9. [PubMed] [Google Scholar]
- 12.Hussein S, Prieto J, O'Shea M, Hoffbrand AV, Baillod RA, Moorhead JF. Serum ferritin assay and iron status in chronic renal failure and haemodialysis. BMJ. 1975;1:546–8. doi: 10.1136/bmj.1.5957.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bentley DP, Williams P. Serum ferritin as an index of storage iron in rheumatoid arthritis. J Clin Pathol. 1974;27:786–8. doi: 10.1136/jcp.27.10.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartels U, Pedersen NS, Jarnum S. Iron absorption and serum ferritin in chronic inflammatory bowel disease. Scand J Gastroenterol. 1978;13:649–56. doi: 10.3109/00365527809181777. [DOI] [PubMed] [Google Scholar]
- 15.Vreugdenhil G, Baltus CAM, van Eijk HG, Swaak AJG. Anemia of chronic disease: diagnostic significance of erythrocyte and serological parameters in iron deficient rheumatoid arthritis patients. Br J Rheumatol. 1990;29:105–10. doi: 10.1093/rheumatology/29.2.105. [DOI] [PubMed] [Google Scholar]
- 16.Bezwoda WR, Derman DP, Bothwell TH, Baynes R, Hesdorffer C, MacPhail AP. Serum ferritin and Hodgkin's disease. Scand J Haematol. 1985;35:505–10. doi: 10.1111/j.1600-0609.1985.tb02820.x. [DOI] [PubMed] [Google Scholar]
- 17.Hansen TM, Hansen NE, Birgens HS, Holund B, Lorenzen I. Serum ferritin and the assessment of iron deficiency in rheumatoid arthritis. Scand J Rheumatol. 1983;12:353–9. doi: 10.3109/03009748309099740. [DOI] [PubMed] [Google Scholar]
- 18.Jakobsen E, Engeset A, Sandstad B, Aas M. Serum ferritin and bone marrow haemosiderin in patients with malignancies and healthy controls. Scand J Haematol. 1982;28:264–71. doi: 10.1111/j.1600-0609.1982.tb00525.x. [DOI] [PubMed] [Google Scholar]
- 19.Thomson ABR, Brust R, Ali MAM, Mant MJ, Valberg LS. Iron deficiency in inflammatory bowel disease: diagnostic efficacy of serum ferritin. Dig Dis. 1978;23:705–9. doi: 10.1007/BF01072356. [DOI] [PubMed] [Google Scholar]
- 20.Smith RJ, Davis P, Thomson ABR, Wadsworth LD, Fackre P. Serum ferritin levels in the anemia of rheumatoid arthritis. J Rheumatol. 1977;4:389–92. [PubMed] [Google Scholar]
- 21.Baynes RD, Bothwell TH, Bezwoda WR, Gear AJ, Atkinson P. Hematologic and iron-related measurements in rheumatoid arthritis. Am J Clin Pathol. 1987;87:196–200. doi: 10.1093/ajcp/87.2.196. [DOI] [PubMed] [Google Scholar]
- 22.Blake DR, Waterworth RF, Bacon PA. Assessment of iron stores in inflammation by assay of serum ferritin concentrations. BMJ. 1981;283:1147–8. doi: 10.1136/bmj.283.6300.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davidson A, van der Weyden MB, Fong H, Breidahl MJ, Ryan PF. Red cell ferritin content: a re-evaluation of indices for iron deficiency in the anaemia of rheumatoid arthritis. BMJ. 1984;289:648–50. doi: 10.1136/bmj.289.6446.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mulherin D, Skelly M, Saunders A, et al. The diagnosis of iron deficiency in patients with rheumatoid arthritis and anemia: an algorithm using simple laboratory measures. J Rheumatol. 1996;23:237–40. [PubMed] [Google Scholar]
- 25.Baumann KS, Seifert B, Michel B, Ruegg R, Fehr J. Prediction of iron deficiency in chronic inflammatory rheumatic disease anemia. Br J Haematol. 1995;91:820–6. doi: 10.1111/j.1365-2141.1995.tb05395.x. [DOI] [PubMed] [Google Scholar]
- 26.Kurer SB, Seifert B, Michel B, Ruegg R, Fehr J. Prediction of iron in chronic inflammatory rheumatic disease anaemia. Br J Haematol. 1995;91:820–6. doi: 10.1111/j.1365-2141.1995.tb05395.x. [DOI] [PubMed] [Google Scholar]
- 27.Coenen JLLM, van Dieijen-Visser MP, van Pelt J, et al. Measurements of serum ferritin used to predict concentrations of bone marrow in anemia of chronic disease. Clin Chem. 1991;37:560–2. [PubMed] [Google Scholar]
- 28.Ali MAM, Luxton AW, Walker WHC. Serum ferritin concentration and bone marrow iron stores: a prospective study. Can Med Assoc J. 1978;118:945–6. [PMC free article] [PubMed] [Google Scholar]
- 29.Mazza J, Barr RM, McDonald JWD, Valberg VS. Usefulness of the serum ferritin concentration in the detection of iron deficiency in a general hospital. Can Med Assoc J. 1978;119:884–6. [PMC free article] [PubMed] [Google Scholar]
- 30.Psaty BM, Tierney WM, Martin DK, McDonald CJ. The value of serum iron studies as a test for iron-deficiency anemia in a county hospital. J Gen Intern Med. 1987;2:160–7. doi: 10.1007/BF02596144. [DOI] [PubMed] [Google Scholar]
- 31.Burns ER, Goldberg SN, Lawrence C, Venz B. Clinical utility of serum tests for iron deficiency in hospitalized patients. Am J Clin Pathol. 1990;93:240–5. doi: 10.1093/ajcp/93.2.240. [DOI] [PubMed] [Google Scholar]
- 32.Punnonen K, Irjala K, Rajamaki A. Serum transferrin receptor and its ratio to serum ferritin in the diagnosis of iron deficiency. Blood. 1997;89:1052–7. [PubMed] [Google Scholar]
- 33.Beutler E, Robson MJ, Buttenwieser E. A comparison of the plasma iron, iron-binding capacity, sternal marrow iron, and other methods in the clinical evaluation of iron stores. Ann Intern Med. 1958;48:60–82. doi: 10.7326/0003-4819-48-1-60. [DOI] [PubMed] [Google Scholar]
- 34.Galen PS, Gambino SR. Beyond Normality: The Predictive Value and Efficiency of Medical Diagnosis. New York, NY: Wiley; 1975. [Google Scholar]
- 35.Lipschitz DA. Iron deficiency anemia, the anemia of chronic disease, sideroblastic anemia and iron overload. In: Stein JH, editor. Internal Medicine. 4th ed. Chicago, Ill: Mosby-Year Book; 1994. pp. 835–45. In: ed. [Google Scholar]
- 36.Witte DL, Angstadt DS, Davis SH, Schrantz RD. Predicting bone marrow iron stores in anemic patients in a community hospital using ferritin and erythrocyte sedimentation rate. Am J Clin Pathol. 1988;90:85–7. doi: 10.1093/ajcp/90.1.85. [DOI] [PubMed] [Google Scholar]
- 37.Cash JM, Sears DA. The anemia of chronic disease: spectrum of associated diseases in a series of unselected hospitalized patients. Am J Med. 1989;87:638–44. doi: 10.1016/s0002-9343(89)80396-1. [DOI] [PubMed] [Google Scholar]