Abstract
The clinical utility of Legionella urinary antigen assays for the diagnosis of Legionnaires' disease was assessed by using samples from 317 culture-proven cases. The sensitivities of the Binax enzyme immunoassay (EIA) and Biotest EIA were found to be 93.7 and 94.4% for travel-associated infection and 86.5 and 76.0% for community-acquired infection but only 44.2 and 45.7% for nosocomially acquired infection, respectively.
Legionella pneumophila has been found worldwide to be a common pulmonary pathogen of severe community-acquired, travel-associated, or nosocomial pneumonia. Where pneumonia is sufficiently severe to require admission to an intensive care unit, L. pneumophila ranked first or second in seven of nine recent studies (17). Since Legionnaires' disease cannot be readily diagnosed by clinical manifestations alone, specialized laboratory tests are essential if the etiology is to be established. Of the various methods available, culture is the most specific and is usually accepted as the “gold standard.” However, in routine and clinical laboratory work, legionellosis is rarely proven by culture whereas detection of urinary antigen is now common (6, 14). All commercially available assays, except the Biotest enzyme immunoassay (EIA) (Dreieich, Germany), are marketed by their manufacturers as kits for the detection of L. pneumophila serogroup (sg) 1 urinary antigen, but some recent studies have shown that in practice the sensitivities of these assays ranged from 14 to 65% for non-sg 1 infection (1, 9, 10, 12). None of these studies, however, provide information on the clinical utility of urinary antigen detection for the diagnosis of community acquired, travel-associated, and nosocomial Legionnaires' disease with regard to the distribution of serotypes among these categories of infections.
For this study an infection was considered to be nosocomial if it fulfilled the criteria established by Joseph et al. (15). That is, the patient must have spent more than 9 days in the hospital before the onset of symptoms of legionellosis or have spent between 1 and 9 days in the hospital prior to the onset of symptoms and either (i) have become ill in a hospital where one or more patients with Legionnaires' disease were being treated or (ii) have yielded an isolate that cound not be distinguished from Legionella organisms in the hospital water supply by using monoclonal antibody (MAb) subgrouping or one genotypic method. Legionellosis was considered to be travel associated if it matched the definitions established by the European Surveillance Scheme for Travel-Associated Legionnaires' Disease (http://www.ewgli.org). That is, the person must have spent at least one night away from home during the 10 days prior to the onset of symptoms, either in the country of domicile or abroad. Cases for which both nosocomial and travel-associated infection could be excluded with confidence were considered to be community acquired. Data were available for 317 cases of culture-proven Legionnaires' disease from across Europe and were subgrouped into community-acquired (n = 148), nosocomial (n = 67), or travel-associated (n = 118) categories of infection.
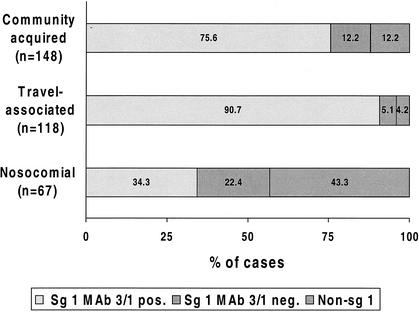
Patient isolates were serotyped into serogroups 1 to 15 by MAbs as described previously (8). Strains belonged to sg 1 (n = 281), sg 3 (n = 20), sg 4 (n = 5), sg 5 (n = 7), sg 6 (n = 9), sg 8 (n = 2), sg 10 (n = 3), sg 12 (n = 1), or sg 13 (n = 1) or were not typeable into the known sgs (n = 4). Monoclonal subgroups of sg 1 were subdivided into those expressing and those not expressing the virulence-associated epitope recognized by MAb 3/1 of the Dresden Panel (11), which corresponds to MAb 2 of the International Panel (13). This epitope is not present on Legionella bacteria belonging to any other serogroups or species. The distribution of MAb 3/1-positive and MAb 3/1-negative (divided into sg 1 or non-sg 1) strains in relation to the category of infection is shown in Fig. 1. The proportion of MAb 3/1-positive isolates was significantly (P < 0.0005) lower for nosocomial cases than for either community-acquired or travel-associated ones. The predominance of this serotype among clinical isolates has been known for more than 15 years (4, 5), but data on the distribution of this serotype by category of infection are few. Immunochemical analysis of the corresponding epitope suggested that it is the increased hydrophobicity (11, 18) which leads to the better survival of these Legionella strains in aerosols (3).
FIG. 1.
Distribution of MAb 3/1 (MAb 2)-positive and negative L. pneumophila serotypes depending on the category of infection.
Depending on the assays used in the participating laboratories, urine samples were tested by Binax Legionella Urinary EIA (Binax, Portland, Maine), by Biotest Legionella Urin Antigen EIA (Biotest, Dreieich, Germany), or by both assays. The results reported here were obtained by testing the first urine specimen available from each patient after onset of their pneumonia. Table 1 shows the results obtained by category of infection. In total, Legionella antigen was detectable in approximately 80% of all culture-proven Legionnaires' disease samples. Among these, the highest sensitivity was found for travel-associated cases (≥93.7%) whereas only about 45% of nosocomial infections were diagnosed by urinary antigen detection. The sensitivities for community-acquired cases amounted to 86.5% (Binax) and 76.0% (Biotest). The observed difference between these assays (P = 0.044) was not a true reflection of their respective sensitivities but rather due to the number of non-sg 1 infections examined in each assay: 8 by Binax EIA and 16 by Biotest. The sensitivity of each assay is significantly lower for non-sg 1 cases than for sg 1 cases, especially for Legionnaires' disease caused by MAb3/1-positive strains (10).
TABLE 1.
Diagnosis of community-acquired, travel-associated, and nosocomial Legionnaires' disease by urinary antigen detection methods using Binax Legionella Urinary and Biotest Legionella Urin Antigen EIAs
| Category of infection | Binax EIA
|
Biotest EIA
|
||
|---|---|---|---|---|
| n | No. positive (%) | n | No. positive (%) | |
| Community acquired | 89 | 77 (86.5) | 125 | 95 (76.0) |
| Travel associated | 79 | 74 (93.7) | 90 | 85 (94.4) |
| Nosocomial | 43 | 19 (44.2) | 46 | 21 (45.7) |
| Total | 211 | 170 (80.6) | 261 | 201 (77.0) |
Due to the high specificity of commercially available Legionella urinary antigen assays, Plouffe et al. (16) proposed in 1995 that the case definition for definitive Legionnaires' disease be expanded to include diagnosis by detection of urinary antigen. Over the last few years this diagnostic method has been used increasingly throughout the world and is without doubt now the primary diagnostic tool for Legionnaires' disease (7, 14). Nevertheless, it should be remembered that only 55.9% of all definitive cases in the reevaluation study conducted by Plouffe and colleagues were urinary antigen positive (16). This sensitivity is much lower than those published in studies evaluating commercially available urinary antigen assays (6), but it supports the assumption that Legionnaires' disease continues to be overlooked because of the nonavailability of diagnostic laboratory testing. Our multinational study reveals the very different clinical utility of urinary antigen detection in relation to the category of infection being investigated. This appears to be caused primarily by the different serotype distribution in these different categories. Since travel-associated and community-acquired cases are caused predominantly by MAb 3/1 (MAb 2)-positive strains (Fig. 1), Legionella urinary antigen detection is clearly the method of choice for their detection, yielding high positive predictive values that are not found with other diagnostic methods. In a previous retrospective study using Binax and Biotest EIAs, sensitivities for MAb 3/1-positive cases were shown to be ≥96% (10). On the other hand, irrespective of the assay used, only about 45% of nosocomial cases can be diagnosed by urinary antigen detection. Taking the low sensitivities of all other diagnostic methods into account, it is clear that nosocomial Legionnaires' disease might easily go unnoticed in hospitals (2). Therefore, in addition to the requirements for routine control of Legionella organisms in hospital water supplies, the clinical microbiologist should always apply the whole spectrum of laboratory methods for the diagnosis of Legionnaires' disease.
Acknowledgments
We thank Ines Wolf, Sigrid Gäbler, Nita Doshi, Ulla Karlsson, and Kerstin Jacobson for excellent technical assistance.
REFERENCES
- 1.Benson, R. F., P. W. Tang, and B. S. Fields. 2000. Evaluation of the Binax and Biotest urinary antigen kits for the detection of Legionnaires' disease due to multiple serogroups and species of Legionella. J. Clin. Microbiol. 38:2763-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Craven, D. E., and K. A. Steger. 1997. Hospital-acquired pneumonia: perspectives for the healthcare epidemiologist. Infect. Control Hosp. Epidemiol. 18:783-795. [DOI] [PubMed] [Google Scholar]
- 3.Dennis, P. J., and J. V. Lee. 1988. Differences in aerosol survival between pathogenic and non-pathogenic strains of Legionella pneumophila serogroup 1. J. Appl. Bacteriol. 65:135-141. [DOI] [PubMed] [Google Scholar]
- 4.Dournon, E. W., F. Bibb, P. Rjagopalan, N. Desplaces, and R. M. McKinney. 1988. Monoclonal antibody reactivity as a virulence marker for Legionella pneumophila serogroup 1 strains. J. Infect. Dis. 157:496-501. [DOI] [PubMed] [Google Scholar]
- 5.Ehret, W., B. U. von Specht, and G. Ruckdeschel. 1986. Discrimination between clinical and environmental strains of Legionella pneumophila by a monoclonal antibody. Isr. J. Med. Sci. 22:715-723. [PubMed] [Google Scholar]
- 6.Fields, B. S., R. F. Benson, and R. E. Besser. 2002. Legionella and Legionnaires' disease: 25 years of investigation. Clin. Microbiol. Rev. 15:506-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Formica, N., M. Yates, M. Beers, J. Carne, G. Hogg, N. Ryan, and G. Tallis. 2001. The impact of diagnosis by Legionella urinary antigen test on the epidemiology and outcomes of Legionnaires' disease. Epidemiol. Infect. 127:275-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helbig, J. H., J. B. Kurtz, M. Castellani-Pastoris, C. Pelaz, and P. C. Lück. 1997. Antigenic lipopolysaccharide components of Legionella pneumophila recognized by monoclonal antibodies: possibilities and limitations for division of the species into serogroups. J. Clin. Microbiol. 35:2841-2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helbig, J. H., S. A. Uldum, P. C. Lück, and T. G. Harrison. 2001. Detection of Legionella pneumophila antigen in urine samples using the BinaxNOW immunochromatographic assay and comparison with both Binax Legionella Urinary Enzyme Immunoassay (EIA) and Biotest Legionella Urin Antigen EIA. J. Med. Microbiol. 50:509-516. [DOI] [PubMed] [Google Scholar]
- 10.Helbig, J. H., S. A. Uldum, P. C. Lück, and T. G. Harrison. 2002. Detection of Legionella pneumophila antigen in urine samples: recognition of serogroups and monoclonal subgroups, p. 204-206. In R. Marre et al. (ed.), Legionella. ASM Press, Washington, D.C.
- 11.Helbig, J. H., P. C. Lück, Y. A. Knirel, W. Witzleb, and U. Zähringer. 1995. Molecular characterization of a virulence-associated epitope on the lipopolysaccharide of Legionella pneumophila serogroup 1. Epidemiol. Infect. 115:71-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horn, J. 2002. Comparison of non-serogroup 1 detection by Biotest and Binax Legionella urinary antigen enzyme immunoassays, p. 207-210. In R. Marre et al. (ed.), Legionella. ASM Press, Washington, D.C.
- 13.Joly, J. R., R. M. McKinney, O. J. Tobin, W. F. Bibb, I. D. Watkins, and D. Ramsay. 1986. Development of a standardized subgrouping scheme for Legionella pneumophila serogroup 1 using monoclonal antibodies. J. Clin. Microbiol. 23:768-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joseph, C. 2002. Surveillance of Legionnaires' disease in Europe, p. 311-317. In R. Marre et al. (ed.), Legionella. ASM Press, Washington, D.C.
- 15.Joseph, C. A., J. M. Watson, T. G. Harrison, and C. L. R. Bartlett. 1994. Nosocomial Legionnaires' disease in England and Wales, 1980-92. Epidemiol. Infect. 112:329-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plouffe, J. F., T. M. File, R. F. Breiman, B. A. Hackman, S. J. Salstrom, B. J. Marston, and B. S. Fields. 1995. Reevaluation of the definition of Legionnaires' disease: use of the urinary antigen assay. Clin. Infect. Dis. 20:1286-1291. [DOI] [PubMed] [Google Scholar]
- 17.Vergis, E. N., E. Abkas, and V. L. Yu. 2000. Legionella as a cause of severe pneumonia. Semin. Respir. Crit. Care Med. 21:295-304. [DOI] [PubMed] [Google Scholar]
- 18.Zähringer, U., Y. A. Knirel, B. Lindner, J. H. Helbig, A. Sonesson, R. Marre, and E. T. Rietschel. 1995. The lipopolysaccharide of Legionella pneumophila serogroup 1 (strain Philadelphia 1): chemical structure and biological significance. Prog. Clin. Biol. Res. 392:113-139. [PubMed] [Google Scholar]