Abstract
OBJECTIVE
To determine the effect of treatment with erythromycin on the resolution of symptoms among adults with pharyngitis not caused by group A streptococcus (GAS).
DESIGN
Randomized, double-blind, placebo-controlled trial.
SETTING
Ambulatory setting (hospital-based general internal medicine practices, walk-in clinic, employee health service, and university health service).
PATIENTS
One hundred and eighty-six adults who met eligibility criteria and whose chief complaint included sore throat. Patients with positive cultures for GAS were excluded.
INTERVENTION
Ninety-three patients received erythromycin (333 mg three times daily for 10 days) and 93 control patients received placebo.
MEASUREMENTS AND MAIN Results
Major outcome measurements included time to improvement in sore throat, time to improvement in cough, time to improvement in activity level, and subjective sense of well-being. The average age of the patients studied was 26.6 years; 35% were men. Patients given erythromycin had more rapid resolution of sore throat symptoms (hazard ratio 1.43; 95% confidence interval [CI] 1.00, 2.03; p = .049). Cough also resolved more rapidly in patients receiving erythromycin (hazard ratio 2.22; 95% CI 1.01, 4.88; p = .05). There were no differences between the two treatment groups in improvement of activity level or how sick patients felt in general. Most of the benefit in resolution of sore throat was conferred on patients who sought medical care within 2 days of onset.
CONCLUSIONS
Our results suggest that the benefit of erythromycin treatment for patients with non–GAS pharyngitis is small and of borderline statistical significance. Because of the small size of the effect and because widespread use of erythromycin could promote drug resistance, we do not recommend routine use of erythromycin in adult patients with this type of pharyngitis.
Keywords: erythromycin; pharyngitis; non–group A streptococcus (GAS); sore throat, resolution of
Pharyngitis is among the more common acute illnesses in the United States.1,2 Rational and practical approaches to the diagnosis of group A streptococcus (GAS) infections exist.3,4 However, GAS accounts only for approximately 10% of all cases of pharyngitis in adults.4 It is widely believed, consequently, that the majority of patients with pharyngitis have viral illnesses.5 Nevertheless, antibiotics are prescribed widely for pharyngitis, independent of the presence of GAS,6,7 although there is great variation in clinical strategies among practitioners.8
Our previous investigations and those of others have suggested that many cases of non-GAS pharyngitis may be caused by nonviral organisms and respond to therapy with antibiotics.9–14 Because treatment practices vary for cases of pharyngitis that are not attributable to GAS, any benefits of antibiotic therapy could have a substantial impact. We conducted a randomized, placebo-controlled trial of erythromycin and placebo in the treatment of pharyngitis in adults not infected with GAS to evaluate the effects of antibiotic treatment on symptoms.
Methods
Study Population
We enrolled patients who came for medical care to employee health services, walk-in clinics, or general internal medicine practices at Beth Israel Hospital, Boston City Hospital, and the Massachusetts Institute of Technology Medical Department. Patients between the ages of 18 and 50 were eligible if a sore throat was among their chief complaints. We excluded patients who had been ill for more than 6 days, whose rhinitis symptoms were more prominent than the complaint of sore throat, whose oral temperature was 40°C or greater, who had symptoms of urinary tract infection or vaginitis, were pregnant or nursing, had a history of rheumatic fever, had significant medical illnesses other than hypertension, had taken antibiotics within the previous 10 days, had a history of erythromycin allergy, did not have a telephone at home, were not literate in English, had previously enrolled in the study, or would be unable to cooperate with the study protocol in the opinion of study personnel. Patients were also excluded if any evidence of pneumonia or otitis media was found during the intake physical examination.
Patients were screened for eligibility between January 1983 and December 1984. Of 998 patients screened, 420 met eligibility criteria and 215 agreed to participate. The major reasons for exclusion: were illness duration greater than 7 days (44%); nasal congestion was the most prominent symptom (22%); temperature above 40°C (1%); dysuria or vaginitis (9%); pregnancy or lactation (3%); history of rheumatic fever (3%); a chronic illness (11%); recent antibiotic use (17%); history of erythromycin allergy (4%); no telephone at home (6%); inability to read English (5%); age less than 18 or greater than 50 (7%). (The percentages add up to more than 100 because patients may have had more than one reason for exclusion.) The major reasons patients refused to participate were unwillingness to take antibiotics, insistence on therapy with an antibiotic, lack of time for follow-up, and unwillingness to have blood drawn. Participating patients were randomized to receive either active drug or placebo. Three patients were dropped from the analysis because their data forms were misplaced before data entry. The final study group therefore consisted of 212 randomized patients.
Treatment Assignment
Patients were randomized to regimens of erythromycin (333 mg) or placebo three times daily. Erythromycin was provided as the delayed-release erythromycin base (E-mycin, The Upjohn Company, Kalamazoo, Mich.). We selected this erythromycin preparation for study because it required dosing three times daily rather than four times daily. Placebo pills were identical in appearance to erythromycin capsules and consisted of lactose primarily in a gelatin capsule (also provided by The Upjohn Company). Both physicians and patients were blinded to treatment group assignment. Patients whose initial throat cultures were positive for GAS were told to discontinue their study medication and begin a 10-day course of penicillin, or erythromycin if there was a history of penicillin allergy.
Data Collection
Patient Evaluation
At intake, we obtained a history of the severity of several upper respiratory symptoms that included sore throat, coryza, sneezing, nasal obstruction, headaches, cough, sputum production, hoarseness, achiness, and feverishness. We asked about the duration of the present illness, use of cigarettes, activity level, and use of other medications. We performed a physical examination to assess temperature and respiratory rate, and abnormalities of the ears, nose, throat, cervical lymph nodes, and chest. To assess the severity of each symptom, we asked patients to report severity on a 4-point categorical scale with 0 if the symptom was absent, 1 if the severity of the symptom was mild, 2 if moderate, and 3 if severe. Physician evaluators recorded their recommendations for symptomatic therapy. We discouraged the use of antipyretics and offered codeine, guaifenesin, and pseudoephedrine as indicated to patients who requested symptomatic remedies.
Patient Follow-up
We asked patients to complete a daily questionnaire for 2 weeks in which they recorded the use of medications other than study drug, the severity of upper respiratory symptoms, and potential adverse reactions to erythromycin such as nausea, anorexia, or diarrhea. We also asked them to record their temperatures and to report activity levels on the questionnaire for the period of follow-up. Patients reported their activity level on a 4-point categorical scale ranging from “able to carry out usual activities with no difficulty” to “unable to carry out usual activities and needing to spend most of time in bed.” They also recorded how sick they felt on a 4-point scale ranging from “not sick at all” to “extremely sick.” Patients completed their first diary form at the enrollment visit.
We called patients on three occasions after enrollment to encourage continued diary completion, to inquire about compliance with the study medication regimen, to ask about use of other medications, and to ask whether patients thought they were taking erythromycin or placebo. We asked patients to return for follow-up 2 to 3 weeks after enrollment for repeated cultures. Patients completed the symptom diary again, and we counted their pills to assess compliance. We collected diaries at the follow-up visit and paid each patient $20 for participation.
Laboratory Evaluation
We obtained throat cultures from all patients using double-tipped dry cotton swabs. The Children's Hospital Microbiology Laboratory (Boston, Mass.) grouped β-hemolytic streptococcal isolates using standard techniques. Isolates were identified as group A, B, C, F. G, or other (nongroupable isolates).
Data Analysis
The final study group consisted of all patients who met inclusion criteria and were randomized to active drug or placebo, had a negative culture for GAS, and returned for follow-up. We compared demographic factors, cigarette use, presence or absence of symptoms, severity of sore throat, activity level, physical findings, and culture results (non-GAS subtypes) in the erythromycin and placebo groups. We used χ2 analysis or Fisher's Exact Test, as appropriate for categorical variables, and the Wilcoxon Rank-Sum Test for continuous variables. We defined statistical significance as p < .05.
We compared outcomes using survival analysis. To avoid multiple comparisons and to focus our analysis on outcomes we considered most important, we constructed Kaplan-Meier 15 curves for the following outcomes: time to improvement in sore throat; time to improvement in cough; time to improvement in activity level; and time to improvement in how sick the patient felt in general. We defined patients with severe sore throat as improved if their sore throats became “mild” or “none” by day 6 following study entry and remained “mild” or “none” for at least 3 days. Similarly, we defined patients with mild or moderate sore throat as improved if they had “no sore throat” by day 6 and remained that way for 3 days. We defined the day of improvement as the day on which the patient reported improvement in symptoms using the above criteria. We used similar definitions for improvement in cough and how sick the patient felt. We truncated survival curves at 7 days following enrollment because of our hypothesis that any clinically important difference in symptoms would occur early. Because previous research has shown that early antibiotic treatment of GAS pharyngitis is more effective than when therapy is delayed,16 we hypothesized that those patients enrolling within 2 days of the onset of symptoms (“early presenters”) would achieve more benefit from treatment than those who enrolled later (“late presenters”). We generated separate survival curves for early presenters and late presenters.
To adjust for potential confounding variables, clinical findings associated with group assignment at p < .2 were entered into a Cox proportional hazards model.17 Candidate variables included demographic factors, medical history, upper respiratory infection symptoms, physical examination findings, and culture results as shown in Tables 12. We tested for an interaction between early presentation and treatment effect in the Cox model by including an interaction term for duration of illness and treatment group as well as independent variables for treatment group and duration of illness in the Cox model. The effect of any individual factor or combination of factors was considered to be significant if the p value was less than .05.
Table 1.
Patient Characteristics at Intake (n=186)
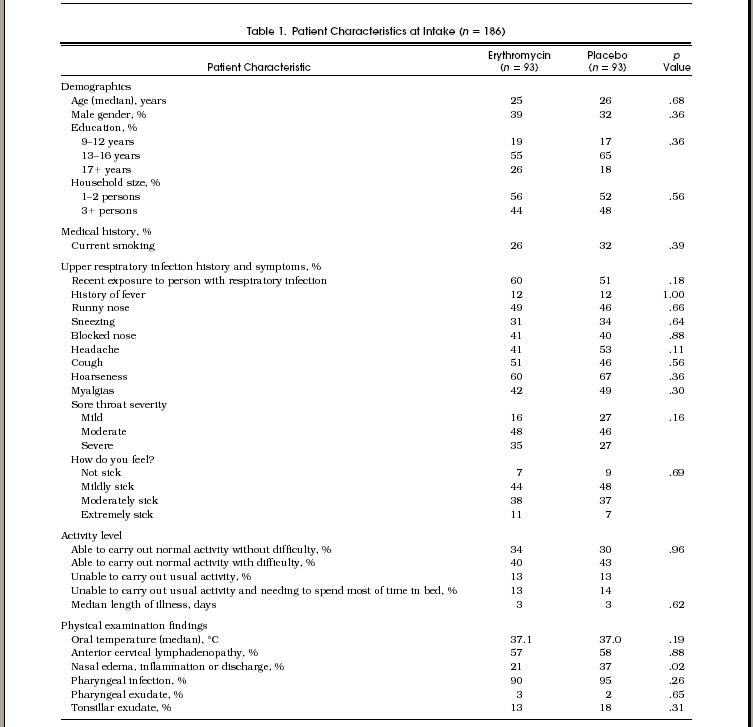
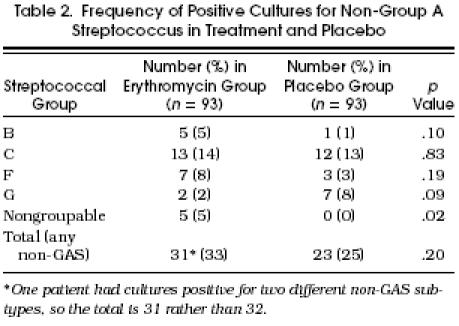
Table 2.
Frequency of Positive Cultures for Non-Group A Streptococcus in Treatment and Placebo
The study protocol was reviewed and approved by Beth Israel Hospital's Committee on Clinical Investigations.
Results
Patient Population
Of 212 randomized patients, 15 were excluded following randomization because their initial throat cultures were positive for GAS. Eleven patients were excluded because they did not return for follow-up and information on the resolution of symptoms was not available. Excluded patients were equally distributed between the treatment groups. Therefore, the number of patients included in the analysis was 186, equally divided between the two groups.
Table 1 shows the characteristics at intake of the two groups. We compared demographic variables, symptom status, use of concomitant medications, smoking history, and physical findings at intake. The average age of the study population was 26.6 years (median 25 years, 25th, 75th percentile = 23, 30 years), and 65% were female. Patients in the two groups were equally distributed among enrollment sites, by season of study entry, and by educational background. Histories of fever and use of over-the-counter medications for symptom relief were equal in the two groups. Patients in the placebo group were more likely to have abnormal nasal findings on physical examination (p = .02). Of 95 patients with a temperature greater than 38°C at intake, 5 (5.4%) were in the erythromycin group and 1 (1.1%) was in the placebo group (p = .10).
Table 2 demonstrates that nearly a third of the patients in the study groups had cultures that were positive for non-GAS. The most common non-GAS groups cultured were C and F. The total number of patients with positive cultures for non-GAS were similar in the two treatment groups (p = .2).
Clinical Outcomes
Of the 186 patients, 179 had sufficient diary data to determine the time to improvement in sore throat as defined above. Patients who took erythromycin improved slightly more rapidly than those who took placebo (hazard ratio 1.43; 95% CI 1.00">hazard ratio 1.43; 95% CI 1.00, 2.03; p = .049; Figure 1). By day 3, 32% of patients taking erythromycin had improved compared with 18% of patients taking placebo. The median time to improvement in the treatment group was 4 days compared with 5 days in the placebo group (25th, 75th percentile = 3, 7 days). Figures 23 demonstrate that most of the benefit was conferred on early presenters (hazard ratio 2.45; 95% CI 1.37, 4.38; p = .003) compared with late presenters (hazard ratio 1.05; 95% CI 0.67, 1.64; p = .25).
Figure 1.
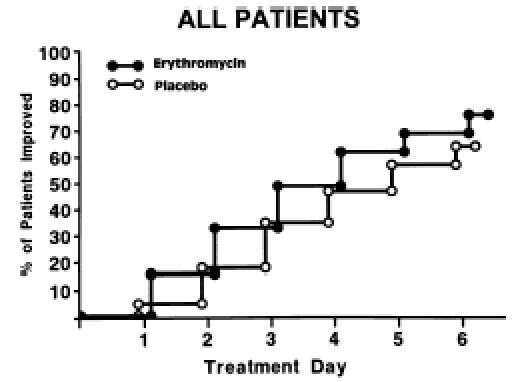
Comparison of days until improvement of sore throat among patients given erythromycin versus placebo (n = 179).
Figure 2.
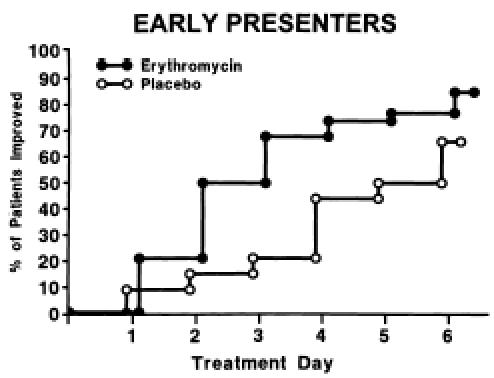
Comparison of days until improvement of sore throat in early presenters (n = 68) for patients given erythromycin versus placebo.
Figure 3.
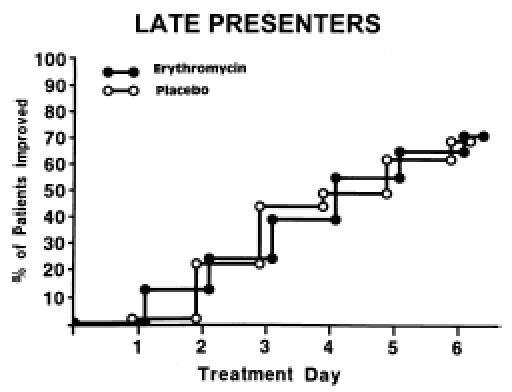
Comparison of days until improvement of sore throat in late presenters (n = 111) for patients given erythromycin versus placebo.
When we included an interaction term for duration of illness prior to enrollment and treatment group in a Cox model, the interaction was significant (p = .025). Adjusting individually for the presence of abnormal nasal findings, sore throat severity, prescription of pseudoephedrine, season of the year, compliance with medication regimen, the presence of non-GAS isolates on initial culture, cough, current smoking, history of fever, lymphadenopathy, or exudate on physical examination did not change the results substantially (i.e., the coefficient associated with treatment group did not change by more than 10% when 9 of these 11 variables were included individually in the model). For the remaining two variables (sore throat severity and temperature at time of enrollment), the coefficient changed by 15% or less. When these latter two variables were included in the model, the p value associated with treatment increased from p = .05 to .08. When we adjusted the model containing the interaction term, the value associated with the interaction term increased from p = .025 to .049.
Of the 186 patients, 87 had sufficient data in the diary for us to assess the time to improvement in cough (n = 41 in placebo group, n = 46 in erythromycin group). Cough improved significantly more rapidly among patients receiving erythromycin (hazard ratio 2.22; 95% CI 1.01, 4.88; p = .05). By day 3, 26% of patients taking erythromycin had improved compared with 17% of patients in the placebo group. Patients who presented within 2 days of illness onset were no more likely to have improvement in their cough with erythromycin treatment than were those who presented later in the illness course. The effect of erythromycin on the resolution of cough was unchanged after adjusting for potential confounders including current smoking, the use of antitussive agents, headache, tonsillar exudate, nasal findings, the presence of either fever or lymphadenopathy or exudate, and the presence of non-GAS by culture.
There was no significant difference between treatment and placebo groups in either time to improvement in activity level or improvement in how sick the patient felt in general.
Compliance with Medication Regimen and Possible Adverse Effects of Treatment
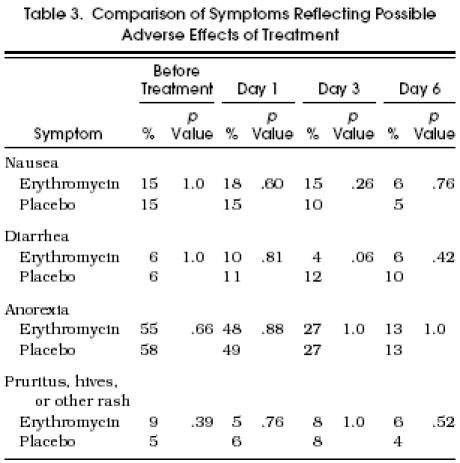
Only 12 of the 186 patients had more than 12 tablets remaining at the time of follow-up, and they were equally distributed between the treatment groups. Throughout the follow-up period, patients reported on potential side effects of treatment including nausea, vomiting, diarrhea, and rash (Table 3). Although patients receiving erythromycin experienced slightly more nausea during the early days of treatment than did patients receiving placebo, this difference was not significant. There were no other significant differences between the two groups in the frequency of other symptoms reflecting possible adverse effects of treatment. Patients’ reports of the treatment they thought they were given (erythromycin vs placebo) were not associated with their actual treatment groups (p > .2).
Table 3.
Comparison of Symptoms Reflecting Possible Adverse Effects of Treatment
Discussion
The benefits of treating pharyngitis caused by GAS are well established. Treatment with antibiotics reduces the attack rate of both suppurative and nonsuppurative sequelae,18 and probably also speeds the resolution of symptoms.16,19,20 Our study assessed the value of a 10-day course of erythromycin in adults with pharyngitis who did not have GAS infection. We found that erythromycin use was associated with a more rapid resolution of sore throat symptoms, primarily in those patients with more recent onset of symptoms. Cough resolved more quickly in patients given erythromycin, but no improvement was seen in their level of activity or in how sick they felt in general. The positive treatment effects we observed were small, and of borderline statistical significance.
Physicians frequently prescribe antibacterial therapy for patients with non-GAS pharyngitis.6,7,21 There is tremendous variation in practice in the United States. Many physicians never obtain throat cultures or order rapid tests for GAS antigen and simply prescribe antibiotics.22 Many other physicians begin antibiotic treatment before culture results become available and may or may not continue antibiotic treatment when the throat culture is negative.22 In addition, patients with pharyngitis frequently request antibiotics.23
A substantial fraction of cases of non-GAS pharyngitis may be caused by nonviral agents. Mycoplasma pneumoniae,9,10,12 Chlamydia species,9,12,14 non–group A β-hemolytic streptococci (particularly groups C and G 11,13,24,25), Arcanobacterium (formerly Corynebacterium) hemolyticum,26,27 Neisseria gonorrhoeae,28 possibly Hemophilus influenzae,29 and other organisms are reported to cause pharyngitis occasionally in adults. Erythromycin acts against many of these organisms, as well as against GAS.
We studied patients who came to several walk-in centers in a large urban area in the northeast region of the United States. They were generally young and healthy. All patients reported a sore throat as the primary complaint. We excluded from the study those patients with coryza and rhinorrhea as their chief complaint in an attempt to minimize the number of patients with acute viral upper respiratory infections.
The results of our study suggest that erythromycin has only a modest effect on symptom duration in adults with non-GAS pharyngitis. In comparison with placebo, erythromycin reduced the median time to improvement of sore throat by 1 day. The beneficial effect of erythromycin was restricted to the subgroup of patients who presented within the first 2 days of their illness. We could not identify any other clinical information that indicated patients who were likely to benefit from treatment. We failed to demonstrate any improvement in patients’ activity level and how sick they felt in general.
Our study is not the first trial of antibiotic use for non-GAS pharyngitis. Marlow et al. performed a small randomized double-blind trial of erythromycin versus placebo in patients aged 12 years and older.30 Erythromycin appeared to decrease the median number of days of feeling ill but did not lead to more rapid resolution of the sore throat itself. McDonald and colleagues performed a randomized controlled study of erythromycin in patients who had had sore throat symptoms for less than 48 hours and had objective evidence of pharyngitis.31 In their study of 98 patients, most symptoms tended to resolve more rapidly with erythromycin therapy, although in only one finding (an overall symptom score) was the shorter duration of symptoms statistically significant. Of interest, McDonald et al. found that the incidence of new illnesses in household members was reduced by the use of erythromycin. These studies, like ours, do not support the widespread use of erythromycin in non-GAS pharyngitis.
There are reasons to be concerned about widespread use of erythromycin in non-GAS illness. Although it may hasten the resolution of pharyngitis, erythromycin sometimes produces adverse gastrointestinal symptoms and might produce other symptoms as well. Expanded use of erythromycin can lead to emergence of erythromycin-resistant organisms. Seppala and colleagues reported the development of resistance of GAS to erythromycin in Finland during a 10-year period in which erythromycin use had tripled.32 Similar effects of widespread use of erythromycin were noted in Japan.33
Our study has a number of important limitations. Because we studied young adults in whom nonviral causes of pharyngitis may be more common, our results may not apply to populations of older adults. Also, although the patients were randomly allocated to the two treatment arms, the randomization did not result in an equal distribution of all potential confounding factors. For example, nasal mucosal inflammation was slightly more frequent in the placebo than in the erythromycin group, and pseudoephedrine was prescribed more frequently in the placebo than in the erythromycin group. We do not think this distorted the results, however, because the relation between treatment group and time to improvement in sore throat symptoms remained significant after we adjusted for these potential confounders using multivariable statistical techniques. Also, because we analyzed the effect of treatment in the subgroup of early presenters in a nonrandomized fashion, we may have biased the results. Although the original data were collected nearly a decade ago, there is little reason to think that the epidemiology of non-GAS pharyngitis or its responsiveness to erythromycin has changed over the past 10 years.34 As the literature suggests that most non-GAS subtypes are susceptible to erythromycin, we did not perform sensitivity testing of non-GAS isolates to erythromycin. A more important limitation of our study was the lack of detailed microbiologic studies that could have supported an underlying premise of the study: namely, that certain potentially treatable, nonviral organisms were responsible for a substantial fraction of cases of non-GAS pharyngitis. Because we lack detailed microbiologic data, the diagnosis of patients responsive to treatment in our study remains uncertain. Of note, we found that the presence of non-GAS by culture was not associated with treatment effect, suggesting that the non-GAS pathogen is not likely to be the primary cause of symptoms in the patients we studied.
In summary, our study showed that adult patients with non-GAS pharyngitis who presented within the first day or two of their illnesses had a modest response of some symptoms to treatment with erythromycin but did not experience a subjective improvement in sense of well-being or return to their usual activity level more quickly than placebo-treated patients. The decision to treat should be based on a number of factors, including the patient's desire for therapy.23 Because widespread use of erythromycin has the potential to promote generation of drug-resistant organisms, and because the benefit we observed was small and of borderline statistical significance, we believe that routine use of erythromycin for patients with non-GAS pharyngitis is not indicated at this time. Our study and others do not offer compelling support for use of erythromycin for routine treatment of non-GAS pharyngitis.30,31
Acknowledgments
The authors thank Joseph Ingelfinger, MD, John Moses, MD, Carmen Alicea, and Linda Hines for their assistance and the PyMah Corp. for contributing Tempa-Dot thermometers for use in the study.
References
- 1.Cypress BK. Office Visits for Disease of the Respiratory System. Hyattsville, Md: U.S. Department of Health, Education and Welfare; 1979. 18. [Google Scholar]
- 2.Finch RG. Epidemiological features and chemotherapy of community-acquired respiratory tract infections. J Antimicrob Chemother. 1990;26(2):53–61. doi: 10.1093/jac/26.suppl_e.53. [DOI] [PubMed] [Google Scholar]
- 3.Hillner BE, Centor RM. What a difference a day makes: a decision analysis of adult streptococcal pharyngitis. J Gen Intern Med. 1987;2:244–50. doi: 10.1007/BF02596449. [DOI] [PubMed] [Google Scholar]
- 4.Komaroff AL, Pass TM, Aronson MD, et al. The prediction of streptococcal pharyngitis in adults. J Gen Intern Med. 1986;1:1–7. doi: 10.1007/BF02596317. [DOI] [PubMed] [Google Scholar]
- 5.Gwaltney JM. In: Principles and Practice of Infectious Disease. New Edition. Mandell GI, Douglas RG Jr, Bennett JE, editors. New York, NY: Churchill Livingstone; 1990. pp. 493–8. Pharyngitis. In: , eds. [Google Scholar]
- 6.Hoffman S. Incidence and management of sore throat in general practice. Scand J Prim Health Care. 1986;4(2):143–50. doi: 10.3109/02813438609014821. [DOI] [PubMed] [Google Scholar]
- 7.Herz MJ. Antibiotics and the adult sore throat—an unnecessary ceremony. Fam Pract. 1988;5(2):196–9. doi: 10.1093/fampra/5.3.196. [DOI] [PubMed] [Google Scholar]
- 8.Slawson DC, Baer LJ, Richardson MD. Antibiotic use in the treatment of non-streptococcal pharyngitis. Fam Med. 1991;23(2):198–201. [PubMed] [Google Scholar]
- 9.Komaroff AL, Aronson MD, Pass TM, Ervin CT, Branch WT, Jr, Schachter J. Serologic evidence of chlamydial and mycoplasmal pharyngitis in adults. Science. 1983;222:927–8. doi: 10.1126/science.6415813. [DOI] [PubMed] [Google Scholar]
- 10.Glezen WP, Clyde WA, Senior RJ, Sheaffer CI, Denny FW. Group A streptococci, mycoplasmas and viruses associated with acute pharyngitis. JAMA. 1967;202:119–24. [PubMed] [Google Scholar]
- 11.Gerber MA, Randolph MF, Martin NJ, et al. Community-wide outbreak of group G streptococcal pharyngitis. Pediatrics. 1991;87:598–603. [PubMed] [Google Scholar]
- 12.Huovinen P, Lahtonen R, Ziegler T, et al. Pharyngitis in adults: the presence and coexistence of viruses and bacterial organisms. Ann Intern Med. 1989;110(2):612–6. doi: 10.7326/0003-4819-110-8-612. [DOI] [PubMed] [Google Scholar]
- 13.Turn JC, Hayden GF, Kisellica D, Lohr J, Fishburne CF, Murren D. Association of group C beta-hemolytic streptococci with endemic pharyngitis among college students. JAMA. 1990;264(2):2644–7. [PubMed] [Google Scholar]
- 14.Grayston JT, Kuo CC, Wang SP, Altman J. A new Chlamydia psit-taci strain, TWAR, isolated in acute respiratory tract infections. N Engl J Med. 1986;315(2):161–8. doi: 10.1056/NEJM198607173150305. [DOI] [PubMed] [Google Scholar]
- 15.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York, NY: Wiley; 1980. [Google Scholar]
- 16.Brink WR, Rammellkamp CH, Jr, Denny FW, Wannamaker LW. Effect of penicillin and aureomycin on the natural course of streptococcal tonsillitis and pharyngitis. Am J Med. 1951;10:300–8. doi: 10.1016/0002-9343(51)90274-4. [DOI] [PubMed] [Google Scholar]
- 17.Cox DR. Regression Methods and life tables (with discussion) J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 18.Peter G, Smith AI. Group A streptococcal infections of the skin and pharynx. N Engl J Med. 1977;297:365–70. doi: 10.1056/NEJM197708182970706. [DOI] [PubMed] [Google Scholar]
- 19.Haight TH. Erythromycin therapy of respiratory infections, I: controlled studies on the comparative efficacy of erythromycin and penicillin in scarlet fever. J Lab Clin Med. 1954;43:15–30. [PubMed] [Google Scholar]
- 20.Nelson JD. The effect of penicillin therapy on the symptoms and signs of streptococcal pharyngitis. Pediatr Infect Dis. 1984;3:10–13. doi: 10.1097/00006454-198401000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Berger PC, Elford RW, Yeo M, Cimolai N, Anand CM. Pharyngitis 1987: a survey of physicians’ attitudes and practices in southern Alberta. Can J Public Health. 1989;80(2):38–41. [PubMed] [Google Scholar]
- 22.Cochi SL, Fraser DW, Hightower AW, Facklam RR, Broome CV. In: Pharyngitis: Management in an Era of Declining Rheumatic Fever. Shulman ST, editor. New York, NY: Praeger Press; 1984. pp. 73–94. Diagnosis and treatment of streptococcal pharyngitis: survey of US medical practitioners. [Google Scholar]
- 23.Herman JM. Patients’ willingness to take risks in the management of pharyngitis. J Fam Pract. 1984;19:767–72. [PubMed] [Google Scholar]
- 24.Benjamin JT, Perriello VA. Pharyngitis due to group C hemolytic streptococci in children. J Pediatr. 1976;89:254–6. doi: 10.1016/s0022-3476(76)80459-3. [DOI] [PubMed] [Google Scholar]
- 25.McCue JD. Group G streptococcal pharyngitis: an analysis of an outbreak at a college. JAMA. 1982;248:1333–6. doi: 10.1001/jama.248.11.1333. [DOI] [PubMed] [Google Scholar]
- 26.Banck G, Nyman M. Tonsillitis and rash associated with Corynebacterium hemolyticum. J Infect Dis. 1986;154:1037–40. doi: 10.1093/infdis/154.6.1037. [DOI] [PubMed] [Google Scholar]
- 27.Miller RA, Brancato F, Holmes KK. Corynebacterium hemolyticum as a cause of pharyngitis and scarlatiniform rash in young adults. Ann Intern Med. 1986;105(2):867–72. doi: 10.7326/0003-4819-105-6-867. [DOI] [PubMed] [Google Scholar]
- 28.Komaroff AL, Aronson MD, Pass TM, Ervin CT. Prevalence of pharyngeal gonorrhea in general medical patients with sore throats. Sex Transm Dis. 1980;7:116–9. doi: 10.1097/00007435-198007000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Kroneman OC, Brody H. Hemophilus influenzae pharyngitis and cellulitis in adults. J Fam Pract. 1980;11(2):865–7. [PubMed] [Google Scholar]
- 30.Marlow RA, Torrez AJ, Jr, Haxby D. The treatment of nonstreptococcal pharyngitis with erythromycin: a preliminary study. Fam Med. 1989;21(2):425–7. [PubMed] [Google Scholar]
- 31.McDonald CJ. A controlled trial of erythromycin in adults with nonstreptococcal pharyngitis. J Infect Dis. 1985;152(2):1093–4. doi: 10.1093/infdis/152.5.1093. [DOI] [PubMed] [Google Scholar]
- 32.Seppala H, Nissinen A, Huovinen S, et al. Resistance to erythromycin in group A streptococci. N Engl J Med. 1992;30(2):292–7. doi: 10.1056/NEJM199201303260503. [DOI] [PubMed] [Google Scholar]
- 33.Maruyama S, Yoshioka H, Fujita K, Takimoto M, Satake Y. Sensitivity of group A streptococci to antibiotics. Am J Dis Child. 1979;133:1143–5. doi: 10.1001/archpedi.1979.02130110051007. [DOI] [PubMed] [Google Scholar]
- 34.Kechrid A, Aissa N, Bouzouaia N, Ben Hassan A, Fendri C, Ben Redjeb S. Septicemia caused by A, B, C and G groups of beta hemolytic streptococci. Presse Med. 1993;22:896–8. [PubMed] [Google Scholar]


