Abstract
OBJECTIVE
To determine the rates of immediate survival and survival to discharge for adult patients undergoing in-hospital cardiopulmonary resuscitation, and to identify demographic and clinical variables associated with these outcomes.
MEASUREMENTS AND MAIN RESULTS
The MEDLARS database of the National Library of Medicine was searched. In addition, the authors' extensive personal files and the bibliography of each identified study were searched for further studies. Two sets of inclusion criteria were used, minimal (any study of adults undergoing in-hospital cardiopulmonary resuscitation) and strict (included only patients from general ward and intensive care units, and adequately defined cardiopulmonary arrest and resuscitation). Each study was independently reviewed and abstracted in a nonblinded fashion by two reviewers. The data abstracted were compared, and any discrepancies were resolved by consensus discussion. For the subset of studies meeting the strict criteria, the overall rate of immediate survival was 40.7% and the rate of survival to discharge was 13.4%. The following variables were associated with failure to survive to discharge: sepsis on the day prior to resuscitation (odds ratio [OR] 31.3; 95% confidence interval [CI] 1.9, 515), metastatic cancer (OR 3.9; 95% CI 1.2, 12.6), dementia (OR 3.1; 95% CI 1.1, 8.8), African-American race (OR 2.8; 95% CI 1.4, 5.6), serum creatinine level at a cutpoint of 1.5 mg/dL (OR 2.2; 95% CI 1.2, 3.8), cancer (OR 1.9; 95% CI 1.2, 3.0), coronary artery disease (OR 0.55; 95% CI 0.4, 0.8), and location of resuscitation in the intensive care unit (OR 0.51; 95% CI 0.4, 0.8).
CONCLUSIONS
When talking with patients, physicians can describe the overall likelihood of surviving discharge as 1 in 8 for patients who undergo cardiopulmonary resuscitation and 1 in 3 for patients who survive cardiopulmonary resuscitation.
Keywords: cardiopulmonary resuscitation, meta-analysis, resuscitation, prognosis
The do-not-resuscitate (DNR) order has become well accepted and widely used in American hospitals, and for the majority of patients who die in the hospital, a DNR order has been written by the time of their death.1–3 The decision to discuss or execute a DNR order is driven by several concerns: the patient's current quality of life, the likelihood that cardiopulmonary resuscitation (CPR) will be successful, the patient's long-term prognosis following successful resuscitation, and his or her anticipated quality of life following successful resuscitation.4, 5
Although judgments about quality of life are best assessed by the patient, physicians have typically been relied on to provide biomedical information and estimates of prognosis; this is consistent with a shared approach to medical decision making. Information about prognosis can either be communicated implicitly (e.g., “I don't think CPR is likely to help you”) or explicitly (“Patients with your condition have a less than 1% chance of surviving to discharge after CPR”). The explicit approach has been shown in two studies to influence patient decisions about DNR orders,6, 7 so it is important that prognostic information be as accurate as possible.
Recent work has shown, however, that physicians are not accurate in predicting the outcome of CPR. In fact, when presented with detailed vignettes of actual patient cases, physician predictions of the likelihood of immediate survival following CPR were no better than random guessing, with an area under the receiver-operating characteristic (ROC) curve not significantly different from 0.5.8 An analysis with the physician prediction of the likelihood of survival as the outcome variable in a multivariate regression shows that physicians appear to have an underlying cognitive model. However, this model overemphasizes the importance of age and omits other important factors such as serum creatinine level, cancer, pneumonia, dependent functional status, and sepsis (unpublished results). Thus, patient decisions about DNR orders may be inappropriate, with the result that some patients will not receive CPR who might benefit from it and others will undergo resuscitation and its associated burden with little chance of benefit.
Pre-arrest variables, which can be measured prior to the onset of cardiopulmonary arrest, appear most useful to patients and physicians in their discussions of DNR orders. Peri-arrest variables such as the initial rhythm, duration of resuscitation, response time, and medications used have significant predictive value but are of little use when discussing DNR orders with a competent patient prior to cardiac arrest. For example, asystole as an initial rhythm and poor response time are both highly predictive of a poor outcome, but there is no way to directly measure these clinical parameters prior to the cardiopulmonary arrest.
Many studies have examined the relation between pre-arrest variables and survival following in-hospital CPR.9–57 Sample size calculations (assuming an overall rate of immediate survival of 42%, one-sided α = 0.05, and β = 0.20)57 show that if an important predictor variable is present in 20% of patients who undergo CPR, 929 patients would have to be included in a study in order to detect an absolute difference of 10% in the rate of immediate survival. Similar calculations assuming an overall rate of survival to discharge of 16% show that 487 patients are needed to detect an absolute difference of 10% for this outcome. However, only six studies were identified in a review of the literature that reported results for more than 500 patients, and only one included more than 1,000 patients.10, 36, 41, 43, 47, 56
Therefore, the majority of studies have had inadequate sample size to identify important predictors of the outcome of in-hospital CPR. Although smaller studies have shown relations between pre-arrest variables and the outcome of CPR, those associations have been inconsistent. For example, several studies have shown that pneumonia is a strong predictor of poor outcome,12, 37 others have not.32, 55
Two previous meta-analyses examined the relation between pre-arrest variables and survival after in-hospital CPR.58, 59 On the one hand, meta-analyses have the advantage of greater power to detect variables associated with the outcome of CPR, and may offer greater generalizability because patients studied are drawn from several institutions. On the other hand, publication bias (studies not submitted or published if they find no association), reporting bias (data for specific variables not reported if no association is found), and interstudy variability are known limitations of this analytic technique.60 These two meta-analyses found that the following variables are associated with a decreased likelihood of survival to discharge: age,58, 59 cancer,58, 59 cerebrovascular accident,59 congestive heart failure,59 homebound status,58, 59 hypotension,59 metastatic cancer,58 pneumonia,58, 59 sepsis,58, 59 and serum creatinine level above 130 μmol/L (1.5 mg/dL).58, 59 In addition, acute myocardial infarction on admission and a history of coronary artery disease were both associated with an increased likelihood of survival to discharge.58, 59
These two previous meta-analyses had several limitations. First, neither addressed the issue of immediate survival following in-hospital CPR. Also, neither considered the issue of study quality or comparability of definitions when combining estimates. In addition, neither used a random effects model, which takes into account interstudy variability.60 Finally, a number of studies published since 1990 were not included in either analysis.
The goal of this study is a meta-analysis of studies since 1980 that utilize pre-arrest variables to predict the outcome of in-hospital CPR. Two outcomes will be considered: immediate survival and survival to discharge (Fig. 1). Postdischarge survival was not studied because data are only sporadically available, and for varying lengths of time after discharge, precluding meta-analysis. Studies prior to 1980 were excluded because of changes in the technique of CPR, and more importantly because of a concern that the characteristics of resuscitated patients have changed owing to advances in technology, changes in reimbursement, and the widespread application of DNR orders.
Figure 1.
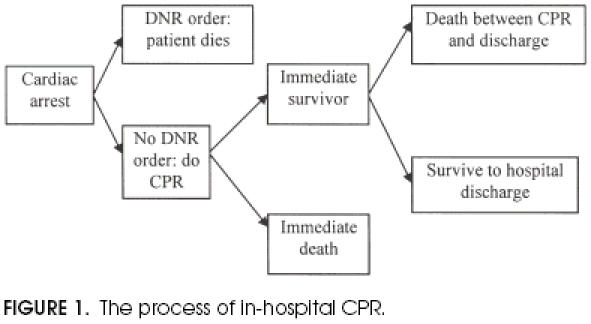
The process of in-hospital CPR.
METHODS
Definitions
Cardiopulmonary arrest is defined as the sudden cessation of spontaneous respiration and circulation leading to loss of consciousness and necessitating CPR, not including syncope, seizures, or respiratory arrest without pulselessness or loss of consciousness that responds to artificial ventilation alone. At a minimum, CPR is defined as the use of chest compressions and rescue breathing.
Survival-to-discharge patients includes those transferred to a rehabilitation facility or extended care facility, and those requiring home nursing services. Adult is defined as having reached the age of 18 years. The patient, rather than arrest, is the unit of analysis in included studies. Otherwise, a patient arresting three times and surviving to discharge would “contribute” three survivals to the analysis. It is not possible to adjust for this problem with aggregate data.
Identification of Studies
Eligible studies were identified from a search of the National Library of Medicine's MEDLARS database using the Medical Subject Heading terms “cardiopulmonary resuscitation/all subheadings” and limited to “abstract online,”“year of publication ≥ 1980,”“human,” and “English language.” A total of 750 studies were identified in this manner. Other studies were identified from a search of each study's list of references, and from one of the author's extensive file of articles on CPR. Studies that did not report at least one of the outcomes of interest (immediate survival or survival to discharge of in-hospital CPR) stratified by at least one pre-arrest variable (e.g., separate estimates of survival to discharge for patients with and without metastatic cancer) were excluded, leaving 49 studies for closer review. No attempt was made to search for unpublished studies; we felt that publication bias was unlikely to be an issue because a “negative” study result is not typically possible in a descriptive study.
Inclusion Criteria
Some authors advocate a detailed analysis of the quality of each study in a meta-analysis, with a score used to assess adherence to ideal research standards.61 Others have questioned this approach because of the inherent subjectivity of weighted scores and variation in how completely study design is reported in published manuscripts.62 In addition, such standards are most appropriate for randomized controlled trials and other studies of intervention. This is a meta-analysis of nonrandomized studies, for which key standards such as blinding, allocation to treatment groups, withdrawals, therapeutic regimen, study administration, and method of randomization are not meaningful.
We have chosen to focus on three key aspects of study design: the population studied, the clarity of definitions, and the setting. Two sets of inclusion criteria were used, minimal and strict. The minimal inclusion criteria were studies of adult patients undergoing in-hospital CPR that measured either immediate survival or survival to discharge. Strict inclusion criteria were the minimal inclusion criteria as well as inclusion only of patients from both the general ward and intensive care unit (ICU), and provision of adequate definitions of cardiopulmonary arrest and CPR. (An adequate definition was felt by consensus of the reviewers to be consistent with the definitions of cardiopulmonary arrest and CPR described above.)
Two sets of criteria were used to better understand the association between predictor variables and key outcomes. The looser minimal criteria identify a much larger group of less homogeneous studies, but also have the benefit of a larger number of both patients and variables studied. The stricter criteria generate a set of studies that are more likely to be homogenous and are therefore more appropriate for meta-analysis, but in which the likelihood of type II error due to inadequate sample size is greater.
Data Abstraction
Each of the 49 articles initially identified was reviewed separately by two experienced family physician researchers; one researcher reviewed all of the articles, and the remaining three researchers each reviewed 16 or 17 articles. Each physician first described the demographic characteristics of each study and whether it met both the minimal and strict inclusion criteria. Then each researcher abstracted data for all variables reporting at least one of the outcomes of interest (survival to discharge or immediate survival) for patients with and without the characteristic (e.g., the rate of survival to discharge for patients with and without metastatic cancer). Data were recorded in a Microsoft Excel spreadsheet (Microsoft Corporation, Redmond, Wash.).
The lead researcher compared the spreadsheets containing the results of the initial data abstraction. Differences were noted and reconciled by discussion among researchers. Studies not meeting minimal inclusion criteria as described above were removed from the spreadsheet and excluded from further analysis. Studies were excluded for the following reasons: included patients with a pulse,10 included children,34, 46 included out-of-hospital arrests,36, 46 or duplicated data from another analysis by the same authors.48, 56 One study was excluded because only a single patient met the inclusion criteria.49 Four of the studies included in the analysis either children 31, 41 or out-of-hospital arrests,41, 42 but presented their data in such a way that these nonqualifying cases could be excluded from the analysis. A total of 41 studies met the minimal inclusion criteria, while only 10 studies met the strict inclusion criteria.19, 24, 26, 27, 32, 43, 45, 47, 53, 55
Analysis
The rates of immediate survival and survival to discharge were graphed against the sample size (funnel plots) to determine if there was any obvious publication bias. The rates of immediate survival and survival to discharge were also graphed by the year of publication to identify any longitudinal trends.
For dichotomous variables, each variable for each study was abstracted to a contingency table; rows represented patients with and without the variable of interest (e.g., with and without cancer), and columns represented patients who survived and did not survive (e.g., survivors to discharge and patients who did not survive to discharge). The DerSimonian and Laird summary odds ratios (ORs) were calculated based on a random effects model using the software package Meta-Analyst (J. Lau, MD, New England Medical Center, 1996). Odds ratios may overestimate the relative risk when the outcome of interest (mortality in this case) is common. When the number of events in a cell was 0, a value of 0.5 was added to the event cell for both treatment and control groups. The Q statistic was used to describe the heterogeneity of studies. A value of Q>(S− 1), where S is the number of studies combined, suggests substantial heterogeneity. When Q≤ (S− 1), the fixed and random effects models should give similar results.63
For continuous variables, summary effect sizes, standard errors, and 95% confidence intervals (CIs) were calculated using software written by one of the authors using Visual Basic 4.0 for Windows (Microsoft Corporation). The effect size is a standardized estimate, calculated by taking the difference between the mean for patients having the characteristic and the mean for patients not having the characteristic, and dividing that by the standard deviation. For example, if the mean age of nonsurvivors was 70 and that of survivors was 60, with SD = 20, the effect size would be (70 − 60)/20 = 0.5. If the SD was equal to the mean difference,10 the effect size would be one, whereas if the mean difference is twice the SD, the effect size is 2. The summary effect size is calculated using the method described by Hasselblad and McCrory.60
If available in the original literature, dichotomous outcomes are also presented for some continuous variables (i.e., age, hematocrit, and serum creatinine level) because these results may be more easily interpreted by clinicians compared with other dichotomous variables. If more than one dichotomous cutpoint is used in the original literature for a variable, both results are presented (i.e., serum creatinine level of 1.5 and 2.5).
RESULTS
Characteristics of Studies
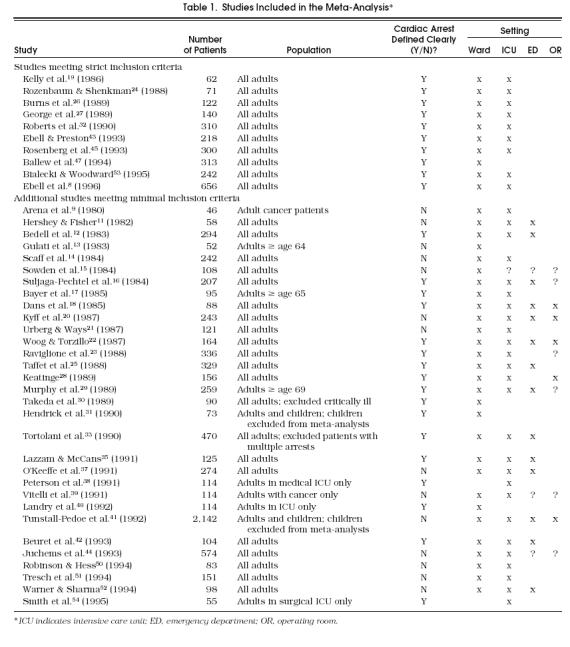
Table 1 presents a summary of the characteristics of studies included in either the minimal or strict analysis. The 41 studies meeting the minimal inclusion criteria included 9,838 patients. The subset of 10 studies meeting the strict inclusion criteria included 2,434 patients. Among the 41 studies that met the minimal criteria, 12 took place in Europe,13, 15, 17, 19, 28, 31, 38, 40123–44, 48 one each in Australia,22 Israel,24 and Japan,30 and the remaining 26 in the United States or Canada.8, 9, 11, 12, 14, 16, 18, 20, 21, 23, 25–27, 29, 32, 33, 35, 38, 39, 43, 45, 50–54
Table 1.
Studies Included in the Meta-Analysis*
For the studies meeting the minimal inclusion criteria, the overall rate of immediate survival was 43.1% (95% CI 42.5%, 43.6%) and the rate of survival to discharge was 14.6% (95% CI 14.3%, 15.0%). For the subset of studies meeting the strict criteria, the overall rate of immediate survival was 40.7% and the rate of survival to discharge was 13.4%. Only five studies reported the setting to which patients were discharged;22, 29, 39, 40, 43 of 93 patients in those studies, 73 (78.5%; 95% CI 74.2%, 82.8%) went home, 18 (19.4%; 95% CI 15.2%, 23.5%) to a nursing home, and 2 (2.1%; 95% CI 0.6%, 3.6%) to “other” settings.
Funnel plots were drawn for both immediate survival and survival to discharge for studies meeting minimal criteria. The plot for immediate survival is shown in Figure 2. The wide portion of the funnel appears to have a paucity of findings in the left side of the distribution at the base (i.e., smaller studies reporting lower rates of survival to discharge). Thus, there may be a publication bias against studies showing a low rate of immediate survival. The funnel plot for survival to discharge in Figure 3 shows a more uniform distribution without a central peak. There were not enough studies meeting strict criteria to draw meaningful funnel plots.
Figure 2.
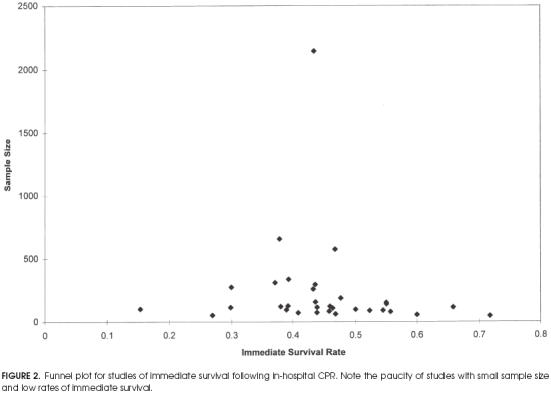
Funnel plot for studies of immediate survival following in-hospital CPR. Note the paucity of studies with small sample size and low rates of immediate survival.
Figure 3.
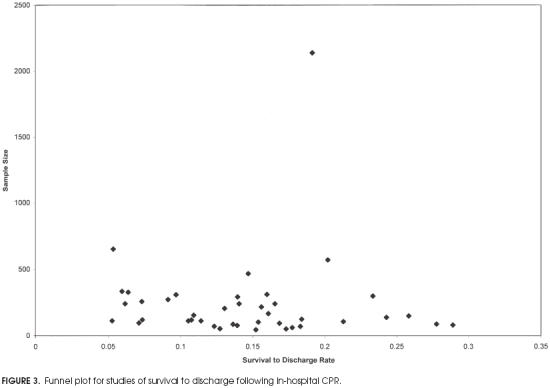
Funnel plot for studies of survival to discharge following in-hospital CPR.
Plots of survival rates by year of publication for immediate survival and survival to discharge are shown in Figures 4 and 5 respectively. They show little change in the mean rate of survival over the past 15 years, although the distribution of reported rates of survival to discharge appears to have broadened since approximately 1988.
Figure 4.
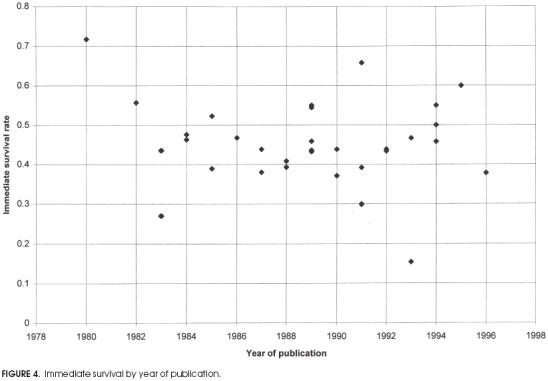
Immediate survival by year of publication.
Figure 5.
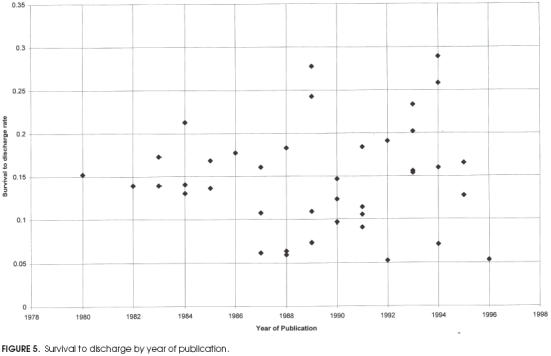
Survival to discharge by year of publication.
Predictors of Survival
Relatively few studies reported on continuous variables associated with survival. Among studies meeting the minimal inclusion criteria, only age was reported by more than two, and for studies meeting the strict inclusion criteria, only age was reported by more than a single study.
To identify outliers, for each predictor variable we examined the distribution of the ORs from the studies that reported information about the predictor variable. For one such variable (the presence of coronary artery disease), the OR of 9.57 from one study 27 was much higher than the ORs in the remaining studies, whether we looked at the five other studies that examined this variable and met strict criteria (range 0.41–0.67; summary OR including the outlier 0.86; 95% CI 0.38, 1.92) or the four other studies that met minimal criteria (range 0 – 0.96). Therefore, we did not include information about this variable from this study in the results.
Immediate Survival
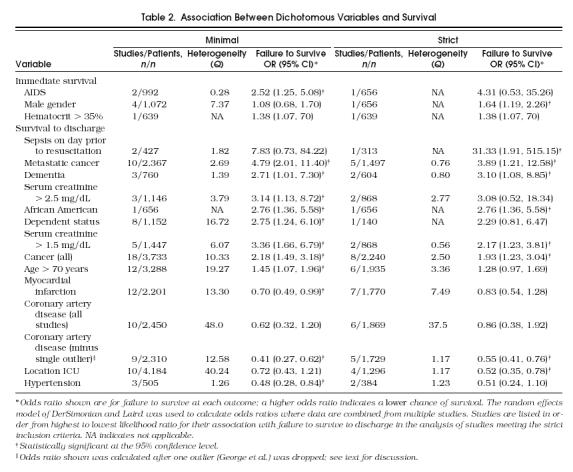
Results for dichotomous variables that were significantly associated with immediate survival are summarized in Table 2. Only three variables were significantly associated with a decreased rate of immediate survival: a diagnosis of AIDS among studies meeting the minimal inclusion criteria, a hematocrit greater than 35%, and male gender among the subset meeting the strict inclusion criteria. The following dichotomous variables were not significantly associated with immediate survival in either group of studies: serum creatinine level, blood urea nitrogen, age over 70 years, presence of a third heart sound, nursing home residence prior to admission, sepsis on admission, location of the resuscitation (ICU vs ward), and diagnoses of congestive heart failure, cerebrovascular accident, cancer, metastatic cancer, pneumonia, coronary artery disease, or myocardial infarction.
Table 2.
Association Between Dichotomous Variables and Survival
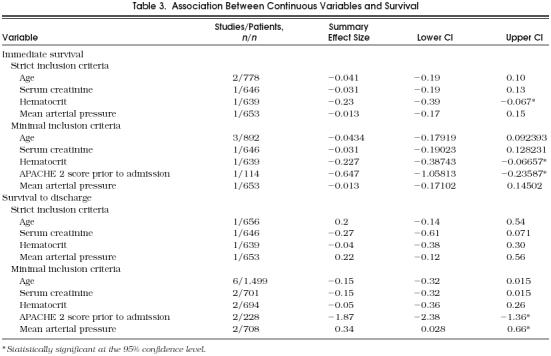
Results for continuous variables are summarized in Table 3. Among studies meeting minimal inclusion criteria, an increased hematocrit was associated with a decreased rate of immediate survival. For the subset of studies meeting the strict inclusion criteria, both an increased hematocrit and a higher APACHE 2 score were associated with a decreased rate of immediate survival.
Table 3.
Association Between Continuous Variables and Survival
Survival to Discharge
Results for dichotomous variables significantly associated with survival to discharge are summarized in Table 2. Among studies meeting strict inclusion criteria, the following variables were associated with decreased survival to discharge: sepsis on the day before resuscitation, cancer, metastatic cancer, dementia, serum creatinine level (at cutpoints of both 1.5 mg/dL and 2.5 mg/dL), African-American race, and dependent status. Patients with coronary artery disease and location of resuscitation in the ICU were more likely to survive to discharge. Among studies meeting the minimal criteria, a similar but not identical group of variables was associated with decreased survival to discharge: cancer, metastatic cancer, dementia, serum creatinine level (at cutpoints of both 1.5 mg/dL and 2.5 mg/dL), African-American race, dependent status, age over 70 years. Patients with myocardial infarction, coronary artery disease, and hypertension had improved prognoses.
Among studies meeting strict inclusion criteria, five of six found age over 70 to be associated with failure to survive to discharge, although this relation was not statistically significant (1.28; 95% CI 0.97, 1.69). Dropping the single outlier 55 resulted in a summary OR of 1.41 (95% CI 1.03, 1.91) for failure to survive to discharge. Although this was a statistically significant association, age over 70 is still not a strong predictor of failure to survive to discharge.
Results for continuous variables are summarized in Table 3. For studies meeting strict inclusion critieria, no variables were associated with survival to discharge. Among studies meeting minimal inclusion criteria, both an increased APACHE 2 score and a decreased mean arterial pressure were associated with a decreased rate of immediate survival.
The following variables were not associated with survival to discharge in either group of studies: blood urea nitrogen level, systolic blood pressure, gender, hematocrit above 35%, nursing home residence prior to admission, and diagnoses of AIDS, congestive heart failure, pneumonia, sepsis on admission, cerebrovascular accident, and diabetes mellitus.
DISCUSSION
This meta-analysis identified patient characteristics associated with both immediate survival and survival to discharge. Because of the greater homogeneity of studies meeting strict criteria, we will focus on the results for studies meeting the strict inclusion criteria.
Overall Rates of Survival
The overall rate of immediate survival for the subset of studies meeting the strict criteria was 40.7%, and the rate of survival to discharge for the same group of studies was 13.4%. When talking to patients, physicians can therefore describe the overall likelihood of immediate survival as “4 out of 10” or “2 out of 5,” and the likelihood of survival to discharge as “1 in 3 for those patients who are revived” or “1 in 8 among all patients who undergo CPR in the hospital.”
Despite major changes in the use of DNR orders, intensive care, and hospital services in general, the rates of immediate survival and survival to discharge have remained relatively constant over the past 15 years (Figs. 4 and 5). One would expect that increasing use of DNR orders would result in a more appropriate use of CPR, and higher survival rates. This assumes that physicians can accurately identify patients who are more likely to benefit from CPR, and that decisions about CPR are based on prognosis alone. Neither may be true; a recent study by one of the authors showed that physicians were unable to predict the outcome of in-hospital CPR when given a series of clinical vignettes for which the outcomes were known to the investigators.8 Also, decisions about CPR are clearly driven by concerns about quality of life that may not be related to prognosis.
Predictors of Immediate Survival
None of the variables associated with survival to discharge, such as metastatic cancer, impaired renal function, and dementia, was associated with immediate survival following in-hospital CPR. Only male gender, a diagnosis of AIDS, an increased hematocrit, and an increased APACHE 2 score (a measure of acute physiologic derangement) were associated with a decreased likelihood of immediate survival. It is unclear why an increased hematocrit or male gender should be associated with a poor outcome. These findings may be an example of a type I error in the single study in which a significant association was reported,55 they may represent a reporting bias (failure of authors to report nonsignificant results), or it may be that gender and increased hematocrit represent intervening variables for tobacco use. Further study is needed to identify whether there is a relation between tobacco use and immediate survival following CPR, and to confirm or refute the association between gender, hematocrit, and immediate survival. Until that time, these findings should be considered preliminary.
Results for immediate survival were reported by only a handful of studies. The ability to predict this outcome distinct from survival to discharge may be valuable to patients who want to have a brief interval to settle affairs or bid farewell to their loved ones. Much more work is needed to identify other predictors of this outcome, and to determine whether an increased hematocrit and male gender are actually associated with a poor outcome.
Predictors of Survival to Discharge
The variable most strongly associated with failure to survive to discharge was sepsis on the day before resuscitation. However, this was only reported by one study meeting the strict criteria, and by two meeting the minimal criteria. Interestingly, sepsis on the day of admission was not a significant predictor of survival. This finding is clinically reasonable because many patients who are not septic on admission develop sepsis later during their hospital stay, as their illness worsens. Clinical prediction rules that include sepsis should therefore recalculate the patient's risk each day, in order to take into account changes in the patient's clinical status.
Cancer, metastatic cancer, dementia, dependent status, location of resuscitation on the general ward (rather than ICU), elevated serum creatinine level, and no history of coronary artery disease were all associated with failure to survive to discharge after in-hospital CPR. The association with coronary artery disease (these patients are more likely to survive to discharge) may be confounded by the fact that these patients are more likely to be placed in the ICU or other monitored unit. Although the association for dependent status among studies meeting strict criteria was not statistically significant, only one such study (with 140 patients) reported data for this variable.21 Patients having these characteristics are less likely to benefit from CPR, and this information should be communicated in a sensitive manner during DNR discussions.
A single study, by one of the authors of this meta-analysis, found an association between African-American race and survival to discharge. This study included over 300 African-American patients, the largest number studied, and was therefore the first study with adequate power to detect such an association. The association persisted after adjustment for age, comorbidities, renal function, severity of illness as measured by the APACHE 3 score, and other clinical variables.55 More study is needed to confirm or refute this disturbing finding, and better understand the reasons for it.
Age is at best a weak predictor of the outcome of in-hospital CPR. This meta-analysis found that age as a continuous variable was not associated with immediate survival. Although age over 70 years as a dichotomous variable was associated with failure to survive to discharge among studies meeting the minimal inclusion criteria, this association was not statistically significant among the subset of studies meeting the strict inclusion criteria. This observation most likely reflects the physiologic and functional heterogeneity among the elderly, and may be confounded by comorbidity. It is therefore important that physicians look beyond a patient's biological age, and consider the presence or absence of variables more strongly associated with the outcome of CPR when discussing DNR orders with patients.
Pneumonia has been variably associated with survival to discharge. For example, Bedell et al. initially found a strong relation between the diagnosis and failure to survive to discharge after CPR,12 although others have not always duplicated this finding.15, 21, 40, 55 In the current meta-analysis, pneumonia was not significantly associated with either immediate survival or survival to discharge. Certainly, differences in the criteria for diagnosis may affect these results. This problem may apply to other clinical diagnoses such as congestive heart failure and sepsis, which have also been variably associated with the outcome of CPR.
A number of variables were significantly associated with survival to discharge in the studies meeting the minimal inclusion criteria, but not in the subset of studies meeting the strict inclusion criteria. In some cases, this may simply be due to type II error (insufficient sample size). For example, only one study each meeting the strict inclusion criteria reported data for systolic blood pressure below 100 mm Hg, third heart sound, or dependent status, and only two studies each for hypertension and dementia. Therefore, future studies should continue to look for a possible association between these variables and survival to discharge after in-hospital CPR.
Limitations of this Study
This meta-analysis has several limitations related to the design and quality of the original studies. First, some of the variables used to predict the outcome of CPR were not clearly defined in many of the studies. Examples include coronary artery disease and cancer. For the former, the vast majority of studies defined coronary artery disease by history as recorded in the medical record. Regarding the diagnosis of cancer, most studies used the medical record, and excluded basal cell carcinomas and patients with distant (less than 5–10 years) diagnoses that are now considered cured. Second, every study of in-hospital CPR included only patients who actually underwent CPR. Patients who had a DNR order written before their first episode of CPR were not included, which might bias the results. However, because these patients did not undergo CPR, we do not know whether they would have survived the intervention, and have no way of including them in calculations of ORs for survival.
Third, although some authors reported all data, others reported only the results for pre-arrest variables significantly associated with survival. This creates a kind of publication bias. Fourth, the nature of the data necessitated the use of ORs rather than relative risk calculations, which may overestimate the OR when the outcome of interest is common. This is especially a concern among studies of immediate survival, in which the frequency of mortality was over 40%. Finally, because some of the studies may have had adjusted OR estimates for potential confounders while others did not, we did not necessarily combine fully adjusted ORs. For example, patients with coronary artery disease are more likely to be admitted to a monitored unit, which may explain part of the “benefit” of this diagnosis. It is therefore important that readers not overinterpret the magnitude of the ORs presented in this meta-analysis.
Guidelines for Future Research on Survival After In-Hospital CPR
During the literature review, we identified many of the limitations of the existing body of literature. These limitations include inadequate sample size, missing or inadequate definitions, variable inclusion and exclusion criteria, differing settings, and a variety of clinical end points. We therefore propose that the sample size of future studies should be at least 500, which is adequate to detect an absolute difference of 10% in survival to discharge (α = 0.05, β = 0.20). Also, researchers should clearly define cardiopulmonary arrest and CPR, and follow the Utstein style recommendations for research on in-hospital resuscitation.64
Meta-analysis would be facilitated by more complete reporting of the results for all predictor variables, and by separate reporting of results for the general ward, ICU, and other locations. Also important is the use of a standard set of clinical end points. The Utstein Style Task Force recommends reporting the duration of return of spontaneous circulation for patients who died in hospital (<20 minutes, 20 minutes to 24 hours, and>24 hours), the rates of survival to discharge, survival to 6 months, and survival at 1 year for patients who survive to discharge.64 Our guidelines for designers of future studies are summarized in Table 4.
Table 4.
Guidelines for Future Studies of Survival After In-Hospital Cardiopulmonary Resuscitation

Acknowledgments
The authors thank Joseph Lau, MD, for the use of his Meta-Analyst software package.
References
- 1.Murphy DJ. Do-not-resuscitate orders: time for reappraisal in long-term care institutions. JAMA. 1988;260:2098–101. doi: 10.1001/jama.260.14.2098. [DOI] [PubMed] [Google Scholar]
- 2.Ruark JE, Raffin TA Stanford University Medical Center Committee on Ethics. Initiating and withdrawing life support: principles and practice in adult medicine. N Engl J Med. 1988;318:25–30. doi: 10.1056/NEJM198801073180106. [DOI] [PubMed] [Google Scholar]
- 3.Jonsson PV, McNamee M, Campion EW. The ‘do-not-resuscitate’ order. Arch Intern Med. 1988;148:2373–5. [PubMed] [Google Scholar]
- 4.Tomlinson T, Brody H. Ethics and communications in do-not-resuscitate orders. N Engl J Med. 1988;318:43–6. doi: 10.1056/NEJM198801073180109. [DOI] [PubMed] [Google Scholar]
- 5.Ebell MH. Practical guidelines for do-not-resuscitate orders. Am Fam Physician. 1994;50:1293–9. [PubMed] [Google Scholar]
- 6.Murphy DJ, Burrows D, Santilli S, et al. The influence of the probability of survival on patients' preferences regarding cardiopulmonary resuscitation. N Engl J Med. 1994;330:545–9. doi: 10.1056/NEJM199402243300807. [DOI] [PubMed] [Google Scholar]
- 7.Schonwetter RS, Walker RM, Kramer DR, Robinson BE. Resuscitation decision making in the elderly: the value of outcome data. J Gen Intern Med. 1993;8:295–300. doi: 10.1007/BF02600139. [DOI] [PubMed] [Google Scholar]
- 8.Ebell MH, Bergus GR, Warbasse LA, Bloomer R. Failure of physicians to predict the outcome of in-hospital cardiopulmonary resuscitation. J Gen Intern Med. 1996;11:16–22. doi: 10.1007/BF02603480. [DOI] [PubMed] [Google Scholar]
- 9.Arena FP, Perlin M, Turner AD. Initial experience with a “code–no code” resuscitation system in cancer patients. Crit Care Med. 1980;8(12):733–5. doi: 10.1097/00003246-198012000-00007. [DOI] [PubMed] [Google Scholar]
- 10.DeBard ML. Cardiopulmonary resuscitation: analysis of six years' experience and review of the literature. Ann Emerg Med. 1981;10(8):408–16. doi: 10.1016/s0196-0644(81)80307-1. [DOI] [PubMed] [Google Scholar]
- 11.Hershey CO, Fisher L. Why outcome of cardiopulmonary resuscitation in general wards is poor. Lancet. 1982;11:31–4. doi: 10.1016/s0140-6736(82)92567-3. [DOI] [PubMed] [Google Scholar]
- 12.Bedell SE, Delbanco TL, Cook EF, Epstein FH. Survival after cardiopulmonary resuscitation in the hospital. N Engl J Med. 1983;309(10):569–75. doi: 10.1056/NEJM198309083091001. [DOI] [PubMed] [Google Scholar]
- 13.Gulati RS, Bhan GL, Horan MA. Cardiopulmonary resuscitation in old people. Lancet. 1983;11:267–9. doi: 10.1016/s0140-6736(83)90244-1. [DOI] [PubMed] [Google Scholar]
- 14.Scaff B, Munson R, Hastings DF. Cardiopulmonary resuscitation at a community hospital with a family practice residency. J Fam Pract. 1984;18(4):561–5. [PubMed] [Google Scholar]
- 15.Sowden GR, Robins DW, Baskett PJ. Factors associated with survival and eventual cerebral status following cardiac arrest. Anesthesia. 1984;39:39–43. doi: 10.1111/j.1365-2044.1984.tb09452.x. [DOI] [PubMed] [Google Scholar]
- 16.Suljaga-Pechtel K, Goldberg E, Strickon P, Berger M, Skovron ML. Cardiopulmonary resuscitation in a hospitalized population: prospective study of factors associated with outcome. Resuscitation. 1984;12:77–95. doi: 10.1016/0300-9572(84)90061-3. [DOI] [PubMed] [Google Scholar]
- 17.Bayer A J, Ang BC, Pathy MS. Cardiac arrests in a geriatric unit. Age Ageing. 1985;14:271–6. doi: 10.1093/ageing/14.5.271. [DOI] [PubMed] [Google Scholar]
- 18.Dans PE, Nevin KL, Seidman CE, McArthur JC, Kariya ST. In-hospital CPR 25 years later: why has survival decreased? South Med J. 1985;78(10):1174–8. [PubMed] [Google Scholar]
- 19.Kelly CA, Watson D, Hutchinson CM, Pole JM. Prognostic factors in cardiac arrest occurring in a district general hospital. Br J Clin Pract. 1986;40(6):251–3. [PubMed] [Google Scholar]
- 20.Kyff J, Puri VK, Raheja R, Ireland T. Cardiopulmonary resuscitation in hospitalized patients: continuing problems of decision-making. Crit Care Med. 1987;15:41–3. doi: 10.1097/00003246-198701000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Urberg M, Ways C. Survival after cardiopulmonary resuscitation for an in-hospital cardiac arrest. J Fam Pract. 1987;25(1):41–4. [PubMed] [Google Scholar]
- 22.Woog RH, Torzillo PJ. In-hospital cardiopulmonary resuscitation: prospective survey of management and outcome. Anaesth Intens Care. 1987;15:193–8. doi: 10.1177/0310057X8701500213. [DOI] [PubMed] [Google Scholar]
- 23.Raviglione MC, Battan R, Taranta A. Cardiopulmonary resuscitation in patients with the acquired immunodeficiency syndrome: a prospective study. Arch Intern Med. 1988;148:2602–5. [PubMed] [Google Scholar]
- 24.Rozenbaum EA, Shenkman L. Predicting outcome of in-hospital cardiopulmonary resuscitation. Crit Care Med. 1988;16(6):583–6. doi: 10.1097/00003246-198806000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Taffet GE, Teasdale TA, Luchi RJ. In-hospital cardiopulmonary resuscitation. JAMA. 1988;260(14):2069–72. [PubMed] [Google Scholar]
- 26.Burns R, Graney MJ, Nichols LO. Prediction of in-hospital cardiopulmonary arrest outcome. Arch Intern Med. 1989;149:1318–21. [PubMed] [Google Scholar]
- 27.George AL, Folk BP, Crecelius PL, Campbell WB. Pre-arrest morbidity and other correlates of survival after in-hospital cardiopulmonary arrest. Am J Med. 1989;87:28–34. doi: 10.1016/s0002-9343(89)80479-6. [DOI] [PubMed] [Google Scholar]
- 28.Keatinge RM. Exclusion from resuscitation. J R Soc Med. 1989;82:402–5. doi: 10.1177/014107688908200711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy DJ, Murray AM, Robinson BE, Campion EW. Outcomes of cardiopulmonary resuscitation in the elderly. Ann Intern Med. 1989;111:199–205. doi: 10.7326/0003-4819-111-3-199. [DOI] [PubMed] [Google Scholar]
- 30.Takeda Y, Mifune J, Taga K, Hifumi S, et al. Survival after sudden cardiac arrest in hospital. Jpn Heart J. 1989;30(5):645–53. doi: 10.1536/ihj.30.645. [DOI] [PubMed] [Google Scholar]
- 31.Hendrick JM, Pijls NH, van der Werf T, Crul JF. Cardiopulmonary resuscitation on the general ward: no category of patients should be excluded in advance. Resuscitation. 1990;20:163–71. doi: 10.1016/0300-9572(90)90051-f. [DOI] [PubMed] [Google Scholar]
- 32.Roberts D, Landolfo K, Light RB, Dobson K. Early predictors of mortality for hospitalized patients suffering cardiopulmonary arrest. Chest. 1990;97:413–9. doi: 10.1378/chest.97.2.413. [DOI] [PubMed] [Google Scholar]
- 33.Tortolani AJ, Risucci DA, Rosati RJ, Dixon R. In-hospital cardiopulmonary resuscitation: patient, arrest and resuscitation factors associated with survival. Resuscitation. 1990;20:115–28. doi: 10.1016/0300-9572(90)90047-i. [DOI] [PubMed] [Google Scholar]
- 34.Lawrence ME, Price L, Riggs M. Inpatients cardiopulmonary resuscitation: is survival prediction possible? South Med J. 1991;84:1462–6. doi: 10.1097/00007611-199112000-00013. [DOI] [PubMed] [Google Scholar]
- 35.Lazzam C, McCans JL. Predictors of survival of in-hospital cardiac arrest. Can J Cardiol. 1991;7(3):113–6. [PubMed] [Google Scholar]
- 36.Marwick TH, Case CC, Siskind V, Woodhouse SP. Prediction of survival from resuscitation: a prognostic index derived from multivariate logistic model analysis. Resuscitation. 1991;22:129–37. doi: 10.1016/0300-9572(91)90003-h. [DOI] [PubMed] [Google Scholar]
- 37.O'Keeffe S, Redahan C, Keane P, Daly K. Age and other determinants of survival after in-hospital cardiopulmonary resuscitation. Q J Med. 1991;296:1005–10. doi: 10.1093/qjmed/81.3.1005. [DOI] [PubMed] [Google Scholar]
- 38.Peterson MW, Geist L J, Schwartz DA, Konicek S, Moseley PL. Outcome after cardiopulmonary resuscitation in a medical intensive care unit. Chest. 1991;100:168–74. doi: 10.1378/chest.100.1.168. [DOI] [PubMed] [Google Scholar]
- 39.Vitelli CE, Cooper K, Rogatko A, Brennan MF. Cardiopulmonary resuscitation and the patient with cancer. J Clin Oncol. 1991;9:111–15. doi: 10.1200/JCO.1991.9.1.111. [DOI] [PubMed] [Google Scholar]
- 40.Landry FJ, Parker JM, Phillips YY. Outcome of cardiopulmonary resuscitation in the intensive care setting. Arch Intern Med. 1992;152:2305–8. [PubMed] [Google Scholar]
- 41.Tunstall-Pedoe H, Bailey L, Chamberlain DA, Marsden AK, Ward ME, Zideman DA. Survey of 3765 cardiopulmonary resuscitations in British hospitals (the BRESUS study): methods and overall results. BMJ. 1992;304:1347–51. doi: 10.1136/bmj.304.6838.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beuret P, Feihl F, Vogt P, Perret A, Romand JA, Perret C. Cardiac arrest: prognostic factors and outcome at one year. Resuscitation. 1993;25:171–9. doi: 10.1016/0300-9572(93)90093-6. [DOI] [PubMed] [Google Scholar]
- 43.Ebell MH, Preston PS. The effect of the APACHE II score and selected clinical variables on survival following cardiopulmonary resuscitation. Fam Med. 1993;25:191–6. [PubMed] [Google Scholar]
- 44.Juchems R, Wahlig G, Frese W. Influence of age on the survival rate of out-of-hospital and in-hospital resuscitation. Resuscitation. 1993;26:23–9. doi: 10.1016/0300-9572(93)90159-n. [DOI] [PubMed] [Google Scholar]
- 45.Rosenberg M, Wang C, Hoffman-Wilde S, Hickham D. Results of cardiopulmonary resuscitation: failure to predict survival in two community hospitals. Arch Intern Med. 1993;153:1370–5. doi: 10.1001/archinte.153.11.1370. [DOI] [PubMed] [Google Scholar]
- 46.Schwenzer KJ, Smit WT, Durbin CG. Selective application of cardiopulmonary resuscitation improves survival rates. Anesth Analg. 1993;76:478–84. doi: 10.1213/00000539-199303000-00005. [DOI] [PubMed] [Google Scholar]
- 47.Ballew KA, Philbrick JT, Caven DE, Schorling JB. Predictors of survival following in-hospital cardiopulmonary resuscitation: a moving target. Arch Intern Med. 1994;154:2426–32. [PubMed] [Google Scholar]
- 48.Ballew KA, Philbrick JT, Caven DWE, Schorling JB. Differences in case definitions as a cause of variation in reported in-hospital CPR survival. J Gen Intern Med. 1994;9:283–5. doi: 10.1007/BF02599658. [DOI] [PubMed] [Google Scholar]
- 49.Fifield DH. Outcomes of resuscitative efforts at Wild Rose Hospital. Wis Med J. 1994;93:55–7. [PubMed] [Google Scholar]
- 50.Robinson GR, Hess D. Postdischarge survival and functional status following in-hospital cardiopulmonary resuscitation. Chest. 1994;105:991–6. doi: 10.1378/chest.105.4.991. [DOI] [PubMed] [Google Scholar]
- 51.Tresch D, Heudebert G, Kutty K, et al. Cardiopulmonary resuscitation in elderly patients hospitalized in the 1990's: a favorable outcome. J Am Geriatr Soc. 1994;42:137–41. doi: 10.1111/j.1532-5415.1994.tb04940.x. [DOI] [PubMed] [Google Scholar]
- 52.Warner SC, Sharma TK. Outcome of cardiopulmonary resuscitation and predictors of resuscitation status in an urban community teaching hospital. Resuscitation. 1994;27:13–21. doi: 10.1016/0300-9572(94)90016-7. [DOI] [PubMed] [Google Scholar]
- 53.Bialecki L, Woodward RS. Predicting death after CPR: experience at a non-teaching community hospital with a full-time critical care staff. Chest. 1995;108:1009–17. doi: 10.1378/chest.108.4.1009. [DOI] [PubMed] [Google Scholar]
- 54.Smith DL, Kim K, Ciarns BA, Fakhry SM, Meyer AA. Prospective analysis of outcome after cardiopulmonary resuscitation in critically ill surgical patients. J Am Coll Surg. 1995;180:402–9. [PubMed] [Google Scholar]
- 55.Ebell MH, Kruse JA, Smith MA, Novak J, Drader-Wilcox J. Failure of three decision rules to predict the outcome of in-hospital cardiopulmonary resuscitation. Med Decis Making. 1997;17:171–7. doi: 10.1177/0272989X9701700207. [DOI] [PubMed] [Google Scholar]
- 56.Ebell MH, Smith MA, Kruse JA, Drader-Wilcox J, Novak J. Effect of race on survival following in-hospital cardiopulmonary resuscitation. J Fam Pract. 1995;40:571–77. [PubMed] [Google Scholar]
- 57.Ebell MH, Neale AV, Hodgkins BA. An expert system for the calculation of sample size. Fam Pract Res J. 1994;14:183–95. [PubMed] [Google Scholar]
- 58.Ebell MH. Pre-arrest predictors of survival following in-hospital CPR: a meta-analysis. J Fam Pract. 1992;34:551–8. [PubMed] [Google Scholar]
- 59.Cohn EB, LeFevre F, Yarnold PR, Arron MJ, Martin GJ. Predicting survival from in-hospital CPR: meta-analysis and validation of a prediction model. J Gen Intern Med. 1993;8:347–53. doi: 10.1007/BF02600069. [DOI] [PubMed] [Google Scholar]
- 60.Hasselblad V, McCrory H. Meta-analytic tools for medical decision-making: a practical guide. Med Decis Making. 1995;15:81–96. doi: 10.1177/0272989X9501500112. [DOI] [PubMed] [Google Scholar]
- 61.Chalmers TC, Smith H, Jr, Blackburn B, et al. A method for assessing the quality of a randomized control trial. Control Clin Trials. 1981;2:31–49. doi: 10.1016/0197-2456(81)90056-8. [DOI] [PubMed] [Google Scholar]
- 62.Hadorn DC, Baker D, Hodges JS, Hicks N. Rating the quality of evidence for clinical practice guidelines. J Clin Epidemiol. 1996;7:749–54. doi: 10.1016/0895-4356(96)00019-4. [DOI] [PubMed] [Google Scholar]
- 63.Fleiss JL, Gross AJ. Meta-analysis in epidemiology, with special reference to studies of the association between exposure to environmental tobacco smoke and lung cancer: a critique. J Clin Epidemiol. 1991;44:127–39. doi: 10.1016/0895-4356(91)90261-7. [DOI] [PubMed] [Google Scholar]
- 64.Writing Group for the Utstein Style Task Force Recommended guidelines for reviewing, reporting, and conducting research on in-hospital resuscitation: the in-hospital “Utstein style.”. Ann Emerg Med. 1997;29:650–72. doi: 10.1016/s0196-0644(97)70256-7. [DOI] [PubMed] [Google Scholar]


