Abstract
OBJECTIVE
To determine the risk factors for aminoglycoside toxicity in the elderly.
DESIGN
Prospective observational study.
SETTING
Acute care teaching hospital serving predominantly veterans.
PARTICIPANTS
Consecutive patients aged 70 years and over receiving aminoglycosides.
INTERVENTIONS
None.
MEASUREMENTS AND MAIN RESULTS
Thirteen (15%) of 88 patients developed aminoglycoside-related nephrotoxicity and 3 (3.4%) developed otovestibular toxicity. Multivariate analysis showed that increasing duration of aminoglycoside therapy was the only factor significantly associated with development of toxicity. Elevated baseline serum creatinine level (p = .02) and use of allopurinol (p = .03) were risk factors specifically for nephrotoxicity. Only 2 (3.9%) of 51 patients receiving aminoglycosides 7 or fewer days developed nephrotoxicity, as compared with 11 (30%) of 37 receiving the drugs for 8 to 14 days and 4 (50%) of 8 treated for more than 14 days.
CONCLUSIONS
Although toxicity is common in elderly patients treated with aminoglycosides, limiting the duration of aminoglycoside therapy to less than a week can substantially reduce risk of toxicity.
Keywords: aminoglycosides, gentamicin, nephrotoxicity, elderly
Aminoglycosides are traditionally administered every 8 hours. However, recent reviews have suggested that administration in larger and less frequent doses (for example, 4–7 mg/kg body weight once per day) offers several advantages, possibly including reduced toxicity.1
Advanced age has long been held to be an important risk factor in the development of aminoglycoside-related toxicity.2, 3 For this reason many physicians avoid use of aminoglycosides in the elderly. However, intolerance to antibiotics of other classes and the emergence of organisms that exhibit resistance to quinolones or β-lactam antibiotics (including the cephalosporins) has necessitated retention of aminoglycosides as part of the clinical armamentarium for this patient group. Furthermore, aminoglycosides remain useful because of their potential for synergy when used in combination with other classes of antibiotics. Examples of clinically demonstrated synergy include lowered mortality when aminoglycosides and β-lactam antibiotics are used in the treatment of significant sepsis due to Pseudomonas aeruginosaKlebsiella pneumoniae.4, 5 Finally, aminoglycosides are significantly cheaper than β-lactam antibiotics or quinolones, and may be preferred in some settings purely for this reason.
Given that aminoglycosides may still be a useful class of antibiotic for the elderly, the purpose of this study was to determine the risk factors for aminoglycoside toxicity in the patients aged 70 years or over. Furthermore, we aimed to develop strategies to reduce aminoglycoside-related toxicity in the elderly. All patients were administered aminoglycosides on an extended-interval (once daily) dosage schedule.
METHODS
All patients receiving an aminoglycoside from September 25 to November 30, 1996, at an acute care teaching facility that serves predominantly veterans were prospectively evaluated. Consecutive patients aged 70 years or over who were receiving aminoglycosides and were not in the intensive care or hemodialysis units were included in this study. Demographic details collected at baseline included age, gender, number of days hospitalized in the last year, previous aminoglycoside use, indication for aminoglycoside, and presence of other factors predisposing to renal disease (such as diabetes mellitus, hypertension, peripheral vascular disease, gout, or renal calculi). Serum creatinine level was measured at the commencement of the aminoglycoside course in all patients, and creatinine clearance was determined by use of the Cockcroft-Gault formula.6 Use of nephrotoxic drugs (for example, vancomycin, diuretics, angiotensin-converting enzyme inhibitors, nonsteroidal anti-inflammatory agents, allopurinol, and amphotericin) or intravenous radiologic contrast medium during the aminoglycoside course was recorded. Other factors that have been linked to nephrotoxicity such as hypotension (defined as systolic blood pressure less than 80 mm Hg) and sepsis occurring during the aminoglycoside course were also recorded. Fluid and hydration status was not formally assessed. The weight of patients was followed daily.
Serum creatinine level was measured every 2 days during the aminoglycoside course and for 7 days after its completion, for patients who remained as hospital inpatients after the course was completed. Nephrotoxicity was defined as a rise in serum creatinine above the baseline level of 0.5 mg/dL (0.045 mmol/L) or more.7 Ototoxicity or vestibular toxicity was evaluated only by means of history and physical examination in the vast majority of patients. Formal audiometric testing was performed only if there was clinical suspicion of hearing loss. Ototoxicity was defined as an increase in auditory threshold of 15 dB or more at any of two or more frequencies.8 Vestibular toxicity was defined as complaint of nausea or vertigo, which commenced after the aminoglycoside course had started and for which no other cause could be found.
Patients were given a starting dose of 4 mg/kg, administered over 30 minutes. Subsequent doses were recommended on the basis of individual pharmacokinetics. This was determined by collecting two serum samples after the first dose and then every other dose of aminoglycoside. Levels were collected by a small team of phlebotomists who were given extensive instruction regarding collection of serum relative to time of aminoglycoside infusion. The first serum sample was collected on completion of the infusion, and the second approximately 6 hours later. These levels were used to calculate the area under the serum aminoglycoside concentration-time curve. The recommended dose was obtained by multiplying the dose that had been given by a target 24-hour area under the curve (that would be expected in a patient with mean population values of volume of distribution and half-life of elimination), divided by the measured 24-hour area under the curve. These calculations were made using a previously described software program.9 Peak levels of at least 12 mg/L were also a target of this pharmacokinetic program. Once-daily dosing was the preferred frequency of administration, except if this was not pharmacokinetically indicated.
Patient demographics and laboratory data were entered into PROPHET Statistics (BBN Systems and Technologies, Cambridge, Mass.). The paired Student's t test was used for continuous variables, and χ2test for binomial data. Potential risk factors for nephrotoxicity were analyzed by a logistic regression model and correlation analysis. The level of statistical significance was regarded as p < .05.
RESULTS
This study included 88 patients aged 70 years or older who had received an aminoglycoside. These patients ranged from 70 to 91 years old (mean 77.9 years): 22 patients were 70–74 years, 36 were 75–79, 24 were 80–85 and 6 were more than 85 years old. Seventy-two (82%) of the patients were male. The patients had spent a mean of 22.6 days in hospital in the last 365 days. Thirty (34%) had previously received an aminoglycoside during a hospital admission in the last year. Indications for aminoglycosides during the current admission included infective exacerbations of chronic obstructive airways disease (31 patients), pneumonia (16 patients), urinary tract infection (8 patients), intra-abdominal infection (6 patients), and other infections (16 patients).
Seventy-three (83%) of the aminoglycoside courses were with gentamicin, and 15 (17%) were with tobramycin. No patient in this series received amikacin. The mean duration of the aminoglycoside course was 7.5 days (range 1–20 days). The percentage of patients receiving aminoglycosides according to the duration of therapy was as follows: 1 to 3 days, 20%; 4 to 5 days, 19%; 6 to 7 days, 18%; 8 to 10 days, 20%; 11 to 14 days, 13%; more than 14 days, 9%.
In all, 15 (17%) of 88 patients who received aminoglycosides developed aminoglycoside-related toxicity. Two patients (one of whom also developed nephrotoxicity) developed vestibular toxicity, and one developed ototoxicity, according to the audiometric definition previously mentioned. Thirteen (15%) of the 88 patients treated with aminoglycosides developed nephrotoxicity during the treatment course. The peak serum creatinine level during or within 1 week after the aminoglycoside course, in those who met the definition of nephrotoxicity, was 0.12 to 0.15 mmol/L (1.4 to 1.7 mg/dL) in three patients, 0.16 to 0.20 mmol/L (1.8 to 2.3 mg/dL) in three patients, 0.21 to 0.3 mmol/L (2.4 to 3.7 mg/dL) in four patients, and above 0.30 mmol/L (3.7 mg/dL) in three patients. One patient whose serum creatinine level rose from 0.23 mmol/L (2.6 mg/dL) at baseline to 0.61 mmol/L (6.9 mg/dL) at the conclusion of his aminoglycoside course died from the effects of uremia.
The study population had a high rate of concomitant factors that may have contributed to poor renal function. Mean baseline creatinine level was 0.125 mmol/L (1.4 mg/dL), and estimated creatinine clearance was 46 mL/min. Almost two thirds (65%) were on another drug known to be nephrotoxic during the aminoglycoside course. These included diuretics (33%), nonsteroidal anti-inflammatory agents (23%), angiotensin-converting enzyme inhibitors (22%), vancomycin (17%), allopurinol (14%), and amphotericin (2.3%). Almost one third (32%) were concomitantly on more than one other nephrotoxic drug. In addition, 21 (24%) of the 88 patients received intravenous radiographic contrast medium, and 12 (14%) suffered a severe hypotensive episode during the aminoglycoside course. Twenty-five (29%) had a history of peripheral vascular disease, 11 (13%) were diabetics, and 5 (5.7%) had a history of structural renal tract abnormalities.
Using univariate analysis, risk factors for nephrotoxicity during the aminoglycoside course included increasingly advanced age (p= .02) and prolonged duration of therapy (p= .004) (Table 1) Variables that approached statistical significance included concomitant allopurinol use (p= .07), significant hypotension during the aminoglycoside course (p= .07), and an elevated baseline creatinine level (p= .095). Factors that did not influence development of nephrotoxicity included type of aminoglycoside used (gentamicin or tobramycin); gender; number of days in hospital in the previous year; number of days of aminoglycoside use in the previous year; and concomitant therapy with vancomycin, nonsteroidal anti-inflammatory agents, diuretics, angiotensin-converting enzyme inhibitors, and intravenous radiographic contrast agents (Table 1).
Table 1.
Association of Variables with Development of Nephrotoxicity (Univariate Analysis)
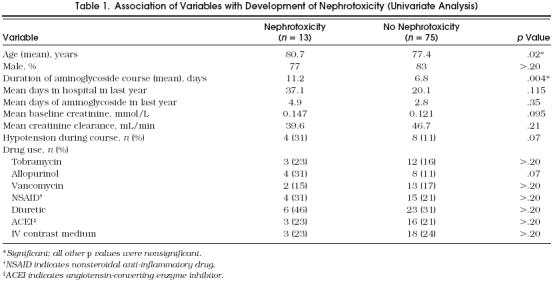
When multivariate analysis was performed, utilizing age, duration of therapy, concomitant use of allopurinol, baseline creatinine level, and hypotension during the aminoglycoside course in the model, duration of therapy (p= .004), baseline creatinine level (p= .02), and use of allopurinol (p= .03) were found to be significantly associated with nephrotoxicity. Increasing age was not significantly related to nephrotoxicity in the multivariate analysis (p= .06).
The relation between duration of aminoglycoside course and development of nephrotoxicity is given in Figure 1. No patient who received aminoglycosides for 3 or fewer days developed nephrotoxicity. Only 2 (3.9%) of 51 patients who received aminoglycosides 7 or fewer days developed nephrotoxicity. In contrast, 11 (30%) of 37 patients receiving aminoglycosides for more than 7 days developed nephrotoxicity. When aminoglycosides were given for more than 14 days, 4 (50%) of 8 patients developed nephrotoxicity.
Figure 1.
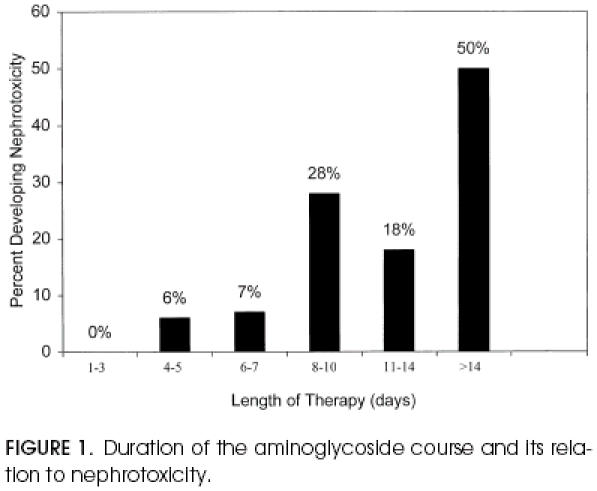
Duration of the aminoglycoside course and its relation to nephrotoxicity.
The relation between baseline serum creatinine level and development of nephrotoxicity is given in Figure 2. Only 8 (12%) of 69 patients with a baseline creatinine level less than 1.7 mg/dL (0.15 mmol/L) developed nephrotoxicity, as compared with 5 (26%) of 19 who had baseline creatinine level of more than 1.7 mg/dL. There was no correlation between baseline creatinine level of more than 1.7 mg/dL and prolonged duration of aminoglycoside therapy (p>.20).
Figure 2.
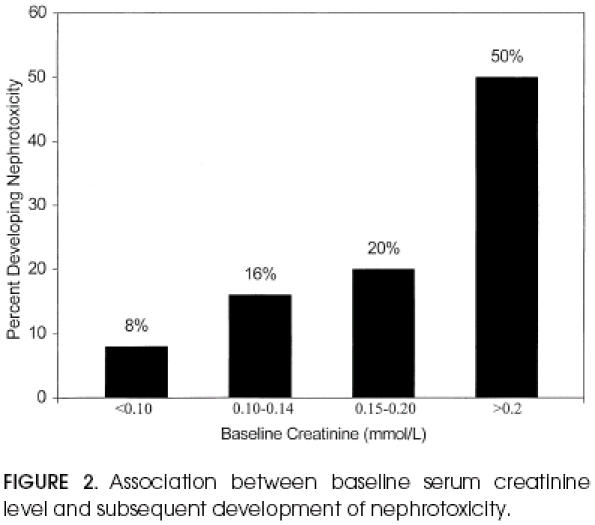
Association between baseline serum creatinine level and subsequent development of nephrotoxicity.
Only three patients had otovestibular toxicity, so it was impossible to assess specific risk factors for this adverse effect of aminoglycosides. When the risk of any toxicity was assessed (that is, either nephrotoxicity or otovestibular toxicity), using both univariate and multivariate analyses, duration of aminoglycoside therapy was the only factor associated with toxicity (p= .01).
DISCUSSION
Animal studies have suggested that the susceptibility to aminoglycoside-related nephrotoxicity in the elderly arises from the declining renal excretory function in this age group.10, 11 It is possible that this is due to the impaired capacity for cellular repair and regeneration in the elderly.12 Some authorities suggest avoiding aminoglycosides completely in all elderly people because of the propensity for toxicity. However, our aim was to determine whether there is a subgroup of this population that can be safely administered aminoglycosides.
We have found that the likelihood of toxicity attributable to aminoglycosides increases with increasing duration of administration of the drug. This was evident for both nephrotoxicity and all toxicities combined. Although only 3.9% of patients developed nephrotoxicity during a course of 7 days or fewer, 30% developed nephrotoxicity when a course of longer than a week was given.
Animal studies have shown that at least several days of aminoglycoside administration are needed before evidence of nephrotoxicity occurs.13, 14 In addition, studies on humans who received multiple daily doses of aminoglycosides have shown the relation between duration of therapy and development of toxicity in some,15 but not all studies.16 Studies that have analyzed a relation between duration of aminoglycoside therapy given once daily and risk of toxicity have been similarly divergent. Examination of the data of Nicolaou et al. shows that 3.0% of patients treated with aminoglycosides for more than 7 days developed nephrotoxicity as compared with 0.8% of those treated for 7 days or fewer (p < .05).17 Their study population was considerably younger than our own (median age, 46 years vs 77.9 years in this report). In contrast, other authors who have examined once-daily therapy have found no statistically significant association between duration of therapy and nephrotoxicity,7 or they found an association for gentamicin but not for tobramycin.18 In one of these studies, sample size was small and a trend was apparent (mean duration of therapy 9.7 days for those who developed nephrotoxicity and 7.9 days for those who did not), but this did not reach statistical significance (p= .14).7
An association between duration of aminoglycoside therapy and development of nephrotoxicity or ototoxicity appears biologically plausible. Aminoglycoside nephrotoxicity can be attributed to absorptive influx of the drug at proximal convoluted tubule cells. This may lead to an accumulation of myeloid bodies within lysosomes, thereby inhibiting lysosomal phospholiases with a subsequent decrease in sphingomyelinase activity.1 The uptake of aminoglycosides may be a saturable process 19; over the course of a number of days sufficient aminoglycoside becomes accumulated in proximal tubule cells to lead to decreased renal tubular function.
In addition to prolonged duration of administration, we found that baseline creatinine level and concomitant use of allopurinol were independent risk factors for development of nephrotoxicity related to aminoglycoside use. Poor baseline renal function has been found to be an independent risk of nephrotoxicity in patients receiving multiple daily dosing.16, 20, 21, 22 In our study, 26% of patients who had a baseline creatinine level of 1.7 mg/dL (0.15 mmol/L) or greater developed nephrotoxicity as compared with 12% of patients with a baseline creatinine level less than 1.7 mg/dL. In a recent study of aminoglycoside toxicity, also in an elderly population, no association was found between baseline serum creatinine level and nephrotoxicity.7 However, patients with baseline serum creatinine level greater than 0.176 mmol/L were excluded from entry into that study, and the mean baseline serum creatinine level of those assessed was 0.088 mmol/L (1.0 mg/dL). In contrast, we had no exclusion criteria based on serum creatinine level and had a greater diversity of baseline serum creatinine levels with a mean level of 0.125 mmol/L (1.4 mg/dL).
Concomitant use of a variety of nephrotoxic drugs (for example, vancomycin or nonsteroidal anti-inflammatory agents) with an aminoglycoside is associated with increased risk of nephrotoxicity.22–24 We found a trend toward greater nephrotoxicity in patients receiving concomitant nonsteroidal anti-inflammatory drugs or diuretics, but this did not reach statistical significance. Given that less than a third of patients received these medications and that our sample size was relatively small (n= 88), the power of our study was too small to detect whether these concomitant drugs really increase the nephrotoxicity of aminoglycosides. We were unable to confirm the previously described association between concomitant vancomycin and aminoglycoside use and development of renal impairment. The duration of concomitant therapy with both vancomycin and an aminoglycoside is probably an important determinant of nephrotoxicity of the antibiotic combination. Patients treated with vancomycin and an aminoglycoside for a prolonged duration (even 14 days or more), such as for sepsis or endocarditis due to methicillin-resistant Staphylococcus aureus(MRSA), appear more likely to develop nephrotoxicity than those with short periods of concomitant therapy. In one study, 22% of those given combination therapy developed nephrotoxicity; combination therapy had been given for a mean of 12.6 days.24 In our study, 2 (25%) of 8 patients receiving both antibiotics for more than 10 days developed nephrotoxicity. None of seven patients receiving dual antibiotic therapy for less than 10 days developed nephrotoxicity. Hence, duration of combination vancomycin-aminoglycoside therapy is probably the most important determinant of the likelihood of nephrotoxicity developing.
Concomitant allopurinol use has not been previously mentioned to be a risk factor for aminoglycoside-related nephrotoxicity. This may in part be because gout is more common in the elderly, and hence this study of elderly patients is more likely to reveal such a risk. Allopurinol is an inhibitor of xanthine oxidase,25 and can in some patients produce renal dysfunction. Our study suggests that in this elderly population aminoglycosides and allopurinol may have an additive effect in producing nephrotoxicity.
In summary, a number of risk factors for aminoglycoside-related toxicity in the elderly receiving once-daily dosing have been identified in this study. Aminoglycosides do not have to be avoided in the elderly. Rather, by limiting the duration of therapy to no more than 1 week, and by avoiding the drug in patients with baseline serum creatinine level greater than 1.7 mg/dL, toxicity can be limited. Aminoglycoside use in the elderly should be prospectively evaluated to validate these guidelines. Our linkage of toxicity associated with allopurinol use remains to be confirmed by other investigators.
References
- 1.Freeman CD, Nicolau DP, Belliveau PP, Nightingale CH. Once-daily dosing of aminoglycosides: review and recommendations for clinical practice. J Antimicrob Chemother. 1997;39:677–86. doi: 10.1093/jac/39.6.677. [DOI] [PubMed] [Google Scholar]
- 2.Cronin RE. Aminoglycoside nephrotoxicity: pathogenesis and prevention. Clin Nephrol. 1979;11:251–6. [PubMed] [Google Scholar]
- 3.Moellering RC. Factors influencing the clinical use of antimicrobial agents in elderly patients. Geriatrics. 1978;33:83–91. [PubMed] [Google Scholar]
- 4.Hilf M, Yu VL, Sharp J, Zuravleff JJ, Korvick JA, Muder RR. Antibiotic therapy for Pseudomonas aeruginosa bacteremia: outcome correlations in a prospective study of 200 patients. Am J Med. 1989;87:540–6. doi: 10.1016/s0002-9343(89)80611-4. [DOI] [PubMed] [Google Scholar]
- 5.Korvick JA, Bryan CS, Farber B, et al. Prospective observational study of Klebsiella bacteremia in 230 patients: outcome for antibiotic combinations versus monotherapy. Antimicrob Agents Chemother. 1992;36:2639–44. doi: 10.1128/aac.36.12.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 7.Koo J, Tight R, Rajkumar V, Hawa Z. Comparison of once-daily versus pharmacokinetic dosing of aminoglycosides in elderly patients. Am J Med. 1996;101:177–83. doi: 10.1016/s0002-9343(96)80074-x. [DOI] [PubMed] [Google Scholar]
- 8.Brummett RE, Fox KE. Aminoglycoside-induced hearing loss in humans. Antimicrob Agents Chemother. 1989;33:797–800. doi: 10.1128/aac.33.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turnidge J, Bell J, Mascaro L. Presented at the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. New Orleans, La: September 1996. The peak-AUC method for aminoglycoside dosage adjustment. Abstract A102. [Google Scholar]
- 10.Marre R, Tarara N, Louton T, Sack K. Age-dependent nephrotoxicity and the pharmacokinetics of gentamicin in rats. Eur J Pediatr. 1980;133:25–9. doi: 10.1007/BF00444750. [DOI] [PubMed] [Google Scholar]
- 11.McMartin DN, Engel SG. Effect of aging on gentamicin nephrotoxicity and pharmacokinetics in rats. Res Commun Chem Pathol Pharmacol. 1982;38:193–207. [PubMed] [Google Scholar]
- 12.Rowe JW. Clinical research on aging: strategies and directions. N Engl J Med. 1977;297:1332–6. doi: 10.1056/NEJM197712152972406. [DOI] [PubMed] [Google Scholar]
- 13.Cuppage FE, Setter K, Sullivan LP, Reitzes E, Melnykovych A. Gentamicin nephrotoxicity. Physiological, biochemical and morphological effects of prolonged administration to rats. Virchows Arch B Cell Pathol. 1977;234:121–38. [PubMed] [Google Scholar]
- 14.Houghton DC, English J, Bennett WM. Chronic tubulointerstitial nephritis and renal insufficiency associated with long-term “subtherapeutic” gentamicin. J Lab Clin Med. 1988;112:694–703. [PubMed] [Google Scholar]
- 15.Ter Braak EW, DeVries PJ, Bouter KP, Van Der Vegt SG, Dorrestein GC, Nortier JW. Once-daily dosing regimen for aminoglycosides plus beta-lactam combination therapy of serious bacterial infections: comparative trial with netilmicin plus ceftriaxone. Am J Med. 1990;89:58–66. doi: 10.1016/0002-9343(90)90099-y. [DOI] [PubMed] [Google Scholar]
- 16.Moore RD, Smith CR, Lipsky JJ, Mellits ED, Lietman PS. Risk factors for nephrotoxicity in patients treated with aminoglycosides. Ann Intern Med. 1984;100:352–7. doi: 10.7326/0003-4819-100-3-352. [DOI] [PubMed] [Google Scholar]
- 17.Nicolau DP, Freeman CD, Belliveau PP, Nightingale CH, Ross JW, Quintiliani R. Experience with a once-daily aminoglycoside program administered to 2184 adult patients. Antimicrob Agents Chemother. 1995;39:650–5. doi: 10.1128/AAC.39.3.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prins JM, Weverling GJ, De Blok K, Van Ketel R, Speelman P. Validation and nephrotoxicity of a simplified once-daily aminoglycoside dosing schedule and guidelines for monitoring therapy. Antimicrob Agents Chemother. 1996;40:2494–9. doi: 10.1128/aac.40.11.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giuliano RA, Verpooten GA, DeBroe ME. The effect of dosing strategy on kidney cortical accumulation of aminoglycosides in rats. Am J Kidney Dis. 1986;8:297–303. doi: 10.1016/s0272-6386(86)80101-9. [DOI] [PubMed] [Google Scholar]
- 20.Lane AZ, Wright GE, Blair DC. Ototoxicity and nephrotoxicity of amikacin: an overview of phase II and phase III experience in the United States. Am J Med. 1977;62:911–8. doi: 10.1016/0002-9343(77)90660-x. [DOI] [PubMed] [Google Scholar]
- 21.Schimpf SC, Gaya H, Klastersky J, Zinner SH. Three antibiotic regimens in the treatment of infection in febrile granulocytopenic patients with cancer. The EORTC International Antimicrobial Therapy Project Group. J Infect Dis. 1978;137:14–29. [PubMed] [Google Scholar]
- 22.Bertino JS, Booker LA, Franck PA, et al. Incidence of and significant risk factors for aminoglycoside-associated nephrotoxicity in patients dosed by using individualized pharmacokinetic monitoring. J Infect Dis. 1993;167:173–9. doi: 10.1093/infdis/167.1.173. [DOI] [PubMed] [Google Scholar]
- 23.Farber BF, Moellering RC. Retrospective study of the toxicity of preparations of vancomycin from 1974–1981. Antimicrob Agents Chemother. 1983;23:138–41. doi: 10.1128/aac.23.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rybak MJ, Albrecht LM, Boike SC, et al. Nephrotoxicity of vancomycin alone and with an aminoglycoside. J Antimicrob Chemother. 1990;25:679–87. doi: 10.1093/jac/25.4.679. [DOI] [PubMed] [Google Scholar]
- 25.Emmerson BT. The management of gout. N Engl J Med. 1996;334:445–51. doi: 10.1056/NEJM199602153340707. [DOI] [PubMed] [Google Scholar]


