Abstract
Objective
To better understand the life expectancy of patients who have an abnormal videofluoroscopic swallowing study.
Design
Retrospective cohort study. The common starting point was the time of the severely abnormal swallowing study. Hospital charts were reviewed for clinical variables of potential prognostic significance by reviewers blinded to the outcome of interest, survival time.
Setting
A university-affiliated, community teaching hospital.
Patients
One hundred forty-nine hospitalized patients who were deemed nonoral feeders based on their swallowing study. Patients excluded were those with head, neck, or esophageal cancer, or those undergoing a thoracotomy procedure.
Measurements And Main Results
Clinical and demographic variables and time until death or censoring were measured. Overall 1-year mortality was 62%. Multivariable Cox proportional hazards analyses identified four variables that independently predicted death: advanced age, reduced serum albumin concentration, disorientation to person, and higher Charlson comorbidity score. Eighty patients (54%) subsequently underwent placement of a percutaneous endoscopic gastrostomy (PEG) tube after their swallowing study.
Conclusions
Mortality is high in patients with severely abnormal swallowing studies. Common clinical variables can be used to identify groups of patients with particularly poor prognoses. This information may help guide discussions regarding possible PEG placement.
Keywords: videofluoroscopic swallowing study, percutaneous endoscopic gastrostomy (PEG), nonoral feeding, survival
When patients cannot meet their nutritional needs by mouth or are at high risk of aspirating food, tube feedings may be required.1 A videofluoroscopic swallowing study (VFSS) is often ordered to determine the risk of aspiration, and the underlying physiologic and anatomic reasons for the swallowing dysfunction.2–4 The prevalence of some swallowing difficulty in inpatients is at least 12%,5 and is in the 20% to 40% range for patients with strokes,6–8 dementia,9,10 or other neurologic conditions.11,12 Traditionally the most common method of tube feeding was via a nasogastric tube. Since 1980, the percutaneous endoscopic gastrostomy (PEG) method has become popular for long-term tube feedings because it is reasonably safe and comfortable for the patient.13
The decision to place a PEG tube is not always straightforward. A recent survey of nursing home residents found only 33% would choose tube feedings if they were no longer able to eat.14 Some clinicians also feel PEG tube placement may be inappropriate for patients who are expected to die soon.13,15–17 Many advanced directives or living wills have options for patients to refuse life-sustaining treatments if they have a terminal illness, or if the “burdens of the treatment outweigh the expected benefits.”18,19 Conversely, if a patient wanted to live longer or allow time for the medical conditions to improve, then prolongation of life could be considered a benefit,20,21 and tube feedings justifiable.
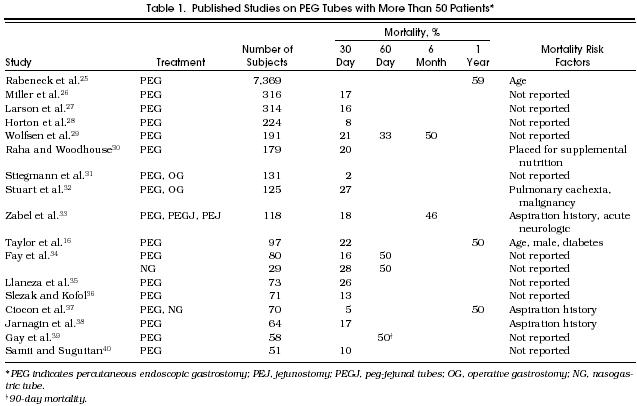
The first step in the decision-making process is to estimate the expected survival of a patient who has abnormal swallowing, then estimate the potential benefit of PEG placement. The medical literature currently provides only part of the necessary information. Of stroke patients with swallowing difficulties, 45% to 68% are dead within 6 months.6,22,23 A recent study reported a survival advantage for stroke patients randomized to feedings by the PEG route versus nasogastric route.24 How such patients would fare without any form of tube feedings is not known. Still less is known for nonstroke conditions. Most investigators study patients after the PEG tube has been placed. As shown in Table 1, the mortality rate for these patients is high: 2% to 27% are dead within 30 days, and approximately 50% or more within 1 year.16,25–40 Although informative, these studies cannot provide survival estimates for those who might not choose PEG feedings.
Published Studies on PEG Tubes with More Than 50 Patients*
Such information could be obtained from a randomized, controlled trial of patients having and not having PEG tubes, with a sample size sufficient to allow identification of prognostic factors. Yet, to conduct such a study would require the participants (and their families) to be sufficiently neutral toward tube feedings to allow randomization in the first place. At this point in time, enrolling such patients in the United States would be difficult. However, some insights might be achieved through a retrospective cohort analysis, starting at the time of an abnormal swallowing study, whether or not a PEG was subsequently placed. We therefore undertook the following study to examine life expectancy in these patients, and to begin identifying important prognostic factors in those eligible to receive tube feedings.
Methods
Study Site
St. Joseph Mercy Hospital (Ann Arbor, Mich.) is a 560-bed, university-affiliated, community teaching hospital. Because swallowing studies could be coded differently on billing data, we obtained lists for all inpatients who had either a barium swallow study or VFSS during years 1990 –1992. This study focused on inpatients, to allow more efficient data collection. Of 1,056 patient records requested, 1,010 were able to be retrieved and reviewed.
Study Exclusion Criteria
Of the 1,010 charts reviewed, 122 of the patients had a barium swallow examination only and so were excluded, leaving 888 patients who had had a VFSS. Of these, 739 patients were not included in the study cohort for the following reasons: they were allowed to eat (usually a modified diet) based on the VFSS results, and so were not candidates for tube feedings (n = 577/888 or 65% of VFSS patients); or they already had a PEG tube placed at the time of the VFSS (n = 79, 9%). Patients were also excluded if they had a thoracotomy on the same hospital admission (for example, coronary artery bypass, n = 33, 4%), because patient variables changed frequently during the postoperative period and PEG placement could be considered part of the postoperative care. Other exclusions were patients for whom the decision to place a PEG tube seemed more straightforward: those with oropharyngeal carcinoma, carcinoma of the thyroid, esophageal cancer, previous radiation therapy to the neck area, or resection of the epiglottis (n = 27, 3%); who had mechanical obstruction from cricopharyngeal spasm (n = 6, 1%); who were intubated or had a cuffed tracheostomy (n = 7, 1%); were uncooperative (n = 8, 1%); or had an esophagectomy or gastrectomy (n = 2, 0.2%).
Inclusion Criteria
One hundred forty-nine patients fulfilled the criteria of nonoral feeders despite compensatory strategies. These were patients who were able to participate in the VFSS evaluation for at least one bolus presentation, and had at least one of the following: inability to sustain arousal for 30 minutes several times per day, combined with neurologic deficits or severe deconditioning; inability to propel more than 25% of the bolus to the pharynx; aspiration of more than 1 consistency, or frank, gross aspiration of 1 consistency, with suboptimal alertness; or moderate-to-severe (greater than 50% of the bolus) stasis in the pharynx that could not be cleared with dry swallows or thin liquids.
Outcome
Charts were reviewed by one of four reviewers (three physicians and a nurse) who were blinded to the primary outcome of interest—survival time. All deaths were assumed to have occurred within Michigan owing to the nature of the hospital admitting patterns. Indices compiled from death certificates from the Michigan Department of Public Health were reviewed for years 1990 –1993 to determine the date and reported principal cause of death. Patients not known to have died, either by death index or by hospital chart, were assumed to be alive as of the last day of the 1993 index, December 31. One year of follow-up was therefore available for all subjects.
Predictors
Data were collected on a number of variables as of the date of the VFSS: patient demographics, level of alertness (normal/impaired), skin decubiti (yes/no), urinary incontinence or catheterization (yes/no), and coexisting comorbidities (counted if currently present or if there was a history of the condition).41 Serum albumin concentration (g/dl) was also recorded on the day of the VFSS, if available. If a level was not available, then the level for the most recent prestudy date was used, or if that was not available, then the first reported level after the study was used. If no serum albumin value could be found (for 18/149 patients), we imputed the mean value of the entire study group. The best level of orientation (person, place, and time) achieved prestudy was also recorded, resulting in a binary variable for being at least oriented to person or not. For 19 of the 149 patients, the level of orientation was indeterminable or missing. In these cases, we chose to err on the side of biasing in favor of the null hypothesis, meaning these patients were assumed to be oriented to person.
The hospital course following the VFSS was reviewed to determine whether or not a PEG tube was placed before hospital discharge. If a PEG was not placed, the reason was listed. This resulted in three categories of patients post-VFSS: PEG tube placed (n = 80), PEG tube not placed because of rapid clinical improvement prior to hospital discharge (“improved” group, n = 18), and PEG tube not placed due to comfort considerations (n = 51). This latter category included patients who refused (or whose families refused) any form of tube feedings (n = 28), patients discharged with some form of tube feeding by the nasogastric route (n = 10), patients who died before receiving a PEG (n = 12), and one patient who was transferred to another hospital.
Statistical Analysis
All analyses were performed using the SAS software package (SAS Institute, Cary, NC). Overall group survival was estimated with a Kaplan-Meier curve. Univariate predictors of mortality risk were identified by the log-rank test for categorical variables, and by single-variable Cox proportional hazards regressions for continuous variables. Multivariable Cox proportional hazards models were constructed from candidate variables related to survival with values of p = .20 or less on univariate analyses. The final multivariable model was determined using backwards elimination, with the criterion for retention set p = .05. In order to detect overfitting that might occur from multiple significance testing with the backwards elimination procedure, the coefficients and standard errors were reevaluated using the bootstrap technique. This involved selecting 1,000 bootstrap samples, each containing 149 “subjects” selected with replacement from our data set. A separate proportional hazards model was developed from each sample, and the mean and standard deviations from the 1,000 sets of regression coefficients were calculated. As the mean regression coefficient and the corresponding standard deviation provide a more valid (less prone to overfitting) reflection of the magnitude and the precision of the effect of each factor in the final model, a comparison of these values with the coefficients and standard errors of the final model provides a mechanism for assessing the validity of that model. Finally, graphs displaying survival patterns for various combinations of the prognostic variables were constructed.
Results
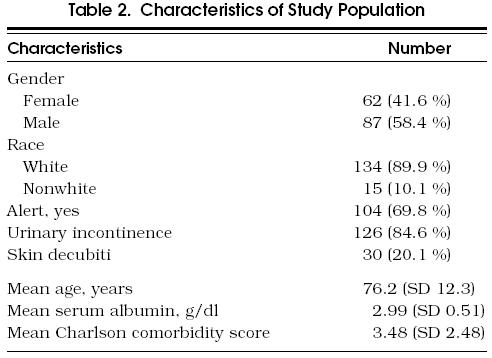
The characteristics of the study population are presented in Tables 23. The mean age was 76.2 years, 41.6% were women, and 89.9% were white. Most of the patients had serious comorbid conditions, with a mean Charlson score of 3.48. The most frequent conditions were cerebrovascular accident (56.4%), hemiplegia (41.6%), congestive heart failure (32.2%), and dementia (20.1%).
Characteristics of Study Population
Univariate Mortality Risk Factors
Overall Group Analysis
The median survival of the study population was 159 days (95% confidence interval [CI] 72, 276 days), estimated 30-day mortality was 27%, 90-day mortality 42%, and 1-year mortality 62%. Causes of death as recorded on the death certificates were cardiac (29.8%), cerebrovascular (18.3%), cancer (10.6%), other neurologic conditions (6.7%), diabetes (5.8%), pneumonia (5.8%), chronic obstructive lung (5.8%), and other (17.3%).
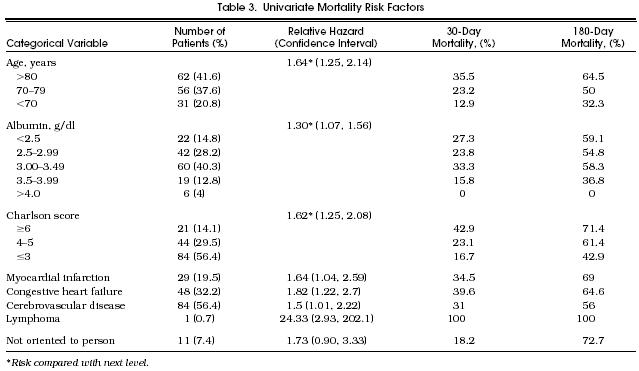
Table 3 summarizes the univariate analyses. The mortality rate ratio (RR), or risk of death, was increased in those with advancing age, lower serum albumin concentration, higher Charlson comorbidity category, and in those who had had a myocardial infarction, congestive heart failure, cerebrovascular disease, or lymphoma. Patients disoriented to person were at borderline increased risk (RR 1.73; 95% CI 0.90 , 3.33).
The multivariable proportional hazards model identified four variables that were independently associated with an increased likelihood of death: advanced age category (RR 1.48 compared with the next younger category, 95% CI 1.10, 2.00), reduced serum albumin category (RR 1.29 compared with the next higher category, 95% CI 1.04, 1.59), disorientation to person (RR 2.29 over those who were oriented, 95% CI 1.16 , 4.53), and higher Charlson comorbidity category (RR 1.82 compared with the next lower category, 95% CI 1.39, 2.37). These remained significant predictors when reevaluated by the bootstrap technique. Figure 1 displays projected survival patterns based on various combinations of the explanatory variables in the multivariable model. As shown, most combinations of the risk factors projected median survivals of less than 6 months.
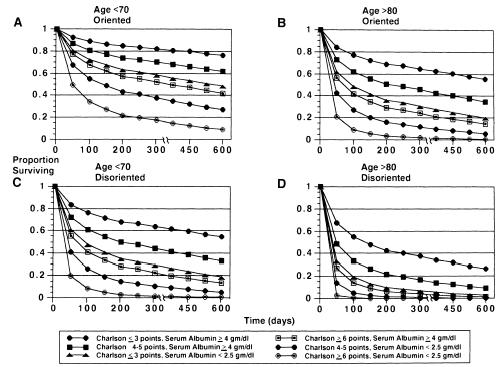
Figure Projected survival estimates based on the multivariable model. For all panels, curves represent combinations of Charlson comorbidity scores and serum albumin levels (g/dl): (A) age less than 70, and oriented to person; (B) age greater than 80, and oriented to person; (C) age less than 70, and disoriented to person; (D) age greater than 80, and disoriented to person.
Subgroup Analysis
Survival curves for the three categories of patients based on subsequent PEG tube status (PEG, comfort care, and improved) are shown in Figure 2 (p = .0001). Unadjusted median survival was 33 days for the comfort group (95% CI 9 , 124 days), and 181 days for the PEG group (95% CI 70, 318 days). Patients in the improved group had their clinical improvement, on the average, within 13.8 days after their VFSS. These patients were younger, more likely to be men, and had higher serum albumin concentrations than the other subgroups. The survival advantage of the improved and the PEG groups compared with the comfort care group remained after adjusting for the four survival determinants in the multivariable model (improved RR 0.09, 95% CI 0.03, 0.31; PEG RR 0.48, 95% CI 0.32, 0.74).
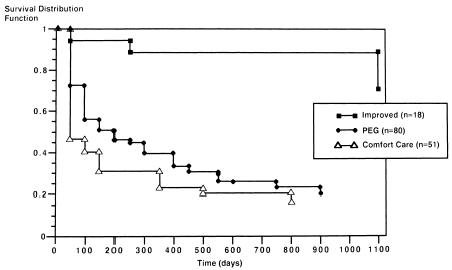
Figure Kaplan-Meier curves according to PEG status. Unadjusted survival curves according to PEG status. The top curve represents the clinically improved group; the middle curve, those who underwent PEG placement; the lower curve, those without PEG placement, comfort care status.
Discussion
The focus of this study was to derive survival estimates for patients with abnormal swallowing studies, in order to help clinicians and patients with the tube feeding decision. Our study group was composed of 17% of all patients who had had a VFSS in our hospital. This is near the midpoint for the reported prevalence of 12% to 32% for severe dysfunction as demonstrated by VFSS.8–11,42 We found these patients to be at high risk of dying in the ensuing year. At particular risk were patients with advanced age, increasing comorbidity, low serum albumin concentration, and disorientation to person. After adjusting for these predictors, patients with PEG tubes placed after their VFSS survived twice as long as those in the comfort care group. A subset of patients, comprising 12% of the study cohort, avoided a PEG tube because of their rapid clinical improvement, occurring on average within 2 weeks of the swallowing study.
Mortality rates and previously identified predictors of death in patients with PEG tubes are shown in Table 1. In our study, we were able to confirm the increased risk associated with advancing age. We were unable to confirm the increased risk associated with male gender and diabetes. The differences in clinical predictors can best be explained by patient selection factors. The other studies began follow-up after patients had been selected for PEG placement for various reasons. Our study began at an earlier time in the clinical course, at the time of an abnormal VFSS, and more closely approximated the point in time when the decision for or against a PEG is made. Other differences in results can be explained by patient inclusion criteria and study settings. For example, we did not include patients with oropharyngeal or esophageal cancer in our study. It is also possible that our study lacked the necessary statistical power to confirm the findings from the other studies.
Despite these differences, the mortality rate is similarly high in these studies and in ours. The 62% one-year mortality of our patients was similar to the 59% rate in patients with Charlson points of 3 or greater.41 For stroke patients, the 56% six-month mortality in our series was nearly identical to mortality rates found in two other recent studies.6,16
The clinical predictors identified in our study are not novel. Age,43 Charlson score,41,43 serum albumin,44–46 and cognitive impairment (our disorientation variable)47,48 have all been previously identified as mortality risk factors. In a sense, we have validated these as predictors for the population of patients with severely abnormal VFSS.
This study has a number of important limitations. It was not a randomized clinical trial comparing survival between patients fed via the PEG route, versus no tube feedings. Our control group was a heterogeneous group that included patients who died in the hospital (12/51), those who had tube feedings by a non-PEG route (10/51), and those who refused any form of tube feedings (28/51). It appears that patients who underwent PEG placement survived approximately twice as long as those who did not, after controlling for the four mortality risk factors. It is likely the survival advantage was due to other prognostic factors which were influencing the decision to place the PEG tube. However, these remain unidentified. We therefore cannot prove the benefit of a PEG tube by this study design.
Another potential limitation is with the identification of the mortality risk factors. The choice of comorbidities was based on the prognostic factors identified by Charlson et al.41 However, we did not distinguish between active versus historical problems for the patient, nor did we identify levels of illness severity within the conditions. As currently defined, the number of comorbidities appears to have prognostic significance in the population of patients with abnormal swallowing. It is unknown if more precise prognostic information would be gained by further stratifying these based on current level of disease activity. Similarly, the variable for cognitive dysfunction was rather crude—disorientation to person or not. If this could not be determined by chart review, then patients were assigned to the default category, orientation to person. One can hypothesize that more precise information would have improved the explanatory ability of this variable. In this same line of reasoning, the serum albumin variable can be criticized because the test was ordered at different times by different clinicians. Another problem is that missing values were assigned the mean value for the group. These flaws would tend to weaken the inverse relation between the serum albumin level and mortality risk. Because a number of factors may influence the serum albumin level,45,46 controlling for the acuity or chronicity of the underlying conditions might further refine this explanatory variable. Many of these discussed limitations are in part due to the retrospective cohort study design. Nevertheless, such a study has merit if it is viewed as an initial step in understanding the prognosis of these patients.
A final limitation is the absence of a “testing” set, a second cohort of patients on which to test the validity of the explanatory variables. We began the validation effort by submitting all of our multivariable regression models to bootstrap testing. This demonstrated that the selected multivariable parameters remained significant through 1,000 replications, suggesting the stability of our findings. However, before the results could be applied with confidence to an individual patient, more definitive validation must be done.49
It is important not to overstate the applicability of our study to individual decision making. At best, it provides a context for patient-physician dialogue. The reported survival estimates are a starting point for estimating the prognosis for patients with severely abnormal swallowing studies, rather than a formal clinical predictive rule. At this time, to extend these estimates for a group to an individual also will require the clinician to consider other prognostic information such as the likelihood of reversibility of the medical conditions,20,21,50 disease-specific predictors found in the literature, individual patient characteristics, and clinical judgment.45 Quality of life, not addressed in our study, also must be considered.20,21,51 The decision for or against tube feedings must be based on all information at hand, imprecise as it might be. Further studies are needed to provide clinicians, patients, and families with clearer guidelines for these difficult decisions.
Acknowledgments
This project was completed with the assistance of the following: E. Francis Cook, ScD, E. John Orav, PhD, Glenn M. Chertow, MD, Dorian D. Moore, MD, Mary Hawley, OTR, and Frances Gronczewski, ART.
References
- 1.Ott DJ, Pikna AL. Clinical and videofluoroscopic evaluation of swallowing disorders. AJR. 1993;161:507–13. doi: 10.2214/ajr.161.3.8352094. [DOI] [PubMed] [Google Scholar]
- 2.Martin BJW, Corlew MM, Wood H, et al. The association of swallowing dysfunction and aspiration pneumonia. Dysphagia. 1994;9:1–6. doi: 10.1007/BF00262751. [DOI] [PubMed] [Google Scholar]
- 3.Logemann JA. Treatment for aspiration related to dysphagia: an overview. Dysphagia. 1986;1:34–8. [Google Scholar]
- 4.Feinberg MJ. Radiographic techniques and interpretation of abnormal swallowing in adult and elderly patients. Dysphagia. 1993;8:356–8. doi: 10.1007/BF01321779. [DOI] [PubMed] [Google Scholar]
- 5.Groher ME, Bukatman R. The prevalence of swallowing disorders in two teaching hospitals. Dysphagia. 1986;1:3–6. [Google Scholar]
- 6.Barer DH. The natural history and functional consequences of dysphagia after hemispheric stroke. J Neurol Neurosurg Psychiatry. 1989;52:236–241. doi: 10.1136/jnnp.52.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordon C, Hewer RL, Wade DT. Dysphagia in acute stroke. BMJ. 1987;295:411–4. doi: 10.1136/bmj.295.6595.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horner J, Massey EW, Riski JE, Lathrop DL, Chase KN. Aspiration following stroke: clinical correlates and outcome. Neurology. 1988;38:1359–62. doi: 10.1212/wnl.38.9.1359. [DOI] [PubMed] [Google Scholar]
- 9.Horner J, Alberts MJ, Dawson DV, Cook GM. Swallowing in Alzheimer's disease. Alzheimer Dis Assoc Disord. 1994;8:177–89. doi: 10.1097/00002093-199408030-00004. [DOI] [PubMed] [Google Scholar]
- 10.Feinberg MJ, Ekberg O, Segall L, Tully J. Deglutition in elderly patients with dementia: findings of videofluorographic evaluation and impact on staging and management. Radiology. 1992;183:811–4. doi: 10.1148/radiology.183.3.1584939. [DOI] [PubMed] [Google Scholar]
- 11.Chen MYM, Peele VN, Donati D, et al. Clinical and videofluoroscopic evaluation of swallowing in 41 patients with neurologic disease. Gastrointest Radiol. 1992;17:95–8. doi: 10.1007/BF01888518. [DOI] [PubMed] [Google Scholar]
- 12.Bird MR, Woodward JC, Gibson EM, Phyland DJ, Fonda D. Asymptomatic swallowing disorders in elderly patients with Parkinson's disease: a description of findings on clinical examination and videofluoroscopy in sixteen patients. Age Ageing. 1994;23:251–4. doi: 10.1093/ageing/23.3.251. [DOI] [PubMed] [Google Scholar]
- 13.Ponsky JL, Gauderer MWL. Percutaneous endoscopic gastrostomy: indications, limitations, techniques, and results. World J Surg. 1989;13:165–70. doi: 10.1007/BF01658394. [DOI] [PubMed] [Google Scholar]
- 14.O'Brien LA, Grisso JA, Maislin G, et al. Nursing home residents’ preferences for life-sustaining treatments. JAMA. 1995;274:1775–9. [PubMed] [Google Scholar]
- 15.Stellato TA, Gauderer MWL. Percutaneous endoscopic gastrostomy in the cancer patient. Am Surg. 1988;54:419–22. [PubMed] [Google Scholar]
- 16.Taylor CA, Larson DE, Ballard DJ, et al. Predictors of outcome after percutaneous endoscopic gastrostomy: a community-based study. Mayo Clin Proc. 1992;67:1042–9. doi: 10.1016/s0025-6196(12)61118-5. [DOI] [PubMed] [Google Scholar]
- 17.American Gastroenterological Association Medical Position Statement. Guidelines for the use of enteral nutrition. Gastroenterology. 1995;108:1280–301. doi: 10.1016/0016-5085(95)90230-9. [DOI] [PubMed] [Google Scholar]
- 18.Emanuel LL, Emanuel EJ. The medical directive. JAMA. 1989;261:3288–93. doi: 10.1001/jama.261.22.3288. [DOI] [PubMed] [Google Scholar]
- 19.Schneiderman LJ, Kronick R, Kaplan RM, Anderson JP, Langer RD. Effects of offering advance directives on medical treatments and costs. Ann Intern Med. 1992;117:599–606. doi: 10.7326/0003-4819-117-7-599. [DOI] [PubMed] [Google Scholar]
- 20.Quill TE. Utilization of nasogastric feeding tubes in a group of chronically ill, elderly patients in a community hospital. Arch Intern Med. 1989;149:1937–41. [PubMed] [Google Scholar]
- 21.Lo B, Dornbrand L. Understanding the benefits and burdens of tube feedings. Arch Intern Med. 1989;149:1925–6. [PubMed] [Google Scholar]
- 22.Schmidt J, Holas M, Halvorson K, Reding M. Videofluoroscopic evidence of aspiration predicts pneumonia and death but not dehydration following stroke. Dysphagia. 1994;9:7–11. doi: 10.1007/BF00262752. [DOI] [PubMed] [Google Scholar]
- 23.Wanklyn P, Cox N, Belfield P. Outcome in patients who require a gastrostomy after stroke. Age Ageing. 1995;24:510–4. doi: 10.1093/ageing/24.6.510. [DOI] [PubMed] [Google Scholar]
- 24.Norton B, Homer-Ward M, Donelly MT, Long RG, Holmes GKT. A randomised prospective comparison of percutaneous endoscopic gastrostomy and nasogastric tube feeding after acute dysphagic stroke. BMJ. 1996;312:13–6. doi: 10.1136/bmj.312.7022.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rabeneck L, Wray NP, Petersen NJ. Long-term outcomes of patients receiving percutaneous endoscopic gastrostomy tubes. J Gen Intern Med. 1996;11:287–93. doi: 10.1007/BF02598270. [DOI] [PubMed] [Google Scholar]
- 26.Miller RE, Castlemain B, Lacqua FJ, Kotler DP. Percutaneous endoscopic gastrostomy. Surg Endosc. 1989;3:186–90. doi: 10.1007/BF02171543. [DOI] [PubMed] [Google Scholar]
- 27.Larson DE, Burton DD, Schroeder KW, DiMagno EP. Percutaneous endoscopic gastrostomy. Gastroenterology. 1987;93:48–52. [PubMed] [Google Scholar]
- 28.Horton WL, Colwell DL, Burlon DT. Experience with percutaneous endoscopic gastrotomy in a community hospital. Am J Gastroenterol. 1991;86:168–9. [PubMed] [Google Scholar]
- 29.Wolfsen HC, Kozarek RA, Ball TJ, et al. Long-term survival in patients undergoing percutaneous endoscopic gastrostomy and jejunostomy. Am J Gastroenterol. 1990;85:1120–2. [PubMed] [Google Scholar]
- 30.Raha SK, Woodhouse K. The use of percutaneous endoscopic gastrostomy (PEG) in 161 consecutive elderly patients. Age Ageing. 1994;23:162–3. doi: 10.1093/ageing/23.2.162. [DOI] [PubMed] [Google Scholar]
- 31.Stiegmann GV, Goff JS, Silas D. Endoscopic versus operative gastrostomy. Gastrointest Endosc. 1990;36:1–5. doi: 10.1016/s0016-5107(90)70911-x. [DOI] [PubMed] [Google Scholar]
- 32.Stuart SP, Tiley EH, Boland JP. Feeding gastrostomy: a critical review of its indications and mortality rate. South Med J. 1993;86:169–72. [PubMed] [Google Scholar]
- 33.Zabel JS, Onstad GR, Cass OW. Long-term follow-up of patients with percutaneous endoscopic gastrostomy (PEG), jejunostomy (PEJ) and peg-jejunal tubes (PEGJ) Gastroenterology. 1989;96:A563. Abstract. [Google Scholar]
- 34.Fay DE, Poplausky M, Gruber M, Lance P. Long-term enteral feeding: a retrospective comparison of delivery via percutaneous endoscopic gastrostomy and nasoenteric tubes. Am J Gastroenterol. 1991;86:1604–9. [PubMed] [Google Scholar]
- 35.Llaneza PP, Menendez AM, Roberts R, Dunn GD. Percutaneous endoscopic gastrostomy. South Med J. 1988;81:321–4. doi: 10.1097/00007611-198803000-00008. [DOI] [PubMed] [Google Scholar]
- 36.Slezak FA, Kofol WH. Combined tracheostomy and percutaneous endoscopic gastrostomy. Am J Surg. 1987;154:271–3. doi: 10.1016/0002-9610(89)90608-9. [DOI] [PubMed] [Google Scholar]
- 37.Ciocon JO, Silverstone FA, Graver M, Foley CJ. Tube feedings in elderly patients. Arch Intern Med. 1988;148:429–33. [PubMed] [Google Scholar]
- 38.Jarnagin WR, Duh QY, Mulvihill SJ, Ridge JA, Schrock TR, Way LW. The efficacy and limitations of percutaneous endoscopic gastrostomy. Arch Surg. 1992;127:261–4. doi: 10.1001/archsurg.1992.01420030023003. [DOI] [PubMed] [Google Scholar]
- 39.Gay F, El Nawar A, Van Gossum A. Percutaneous endoscopic gastrostomy. Acta Gastroenterol Belg. 1992;55:285–94. [PubMed] [Google Scholar]
- 40.Samii AM, Suguitan EA. Comparison of operative gastrostomy with percutaneous endoscopic gastrostomy. Mil Med. 1990;155:534–5. [PubMed] [Google Scholar]
- 41.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 42.Hodge RG, Pikna LA, Ott DJ, et al. Modified barium swallow: clinical indication and radiographic results in determining feeding recommendations. Gastroenterology. 1992;103:1410. Abstract. [Google Scholar]
- 43.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–51. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 44.Corti MC, Guralnik JM, Salive ME, Sorkin JD. Serum albumin level and physical disability as predictors of mortality in older persons. JAMA. 1994;272:1036–42. [PubMed] [Google Scholar]
- 45.Knaus WA, Harrell FE, Lynn J, et al. The SUPPORT prognostic model. Ann Intern Med. 1995;122:191–203. doi: 10.7326/0003-4819-122-3-199502010-00007. [DOI] [PubMed] [Google Scholar]
- 46.Klonoff-Cohen H, Barrett-Connor EL, Edelstein SL. Albumin levels as a predictor of mortality in the healthy elderly. J Clin Epidemiol. 1992;45:207–12. doi: 10.1016/0895-4356(92)90080-7. [DOI] [PubMed] [Google Scholar]
- 47.Tatemichi TK, Paik M, Bagiella E, Desmond DW, Pirro M, Hanzawa LK. Dementia after stroke is a predictor of long-term survival. Stroke. 1994;25:1915–9. doi: 10.1161/01.str.25.10.1915. [DOI] [PubMed] [Google Scholar]
- 48.Kelman HR, Thomas C, Kennedy GJ, Cheng J. Cognitive impairment and mortality in older community residents. Am J Public Health. 1994;84:1255–60. doi: 10.2105/ajph.84.8.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Braitman LE, Davidoff F. Predicting clinical states in individual patients. Ann Intern Med. 1996;125:406–12. doi: 10.7326/0003-4819-125-5-199609010-00008. [DOI] [PubMed] [Google Scholar]
- 50.Hodges MO, Tolle SW, Stocking C, Cassel CK. Tube feedings. Arch Intern Med. 1994;154:1013–20. doi: 10.1001/archinte.154.9.1013. [DOI] [PubMed] [Google Scholar]
- 51.Meyers RM, Grodin MA. Decision making regarding the initiation of tube feedings in the severely demented elderly: a review. J Am Geriatr Soc. 1991;39:526–31. doi: 10.1111/j.1532-5415.1991.tb02501.x. [DOI] [PubMed] [Google Scholar]


