Abstract
Migrating cells are polarized with a protrusive lamella at the cell front followed by the main cell body and a retractable tail at the rear of the cell. The lamella terminates in ruffling lamellipodia that face the direction of migration. Although the role of actin in the formation of lamellipodia is well established, it remains unclear to what degree microtubules contribute to this process. Herein, we have studied the contribution of microtubules to cell motility by time-lapse video microscopy on green flourescence protein-actin- and tubulin-green fluorescence protein–transfected melanoma cells. Treatment of cells with either the microtubule-disrupting agent nocodazole or with the stabilizing agent taxol showed decreased ruffling and lamellipodium formation. However, this was not due to an intrinsic inability to form ruffles and lamellipodia because both were restored by stimulation of cells with phorbol 12-myristate 13-acetate in a Rac-dependent manner, and by stem cell factor in melanoblasts expressing the receptor tyrosine kinase c-kit. Although ruffling and lamellipodia were formed without microtubules, the microtubular network was needed for advancement of the cell body and the subsequent retraction of the tail. In conclusion, we demonstrate that the formation of lamellipodia can occur via actin polymerization independently of microtubules, but that microtubules are required for cell migration, tail retraction, and modulation of cell adhesion.
INTRODUCTION
Cell motility plays a central role in a variety of biological processes, including embryonic development, wound healing, and tumor cell metastasis (Lauffenburger and Horwitz, 1996; Hangan et al., 1997; Shattil and Ginsberg, 1997; Montell, 1999). The driving force for cell migration is directed by the reorganization of the actin cytoskeleton, which includes the protrusion of the lamellipodium at the cell front and the retraction of the cell rear. The protrusion of the lamellipodium is provided by continuous growth of actin filaments toward the leading edge of the lamellipodium, the retraction of the rear is regulated by the release of adhesive contacts from extracellular matrix proteins (Lauffenburger and Horwitz, 1996).
Microtubules have been suggested to play a role in regulating cell migration because destruction of microtubules in fibroblasts resulted in inhibition of protrusive lamellipodial activity (Vasiliev and Gelfand, 1976; Bershadsky et al., 1991). More recently there has been evidence to suggest that microtubules regulate adhesive or protrusive events through pathways involving the small GTPases Rho and Rac (Nobes and Hall, 1999; Waterman-Storer and Salmon, 1999). Rho induces assembly of stress fibers and focal contacts, and Rac activates actin-dependent lamellipodium formation and ruffling (Ridley and Hall, 1992b; Ridley et al., 1992). It has been shown that disrupting microtubules led to Rho activation, which resulted in an increased size of focal contacts and enhanced phosphorylation of paxillin and focal adhesion kinase (Bershadsky et al., 1996; Enomoto, 1996). Direct targeting of microtubules to focal contacts followed by their dissociation from the substrate has recently been demonstrated in fibroblasts, and it was hypothesized that microtubules deliver a relaxing impulse to substrate contacts, thus facilitating the turnover of adhesive contact sites (Kaverina et al., 1999).
Other studies indicate that microtubules exert their control on the reorganization of the actin cytoskeleton via a Rac-dependent pathway at the cell front. Rac1-guanosine 5′-triphosphate (GTP) has been shown to bind to tubulin dimers (Best et al., 1996), and hence it was proposed that the polymerization of microtubules at the cell front liberates Rac1-GTP, thereby inducing actin polymerization (Waterman-Storer et al., 1999). Furthermore, it has been shown that the growth of microtubules induced in fibroblasts after the removal of the microtubule disrupter nocodazole activates Rac1 GTPase. Waterman-Storer and Salmon (1999) suggested a model of positive feedback interactions between microtubules and actin. In this model the authors propose that microtubule disassembly in the main cell body activates RhoA, which is responsible for stress fiber and focal contact formation and contraction of the cell. In contrast, microtubule assembly at the leading edge results in Rac1 activation and lamellipodium formation (Waterman-Storer and Salmon, 1999; Waterman-Storer et al., 1999).
However, this model does not address the following questions: Can microtubules regulate the activation of Rac1 induced by external signals such as growth factors? Do microtubules influence cell migration by regulation of cell adhesion? Are microtubules implicated in mechanisms of tail retraction? To address these issues, we used B16 melanoma cells and Melb-a melanoblasts. In contrast to fibroblasts these cells are highly motile with a high frequency of lamellipodia formation. Green fluorescence protein (GFP)-actin- or tubulin-GFP–transfected cells were used for time lapse experiments to visualize cytoskeletal reorganization. Lamellipodial and ruffling events were quantified by kymograph analysis. Phorbol 12-myristate 13-acetate (PMA) was used to induce cell motility in B16 cells, and stem cell factor (SCF) was used for melanoblasts.
MATERIALS AND METHODS
Cell Lines, Plasmids, and Reagents
Mouse melanoma cells (B16F1) were kindly provided by G. Nicholson (M.D. Anderson Cancer Center, Houston, TX); melb-a melanoblasts were from Dr. Dot Bennett (St. George Hospital, London, UK) (Sviderskaya et al., 1995). B16 cells were grown in DMEM (Life Technologies, Paisley, Scotland) supplemented with 10% fetal calf serum (FCS) (PAA Laboratories, Linz, Austria), 2 mM glutamine, 100 international units/ml penicillin, and 100 μg/ml streptomycin (= complete medium; all Life Technologies). Melb-a cells were grown in complete RPMI additionally supplemented with stem cell factor (SCF; 20 ng/ml) and basic fibroblast growth factor (20 ng/ml).
The construction of the GFP-actin plasmid has been described elsewhere (Ballestrem et al., 1998). The original β5 tubulin-GFP plasmid was kindly provided by Dr. Matus (FMI, Basel, Switzerland) and was modified as follows: to enhance tubulin-GFP expression, the promoter region was replaced by a longer form of the β-actin promoter containing serum response elements as described for the GFP-actin construct (Ballestrem et al., 1998). Plasmids containing myc-tagged Rac1 were kindly provided by Dr. Ballmer-Hofer (Paul Scherrer Institute, Villigen, Switzerland).
Human fibronectin (FN) was purchased from collaborative Biomedical Products (Bedford, MA). Taxol (paclitaxel) and nocodazole were purchased from Sigma (Sigma Chemical Co., St. Louis, MO). Rhodamine-phalloidin was obtained by Fluka (Buchs, Switzerland); antibodies against vinculin (clone V-9131) or tubulin (clone T-5168) were obtained from Sigma; 9E-10 antihuman myc hybridoma was from American Type Culture Collection.
Transfections
Transient and stable protein expression was obtained by transfection of cells with Fugene 6 (Roche, Basel, Switzerland) according to the manufacturer's recommendation. Briefly, 2.5 μg of plasmid DNA and 3 μl of Fugene 6 were incubated for 15 min in 100 μl of OPTIMEM (Life Technologies). This solution was added to cells at 30–60% confluence cultured in complete DMEM in a 35-mm tissue culture plate (Falcon, Becton Dickinson, Basel, Switzerland). After 10 h cells were detached by trypsinizing, washed with phosphate-buffered saline (PBS), and transferred to a 10-cm culture dish. Stable clones were obtained by treatment of cells with 1.5 mg/ml G418 (Geneticin; Life Technologies). For experiments with rac1, transiently transfected cells were plated onto FN (5 μg/ml)-coated glass coverslips. Two days after transfection, expression of the construct was evaluated by fluorescence microscopy by using an anti-myc antibody.
Time-Lapse Studies
Time-lapse studies were preformed as described previously (Ballestrem et al., 1998). B16 cells were detached from plastic tissue culture plates by trypsin/EDTA treatment for 5 min, washed twice in complete DMEM, and plated in Ham's F12 containing 10% FCS on glass coverslips previously coated with 5 μg/ml FN. After a 4- to 12-h incubation cells were treated as indicated with nocodazole (10 μg/ml final), taxol (10 μM final), PMA (100 ng/ml final), or the combination of taxol/PMA, nocodazole/PMA.
SCF-induced lamellipodium formation was analyzed in c-kit expressing melb-a cells plated on serum-coated glass coverslips. After overnight culture in complete medium supplemented with 20 ng/ml SCF, cells were serum and SCF starved for 4 h prior to treatment with nocodazole (10 μg/ml) or taxol (10 μM). One hour later SCF (final concentration of 50 ng/ml) was added to induce lamellipodium formation and images were recorded at 1-min intervals.
Living cells were observed under an inverted fluorescent microscope (Zeiss-Axiovert 100) equipped with Plan-Neofluar 40×, 63×, 100× fluar oil immersion objectives (Zeiss, Oberkochen, Germany), and an incubation chamber for constant temperature and CO2 regulation. GFP fluorescence was visualized by using a fluorescein isothiocyante filter set (450-490, FT 510, LP 520). Single or time-lapse pictures were acquired with a Hamamatsu C4742-95-10 digital charge-coupled device camera (Hamamatsu Photonics, Shizuoka, Japan) controlled by the Openlab software (Improvision, Oxford, UK). For time-lapse recordings, cells expressing GFP-constructs were kept at constant temperature of 37°C and 10% CO2.
Fluorescence Microscopy
B16 cells were cultured on FN (5 μg/ml)-coated glass coverslips at 37°C and 10% CO2 for indicated periods of time. Cells were then fixed with 4% paraformaldehyde for 10 min at room temperature. After 3 washes with PBS cells were permeabilized with 0.5% Triton X-100 in PBS for 5 min and washed again. For actin staining, cells were subsequently incubated with 100 nM Rhodamine-Phalloidin (Fluka) at room temperature; for tubulin staining and myc staining, cells were incubated with primary antibody dilutions of 1/400 in PBS/1% BSA for 30 min. Samples were then washed three times with PBS followed by staining with the secondary fluorescein isothiocyante- or Texas-Red–linked antibody diluted in PBS/1% BSA. For two-color staining with rhodamine-phalloidin and anti-myc antibody, the cells were always stained first with the phalloidine-coupled dye. After three final washes with PBS, cells were analyzed by using a Zeiss-Axiovert 100 microscope.
Quantification of Lamella Dynamics by Kymograph Analysis
B16 cells were seeded at 20,000 cells/ml in glass chambers coated with 5 μg/ml FN. Cells were grown for 18 h in DMEM/10% FCS and shifted to carbonate-free complete F12 medium 2 h prior to commencement of experiments. Cells were then observed under an inverted microscope (Zeiss, Jena, Germany), equipped with a 63× 1.4 NA Ph3 plan apochromat objective. Cell movements were monitored with a low-light video camera (AVT Horn BC-5, Aalen, Germany). Taxol (10 μM final concentration) and nocodazole (10 μg/ml final concentration) were added 60 min, and PMA (100 ng/ml final concentration) 15 min prior to the start of recording.
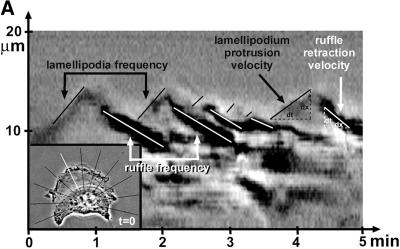
To quantify lamella dynamics, phase contrast images of living B16 cells were digitized by using a video frame grabber card and analyzed by computer-assisted stroboscopic analysis (SACED) as recently described (Hinz et al., 1999).
To monitor dynamics of isolated regions of the cell, the area of interest was selected on the phase contrast image. This area was digitally recorded, producing a gray value image with the width of one pixel encompassing structures along a single line drawn transversally over the cell edge. Dynamics of this selected cell region was studied at intervals of 1 s over the course of 5 min. The digital snapshots were lined up on a time scale in order of their acquisition. The resulting composite phase contrast picture allowed us to continuously follow the translocation of recorded structures over time. In total 11 lines/cell was created, resulting in stroboscopic images randomly distributed across the entire cell perimeter (Figure 6A). The described process was automated by KS 400 software (Zeiss). Ruffles were identified by their dark gray appearance and characteristic centripetal movement, beginning at the lamella edge; protruding cell edges and retracting ruffles were marked (Figure 6A). The main parameters characterizing cell motility were the velocity of lamellipodium protrusions, ruffle retraction rate (μm/min), and the frequency of these events (min−1). Mean values were calculated from 15 cells/condition and analyzed by SACED on 11 lamella regions/cell. At least five independent experiments were performed to calculate mean values (± SD). To determine significant differences between averages, unpaired t tests assuming equal variance were performed, and differences were considered as significant when p < 0.01.
Figure 6.
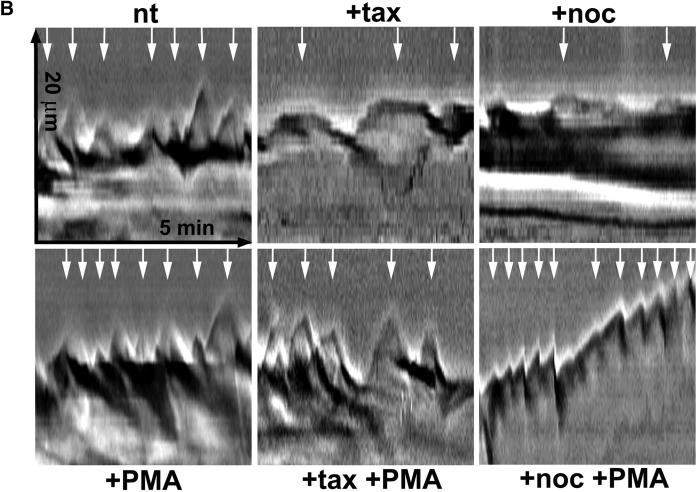
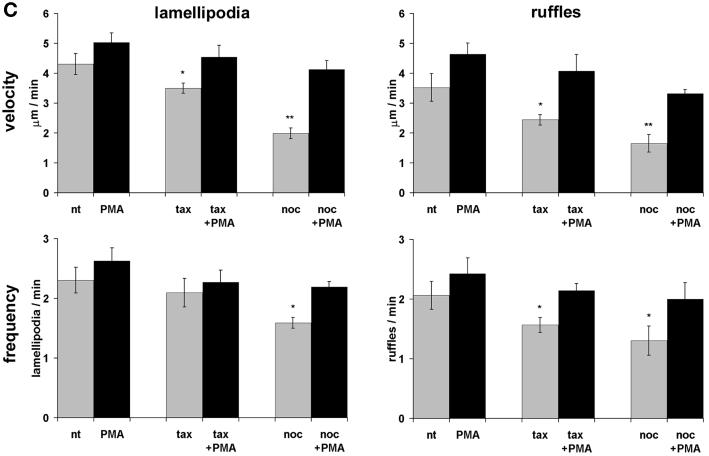
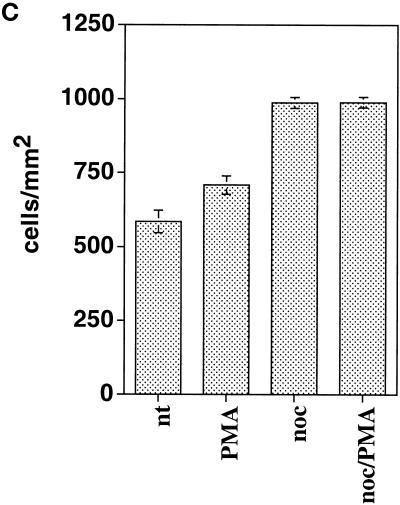
Kymograph analysis. (A) Motility of B16 melanoma cells was analyzed by measuring cell edge movements along regions of interest (insert, white line). Movements at these regions (20-μm line) were recorded in 1-s intervals for a period of 5 min. Pictures were assembled resulting in a stroboscopic image, which displays lamellipodia protrusion (black lines) and ruffle retraction (white lines). Lamellipodia protrusion velocity and ruffle retraction rate in these time/space images are indicated by the ascent (dx/dt, μm/min) of protrusive or retracted structures with notable gray values; frequencies are measured counting the number of lamellipodia or ruffles per minute (1/period, min−1). (B) Stroboscopic images under different conditions. Taxol (+tax) and nocodazole (+noc) treatment for 60 min reduced velocity and frequency of lamellipodia protrusions and ruffle retractions. Protrusions and retractions are indicated by white arrows. Cell edge motility was enhance by PMA (+PMA); stimulation with PMA restored the motility of cells that were pretreated with taxol (+tax +PMA), or nocodazole (+noc +PMA). (C) Quantification of B16 cell motility by kymograph analysis. B16 melanoma cells were left untreated (nt) or were treated with taxol (tax), nocodazole (noc), or/and stimulated with PMA. Cell motility was recorded by SACED. The following cell motility parameters were quantified from stroboscopic images: 1) lamellipodia protrusion velocity, 2) ruffle retraction velocity (retraction rate), 3) lamellipodia frequency, and 4) ruffle frequency. Compared with nt cells, noc and tax decreased cell motility. Stimulation by PMA restored lamellipodia and ruffle velocity and frequency of cells pretreated with taxol (tax +PMA) or nocodazole (noc +PMA). At least 15 cells were analyzed per experimental condition. Error bars indicate SD of mean values, calculated from five independent experiments. p ≤ 0.01, p ≤ 0.001 compared with untreated control B16 cells..
Cell Migration Assay
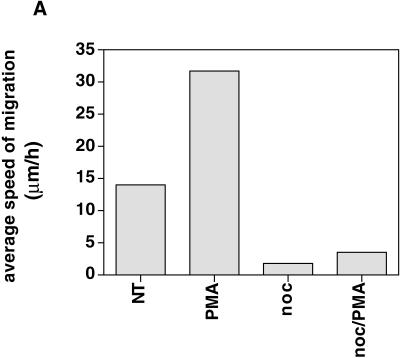
B16 melanoma cells were plated at a density of 5000 cells/well (20% confluency) on serum-coated 24-well culture dishes and incubated overnight in complete medium at 37°C. Cells were placed under an Axiovert 100TV (Zeiss) inverted microscope equipped with an incubation chamber and a 10× objective (CP-Achromat 10×/0.25 Ph1 Var1). The distance of cell migration was measured from recordings over 2 h by using Openlab software. From measurements of all cells (n > 50) in the field covered by the objective, the average speed of migration per cell per hour was calculated for each indicated condition. The average speed from one representative experiment (n = 5) is shown in Figure 9A.
Figure 9.
(A) B16 cell migration is inhibited upon disruption of microtubules. Cells plated on serum-coated plastic dishes were treated with control medium (nt), 100 ng/ml PMA, 10 μg/ml nocodazole, or nocodazole and PMA. The migration distance of at least 50 cells/condition was measured and calculated as average speed of migration per hour (μm/h). The histogram represents one of five independent experiments with similar results. (B) Cell fragment migration. GFP-actin–transfected B16 cells were plated on FN and were subsequently treated with nocodazole and PMA. Time-lapse images show the separation of a part of the cell from the main cell body. The advancing cell fragment leaves a trace of actin-containing membrane behind (arrowhead). Bar, 15 μm. (C) Adhesion of B16 melanoma cells to FN (2.5 μg/ml) is significantly enhanced by addition of nocadozole or the combination of nocadozole with PMA. Stimulation of B16 with PMA alone leads only to little increase in adhesion.
Adhesion Assays
Cell adhesion assays on FN were performed as described previously (von Ballestrem et al., 1996) with slight modifications. Briefly, cells were trypsinized, washed once in complete DMEM, and stained with calcein according to manufacturer's recommendations (Molecular Probes, Eugene, OR). After two washes with RPMI/1% BSA, 5 × 104 cells were added to each well coated with the indicated concentrations of matrix proteins. Cells were allowed to spread for 1 h prior to treatment with taxol, nocodazole, PMA, or combinations of taxol/PMA, nocodazole/PMA at the indicated final concentrations. After 1 h incubation for taxol- and nocodazole-treated cells, 30 min for PMA treatment (combination taxol/PMA, nocodazole/PMA: 1 h taxol or nocodazole followed by 30 min taxol/PMA or nocodazole/PMA) the plate was washed a minimum of three times with 200 μl of prewarmed RPMI/1% BSA. Adherent fluorescent cells were measured by using a Cytofluor fluorescence reader (Stehlin, Basel, Switzerland). Cell adhesion was enumerated as cells bound per unit area based on the fluorescence measured for the total input (50,000 cells/well) of calcein-labeled cells. Adhesion to BSA-coated wells was used as control to provide the background fluorescence, which was subtracted from both, the bound and total input fluorescence.
RESULTS
Microtubule Dynamics in Migrating Melanoma Cells
Migrating cells show a large protruding lamellipodium followed by the lamella and the main cell body. To investigate the contribution of microtubules to lamellipodia formation during migration, melanoma cells were stably transfected with β5-tubulin-GFP and plated on FN. B16 melanoma cells were used for our studies because of their high motility in comparison to the slowly migrating fibroblasts or epithelial cells. Using time-lapse fluorescence microscopy we show that the dynamic behavior of microtubules in the lamella of migrating B16 cells was similar to that observed by other studies with epithelial cells (Waterman-Storer and Salmon, 1997; Wadsworth, 1999).
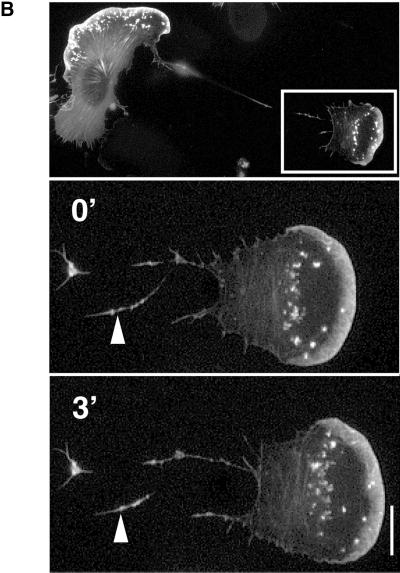
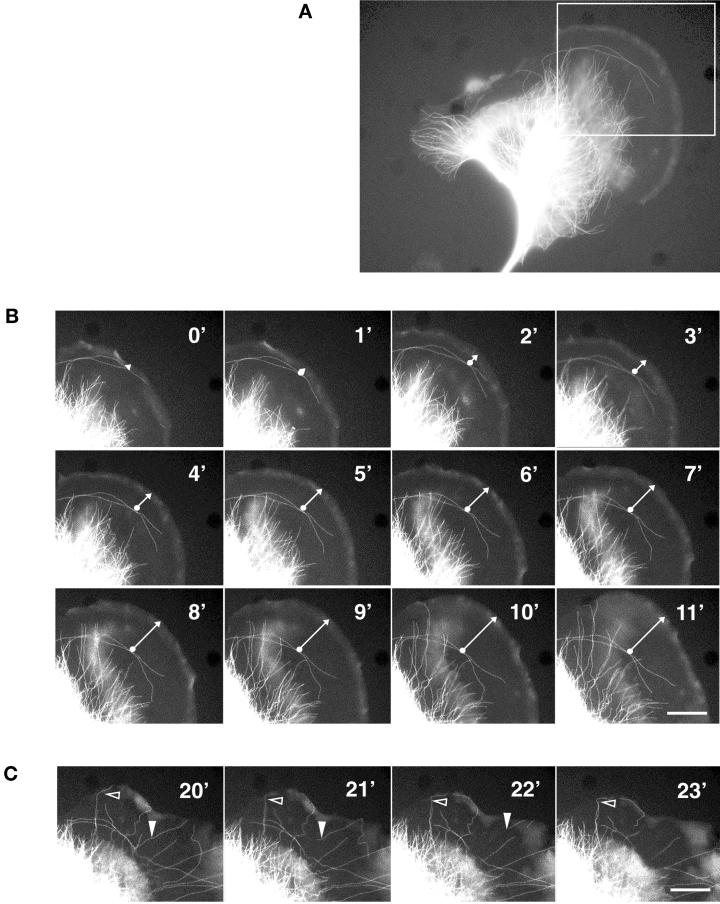
A typical illustration of the distribution of microtubules in a lamella of a migrating melanoma cell is shown in Figure 1. The main cell body contains a dense network of microtubules, whereas the lamella is almost devoid of filamentous tubulin (Figure 1A). The protruding leading edge can be identified by the presence of unpolymerized tubulin-GFP versus the polymerized form. Time-lapse fluorescence microscope images of this cell were taken at 1-min intervals (Figure 1, B and C). A few rare microtubules are visible along the rear of the lamellipodium (Figure 1B, 0′-3′). These tubules become stabilized and stationary while the lamellipodium continues to advance; hence the lamellipodium and part of the lamella advance although they contain no microtubules (Figure 1B, 3′-11′). The tubules of the cell body grow perpendicular to the lamellipodium but remain at a constant distance of ∼10 to 15 μm from the protruding leading edge (Figure 1B, 0′-11′). Generally, we observe that the distance between the perpendicular growing microtubules and the leading edge becomes larger at higher speed of cell migration. When the speed of lamellipodium protrusion slows down, many microtubules grow until they reach the edge of the cell (Figure 1C, open arrowhead). They then become stabilized and eventually depolymerize from the rear end of the microtubule (Figure 1B, filled arrowhead).
Figure 1.
Dynamics of microtubules in a migrating melanoma cell. (A) Distribution of microtubules in a migrating B16 melanoma cell transfected with β5-tubulin-GFP. Note that the lamella is almost devoid of microtubules. (B) Consecutive fluorescence micrographs of the protruding lamella and lamellipodium were taken at 1-min intervals and show the dynamics of microtubules in the advancing lamella. Microtubules perpendicular to the leading edge barely enter the lamella, whereas microtubules growing parallel to the lamellipodium remain fixed with respect to the substrate. The arrow indicates the increasing distance between microtubules oriented parallel to the lamellipodium and the protruding leading edge. (C) As in B after stop of lamellipodium protrusion. Many microtubules reach the leading edge where they become stabilized (empty arrowhead) and eventually depolymerize from the minus end in the perinuclear region (filled arrowhead). Bar, 15 μm.
Are Microtubules Involved in Regulation of Ruffling and Formation of Lamellipodia?
Several studies have proposed a role for microtubules in mediating signals, which control cell migration. Because it became evident that part of the lamella in migrating cells was devoid of microtubules, we wished to investigate whether microtubule assembly would be necessary for actin-dependent ruffling and lamellipodium formation.
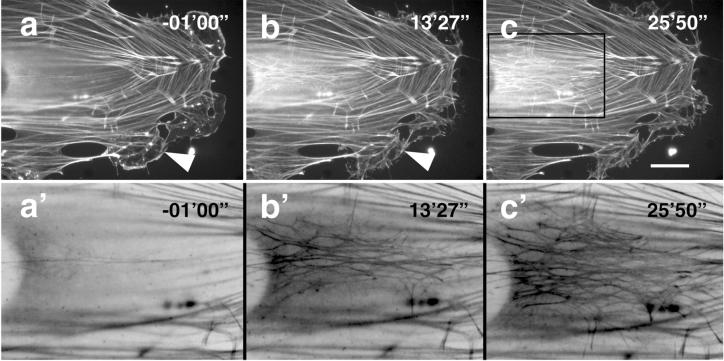
Treatment of cells with 10 μg/ml of the microtubule-disrupting reagent nocodazole resulted in a loss of essentially all polymerized tubulin filaments within 30 min (our unpublished results). To examine the effect of microtubule disruption on the dynamics of the actin cytoskeleton, GFP-actin–transfected cells were plated on coverslips and time-lapse images were recorded after the addition of nocodazole at time 00′00". Prior to microtubule disruption the stationary cell exhibited prominent stress fibers and some lamellipodial extensions (Figure 2, arrows in –01′00"). At time point 13′27" these extensions were retracted and new actin stress fibers and focal contacts were formed in the middle of the cell (Figure 2, bottom time frame 13′27" and 25′50"). These results are similar to those obtained by other groups by using fibroblasts (Danowski, 1989; Bershadsky et al., 1991), and clearly suggest that microtubules are involved in the regulation of ruffling and the formation of lamellipodia.
Figure 2.
Actin dynamics in B16 cells during disruption of microtubules. GFP-actin–transfected cells were plated on FN and treated with 10 μg/ml nocodazole at time 00′00". Images at time 13′27" and 25′50" demonstrate the inhibition of cell edge ruffling, (filled arrowheads) and the formation of new stress fibers and focal contacts (inserts). A′–c′) are high-power images of a–c, respectively, the depicted region is indicated in c. Improved visibility of the newly formed dense actin network was achieved by inverting colors. Bar, 10 μm.
PMA-induced Ruffling Is Rac-dependent
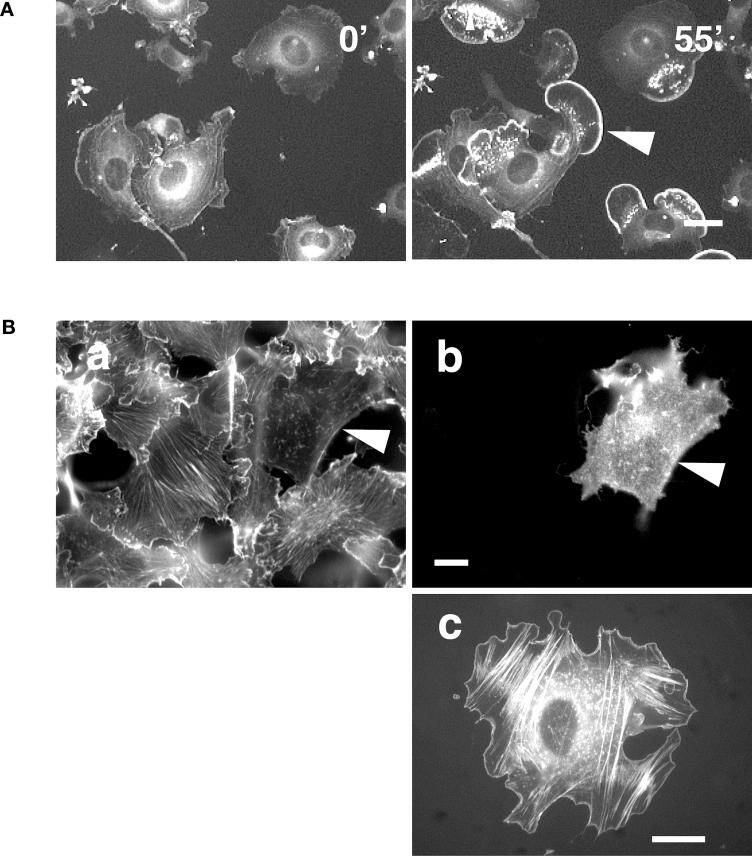
Our findings thus far confer with those obtained in fibroblasts demonstrating that disruption of microtubules leads to inhibition of spontaneous ruffling and lamellipodium formation. It was therefore interesting to investigate whether this disruption also would inhibit stimulation-induced ruffling and lamellipodium formation. Because PMA has been shown to induce the reorganization of the actin cytoskeleton (Schliwa et al., 1984; Bershadsky et al., 1990; Downey et al., 1992), we used this agent to study ruffle and lamellipodium formation in the presence or absence of microtubules. Upon PMA treatment of GFP-actin–transfected cells, it was possible to induce extensive ruffling and lamellipodium formation (Figure 3A). One hour after addition of PMA all cells showed ruffling or lamellipodia (Figure 3A, 55′).
Figure 3.
Lamellipodium formation after stimulation with PMA is Rac1 dependent. (A) GFP-actin–transfected B16 cells were plated on FN and treated with 100 ng/ml PMA at time 0′. Cells show intense ruffling and lamellipodium formation 55′ after addition of PMA (arrowheads). Bar, 40 μm. (B) B16 melanoma cells transiently transfected with N17Rac (dominant negative) were plated on FN and stimulated with 100 ng/ml PMA for 30 min. Fixed cells were stained for actin (a) and double labeled for N17Rac expression (b). In contrast to nontransfected cells, the N17Rac-transfected cell does not show stress fibers and lamellipodia (a, b). Bar, 20 μm. B16 cell cotransfected with GFP-actin and L61Rac (constitutively active) plated on FN (c) exhibits stress fibers and a smooth rim around the cell edge. Bar, 20 μm.
Because the small GTPase Rac is reportedly responsible for ruffling and lamellipodium formation in cells (Hall, 1998), we examined whether our PMA-induced ruffling is Rac dependent. Therefore, B16 cells were transiently transfected with a dominant-negative form of Rac (N17Rac). Cells were plated 5 h after transfection on FN-coated coverslips and treated 42 h later with PMA to induce ruffling. Thirty minutes after treatment, cells were fixed and stained for actin, and N17Rac expression. As shown in Figure 3B (a, b), N17Rac-transfected cells had no lamellipodia. In contrast, nontransfected cells showed an actin-rich rim, indicating active wild-type Rac in these cells. As a further control B16 cells were doubly transfected with a constitutively active form of Rac (L61Rac) and GFP-actin. These cells also showed an actin-rich rim similar to those treated with PMA (Figure 3B, c). The cells transfected with a constitutively active form of Rac no longer responded to PMA treatment, confirming that signals mediated by PMA are upstream of Rac.
Are Microtubules “Essential” for Ruffling and Formation of a Lamellipodium?
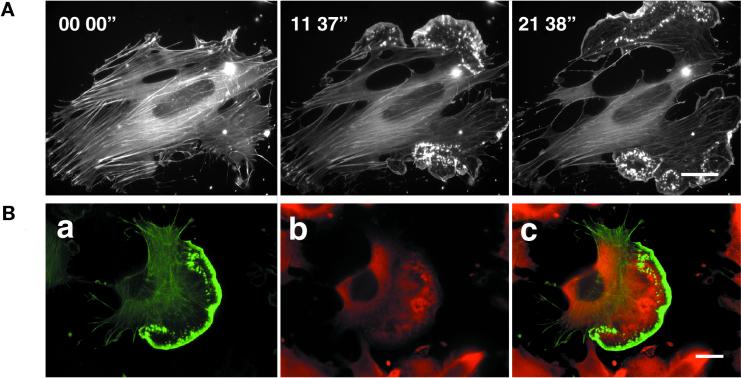
To investigate whether PMA treatment is still able to induce ruffle formation in cells devoid of microtubules, cells pretreated with 10 μg/ml nocodazole, were stimulated with 100 ng/ml PMA and time lapse images were recorded. Nocodazole-treated cells contained prominent stress fibers and showed no ruffles and lamellipodia (Figure 4A, time 00′00"). Within 5 min of application of PMA, however, ruffles began to develop, which extended in the direction of their leading edges to form large lamellipodia (Figure 4A, time 05′00"-21′38"). Despite extensive actin polymerization at the cell periphery, focal contacts and stress fibers remained stable. Consequently, the cell edges advanced tearing the cell apart. PMA stimulation in the absence of nocodazole lead to the expected lamellipodium formation and subsequent displacement of the cell (our unpublished results). At the completion of experiments conducted in presence of nocodazole and PMA, GFP-actin cells were fixed and stained for tubulin, revealing the absence of microtubules throughout the entire cell (Figure 4B).
Figure 4.
PMA-induced lamellipodium formation in cells pretreated with nocodazole. (A) GFP-actin–transfected cells were plated on FN and treated with 10 μg/ml nocodazole for 1 h prior to stimulation with 100 ng/ml PMA (time 00′00"). Time-lapse images show lamellipodia formation resulting in disruption of the cell after PMA stimulation (time 05′00"-21′38"). Bar, 20 μm. (B) Tubulin and actin distribution in cells treated with nocodazole and PMA. GFP-actin–expressing cells were plated on FN and treated subsequently with nocodazole and PMA. Cells were then fixed and stained for tubulin. a, actin distribution; b, tubulin distribution; and c, overlay of tubulin (red) and actin (green) within the cell. Note the cells show lamella and actin-rich lamellipodium (a, c) without any filamentous tubular structures (b, c). The red staining in lamella and lamellipodium of the cell in b and c represents unpolymerized tubulin. Bar, 20 μm
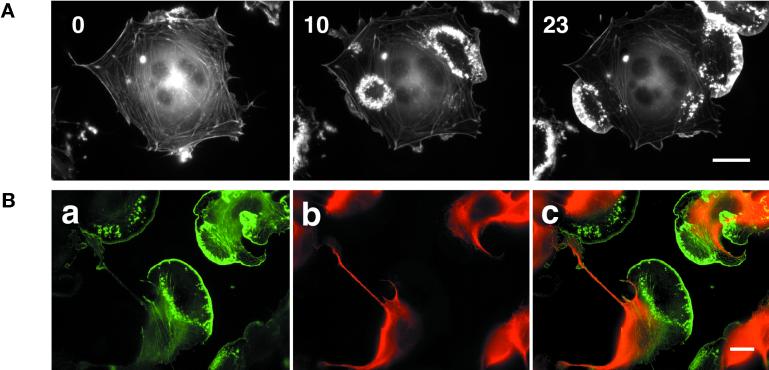
Stimulation of taxol-pretreated cells with PMA resulted in enhancement of ruffling (Figure 5A). Ruffles often appeared as circular structures, which eventually extended toward the cell edge to form lamellipodia (Figure 5A, 10′ and 23′). Interestingly, similar to induction of lamellipodia after exchange from nocodazole-to-taxol–containing medium (Waterman-Storer et al., 1999), taxol-stabilized microtubules did not enter the newly formed lamella (Figure 5B). Lamellipodium formation also was observed in nocodazole- and taxol-treated cells in the absence of PMA although with a lower frequency, suggesting that spontaneous lamellipodia formation, as well as PMA-induced lamellipodia formation can occur in these cells. These results indicate that microtubules do not appear to be essential for actin-dependent ruffling and lamellipodium formation.
Figure 5.
PMA-induced lamellipodium formation in B16 cells pretreated with taxol. (A) Melanoma cells transfected with GFP-actin were plated on FN and treated with 10 μM taxol for 1 h prior to 100 ng/ml PMA at time 0′. Subsequent images show circular actin ruffles and lamellipodium formation after addition of PMA (time 10′ and 23′). Bar, 20 μm. (B) Tubulin and actin distribution in cells treated with taxol and PMA. GFP-actin–expressing cells were plated on FN and treated subsequently with taxol and PMA. Cells were then fixed and stained for tubulin. a, actin distribution; b, tubulin distribution; and c, overlay of tubulin (red) and actin (green) within the cell. Note the cells show lamella and actin-rich lamellipodium (a, c) without any tubular structures (b, c). Bar, 20 μm.
To quantify the ruffling events we analyzed kymographs obtained by SACED (Hinz et al., 1999). This method allows recording of areas of interest along a line with the width of one pixel (Figure 6A). Individual line scans were assembled in sequence of their acquisition, resulting in a composite phase contrast picture (Figure 6, A and B). The activity of cell motion was measured for a period of 5 min and subdivided into lamellipodium protrusion velocity, lamellipodium frequency, ruffle retraction rate, and ruffle frequency. Lamellipodium protrusion velocity in nontreated cells was ∼4.3 μm/min, and was significantly inhibited by taxol (20%) and by nocodazole (50%; Figure 6, B and C). Similarly, the ruffle retraction rate was significantly inhibited by taxol (25%) and by nocodazole (50%) (Figure 6, B and C). Both, ruffle retraction rate and lamellipodium protrusion velocity were fully restored by PMA treatment of the cells (Figure 6, B and C). Frequency of lamellipodium protrusion and ruffle formation remained almost constant after taxol but was significantly inhibited by nocodazole treatment. Lamellipodium and ruffling frequency were fully restored after addition of PMA. These results indicate that it is possible to induce cell motility events despite the disturbance of the microtubule dynamics.
SCF Induces Ruffling in Melanoblasts Pretreated with Nocodazole or Taxol
Although we demonstrated clearly that PMA induces Rac-dependent ruffling in the absence of microtubules, this does not represent a physiological situation. Therefore, we wanted to investigate whether it was possible to reproduce these findings after stimulation of cells via a surface receptor tyrosine kinase.
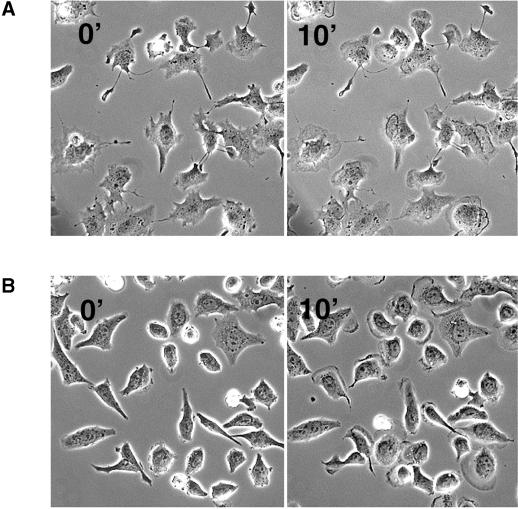
Melb-a is a melanoblast cell line expressing the receptor tyrosine kinase c-kit. Serum starved-melb-a cells do not show any ruffling and lamellipodia. Upon addition of the c-kit ligand SCF these cells immediately form lamellipodia and begin to migrate. In our experiments we plated melb-a cells on serum-coated glass coverslips, serum starved the cells for 4 h, and incubated them for 1 h in presence of 10 μg/ml nocodazole or 10 μM taxol prior to adding 50 ng/ml SCF (final concentration). Nocodazole- and taxol-pretreated cells began to form large lamellipodia within 10 min of SCF treatment (Figure 7, A and B). These observations confirm results obtained with PMA-stimulated melanoma cells, showing that microtubules are not essential for the activation of the actin machinery to form lamellipodia.
Figure 7.
Lamellipodium formation in melanoblasts (melb-a) after c-kit stimulation with SCF. Starved melanoblasts pretreated with nocodazole (A) or taxol (B) were stimulated with 50 ng/ml SCF. Both taxol- and nocodazole-pretreated cells show lamellipodium formation 10 min after addition of SCF (10′).
Microtubules Regulate Adhesion and Tail Retraction in Migrating Cells
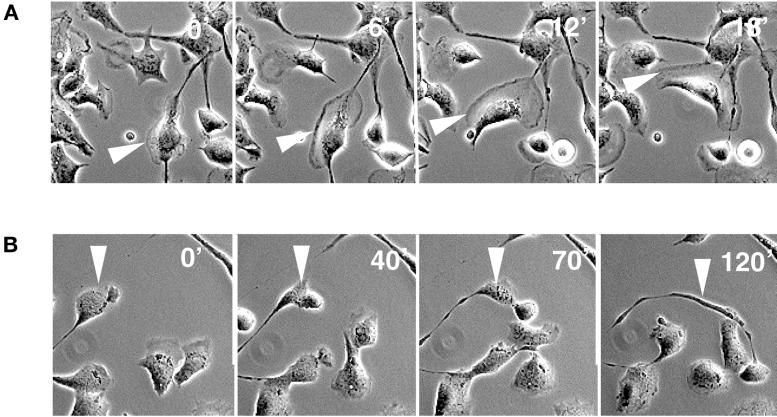
In contrast to cells treated with SCF alone (example in Figure 8A) most of the melb-a cells (90%) treated with nocodazole prior to SCF addition displayed virtually no cell migration, despite the formation of lamellipodia (our unpublished results; Figure 8B). The remaining 10% that were able to advance moved with ∼10 times slower kinetics compared with cells stimulated with SCF alone. Perhaps even more strikingly, these cells were unable to retract their tail (example in Figure 8B).
Figure 8.
Tail retraction is inhibited in cells devoid of microtubules. (A) Migration of a melb-a melanoblast after stimulation with SCF. Cell form lamellipodia and advance quickly (arrowhead). (B) In contrast cells treated with nocodazole prior to SCF form lamellipodia but are inhibited in cell migration. The depicted cell advances very slowly and leaves a trace of membrane behind, indicating inhibition of tail retraction (arrowhead).
Similarly, B16 cells treated with nocodazole in combination with PMA were inhibited in cell migration (Figure 9A). However, probably because of the driving force created by polymerizing actin at the leading edge and the stable focal contact in the main cell body, cells were sometimes torn apart, resulting in fragments separated from the main cell body. These “breakaway” fragments continued to migrate autonomously leaving a trace of actin-containing membrane behind (Figure 9B). These observations and the finding that focal contacts in the main cell body remain stable (cf. Figure 4A) suggest that microtubules might regulate the strength of cell adhesion to extracellular matrix.
To verify this hypothesis we tested adhesion of B16 cells to FN under the different conditions. As shown in Figure 9C, adhesion of cells increased little when stimulated with PMA. However, an increase of cell adhesion (45%) was observed upon treatment of cells with nocodazole alone or with nocodacole and PMA. Thus, these results indicate that microtubules regulate cell-substrate adhesion, which is required for tail retraction of advancing cells.
DISCUSSION
The cytoskeleton is the key modulator of cell motility. The organization of the actin cytoskeleton determines whether a cell moves or remains stationary. It has been shown that disruption of microtubules in fibroblasts leads to the loss of lamellipodium protrusions, cell ruffling, and cell migration, thus indicating a functional link between the actin and the microtubular cytoskeleton (Vasiliev et al., 1970; Liao et al., 1995; Bershadsky et al., 1996; Waterman-Storer et al., 1999). In contrast, other studies demonstrated that microtubules were not required for actin-based cell movements (Zigmond et al., 1981; Euteneuer and Schliwa, 1984). These observations led us to investigate more closely the contribution of microtubular dynamics to cell movements. Using GFP constructs and video microscopy we confirmed that destruction or stabilization of the microtubular network blocked the formation of lamellipodia and inhibited cell migration. However, protrusion of lamellipodia was recovered upon stimulation of the cells with PMA or SCF.
Recent publications have proposed an involvement of microtubules in activation of the small GTPase Rac1 (Best et al., 1996; Waterman-Storer et al., 1999). This has led to the hypothesis that microtubules are directly involved in the polymerization of actin at the cell periphery inducing lamellipodial protrusions. It was shown that Rac1-GTP directly binds to tubulin dimers (Best et al., 1996), and it was hypothesized that tubulin polymerization liberates activated Rac1, which can then result in lamellipodium formation (Waterman-Storer and Salmon, 1999). Furthermore, it was demonstrated that destruction of microtubules results in activation of RhoA, a small GTPase involved in stress fiber formation (Ridley and Hall, 1992a; Nobes and Hall, 1995). These observations led to a model of positive feedback interactions between microtubule and actin dynamics in cell motility (Waterman-Storer and Salmon, 1999). This model proposes that microtubule disassembly in the perinuclear region activates RhoA, leading to a contractile network of actin fibers in the main cell body, thereby regulating contraction of the cell. Simultaneously, microtubule growth at the cell periphery activates Rac1, promoting lamellipodium formation and subsequent advancement of the leading edge. These concerted events, Rho and Rac activation, finally regulate cell migration. In this model, microtubules feature as key players in Rac1-mediated lamellipodia formation. However, our results indicate that although they may play a role (and indeed we see less ruffling and lamellipodium formation in the presence of nocodacole or taxol) they are not essential: First, protruding cell edges in rapidly locomoting cells have only few associated microtubules and the distance between tips of growing microtubules to the leading edge increases with augmented speed of cell migration (our observations; Euteneuer and Schliwa, 1984; Wadsworth, 1999). Second, spontaneous ruffle and lamellipodium formation was still observed in nocodazole- and taxol-treated cells. However, this did not address the issue of whether microtubules may be essential in stimulated ruffle formation. PMA and growth factors are known to be potent stimulators for actin reorganization (Schliwa et al., 1984; Blume-Jensen et al., 1991; Nobes et al., 1995; Vosseller et al., 1997; Timokhina et al., 1998). Melanoma cells treated with PMA showed ruffling and lamellipodium formation in a Rac-dependent manner because ruffling was not observed in cells expressing the dominant-negative form of Rac. In our experiments it was possible to stimulate actin-dependent ruffling and lamellipodium formation in taxol- and nocodazole-pretreated cells to levels comparable with that obtained in untreated cells. Interestingly, lamella of taxol-pretreated cells were devoid of microtubules. Growth factor stimulation of the actin machinery provided physiological evidence that microtubules are not necessary for the transduction of signals leading to Rac1-dependent lamellipodium protrusion. Neither disruption nor stabilization of microtubules resulted in inhibition of SCF-induced lamellipodium formation.
Vasiliev et al. (1970) suggested that microtubules might be required for proper placement of ruffles. In B16 cells circular ruffle emergence was irrespective of the region, but formed lamellipodia when localized close to the cell edge (Ballestrem et al., 1998). No differences in ruffle localization were apparent in cells treated with the combination of nocodacole/taxol and PMA with respect to cells stimulated with PMA only. In both cases big ruffles were formed, often starting as circular structures in the cell body extending toward the cell edges forming lamellipodia. Thus, microtubules seem not to play a role in localization of ruffle formation in PMA-stimulated melanoma cells in contrast to findings in nonstimulated fibroblasts (Vasiliev et al., 1970).
One current hypothesis for lamellipodium formation is that endocytosed membrane vesicles in the cell center are transported via microtubules to the front of the cell were they reinsert, thereby enlarging the leading edge (Rodionov et al., 1993; Bretscher, 1996a,b; Bretscher and Aguado-Velasco, 1998b). There are several aspects that suggest that this may be the case. Because both the actin and the microtubule network are involved in axonal vesicle transport they may have overlapping roles. Interestingly, it has been shown that either destruction of actin filaments or microtubules leads to partial inhibition of neurite outgrowth (Marsh and Letourneau, 1984; Lamoureux et al., 1990). Similarly, in our present study, kymograph analysis demonstrated only partial inhibition of ruffling and lamellipodium formation after disruption or stabilization of microtubules in B16 cells. That microtubules were not essential for ruffling and lamellipodium formation could therefore be explained by an actin-dependent transport of vesicles that is up-regulated upon PMA stimulation. Indeed actin-dependent transport of endocytotic vesicles in mast cells was recently reported by Merrifield et al. (1999). Enhanced transport of melanophore-containing vesicles to the membrane has been shown after addition of PMA in melanoma cells (Reilein et al., 1998). Furthermore, Bretscher and Aguado-Velasco (1988a) demonstrated that epidermal growth factor-induced ruffles arise by exocytosis of internal membrane from the endocytotic cycle in a Rac-dependent manner. It may be possible that lamellipodium formation after PMA or SCF stimulation is based upon a similar mechanism.
Although microtubules were clearly not necessary for ruffling and lamellipodia formation, nocodazole-treated cells did not migrate even after PMA or SCF stimulation. Therefore, they evidently play a role in cell translocation. A recent publication demonstrated that focal contacts were released after multiple targeting by microtubules (Kaverina et al., 1999). It has been proposed that microtubules may deliver relaxing signals to focal contacts, resulting in the release of focal adhesion sites that would enable the cell to move forward, rather than remaining anchored to one spot (Kaverina et al., 1999; Small et al., 1999). Our observations are consistent with these findings in that migration is inhibited in cells devoid of microtubules. Even stimulation with PMA or SCF, although leading to lamellipodium formation and surface actin-dependent ruffling, did not result in cell migration. In PMA-treated cells, focal adhesion contacts remained stable in the main cell body, whereas fragments of cells separated away from the main cell body, apparently under the driving force created by actin polymerization in the continuously protruding lamellipodium. Furthermore, we demonstrate that treatment of cells with nocodazole leads to an increase in cell adhesion to extracellular matrix. Thus, one task of microtubules may be to regulate the turnover of focal contacts and modulate the adhesive strength to extracellular matrix.
In conclusion, we have shown herein that microtubules influence cell motility events, such as stress fiber formation, ruffling, and lamellipodium formation in nonstimulated cells. We demonstrated that microtubules are not essential for actin-dependent lamellipodium formation upon activation of Rac through stimulation with PMA or growth factors such as SCF. In addition we showed that the formation of lamellipodia is actin dependent but microtubules are essential for tail retraction, the release of focal contacts, and hence regulation of coordinated cell migration.
ACKNOWLEDGMENTS
We thank Dr. C. Johnson-Léger and Prof. Dr. G. Gabbiani for critical reading of this article. We are grateful to M.C. Jacquier for excellent technical support, and J. Ntah for secretarial assistance. This work has been supported by the Schweizerische Krebsliga grant KFS 412-1-1997; grants from the Swiss National Science Foundation 31-49241-96, 31-052727.97, 31-50568.97; and grants from the Fondation Gabrielle Giorgi-Cavaglieri and Helmut Horten Stiftung.
Abbreviations used:
- FN
fibronectin
- GFP
green fluorescence protein
- PMA
phorbol 12-myristate 13-acetate
- SACED
stroboscopic analysis of cell dynamics
- SCF
stem cell factor
REFERENCES
- Ballestrem C, Wehrle-Haller B, Imhof BA. Actin dynamics in living mammalian cells. J Cell Sci. 1998;111:1649–1658. doi: 10.1242/jcs.111.12.1649. [DOI] [PubMed] [Google Scholar]
- Bershadsky A, Chausovsky A, Becker E, Lyubimova A, Geiger B. Involvement of microtubules in the control of adhesion-dependent signal transduction. Curr Biol. 1996;6:1279–1289. doi: 10.1016/s0960-9822(02)70714-8. [DOI] [PubMed] [Google Scholar]
- Bershadsky AD, Ivanova OY, Lyass LA, Pletyushkina OY, Vasiliev JM, Gelfand IM. Cytoskeletal reorganizations responsible for the phorbol ester-induced formation of cytoplasmic processes: possible involvement of intermediate filaments. Proc Natl Acad Sci USA. 1990;87:1884–1888. doi: 10.1073/pnas.87.5.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bershadsky AD, Vaisberg EA, Vasiliev JM. Pseudopodial activity at the active edge of migrating fibroblast is decreased after drug-induced microtubule depolymerization. Cell Motil Cytoskeleton. 1991;19:152–158. doi: 10.1002/cm.970190303. [DOI] [PubMed] [Google Scholar]
- Best A, Ahmed S, Kozma R, Lim L. The Ras-related GTPase Rac1 binds tubulin. J Biol Chem. 1996;271:3756–3762. doi: 10.1074/jbc.271.7.3756. [DOI] [PubMed] [Google Scholar]
- Blume-Jensen P, Claesson-Welsh L, Siegbahn A, Zsebo KM, Westermark B, Heldin CH. Activation of the human c-kit product by ligand-induced dimerization mediates circular actin reorganization and chemotaxis. EMBO J. 1991;10:4121–4128. doi: 10.1002/j.1460-2075.1991.tb04989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher MS. Getting membrane flow and the cytoskeleton to cooperate in moving cells. Cell. 1996a;87:601–606. doi: 10.1016/s0092-8674(00)81380-x. [DOI] [PubMed] [Google Scholar]
- Bretscher MS. Moving membrane up to the front of migrating cells. Cell. 1996b;85:465–467. doi: 10.1016/s0092-8674(00)81246-5. [DOI] [PubMed] [Google Scholar]
- Bretscher MS, Aguado-Velasco C. EGF induces recycling membrane to form ruffles. Curr Biol. 1998a;8:721–724. doi: 10.1016/s0960-9822(98)70281-7. [DOI] [PubMed] [Google Scholar]
- Bretscher MS, Aguado-Velasco C. Membrane traffic during cell locomotion. Curr Opin Cell Biol. 1998b;10:537–541. doi: 10.1016/s0955-0674(98)80070-7. [DOI] [PubMed] [Google Scholar]
- Danowski BA. Fibroblast contractility and actin organization are stimulated by microtubule inhibitors. J Cell Sci. 1989;93:255–266. doi: 10.1242/jcs.93.2.255. [DOI] [PubMed] [Google Scholar]
- Downey GP, Chan CK, Lea P, Takai A, Grinstein S. Phorbol ester-induced actin assembly in neutrophils: role of protein kinase C. J Cell Biol. 1992;116:695–706. doi: 10.1083/jcb.116.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto T. Microtubule disruption induces the formation of actin stress fibers and focal adhesions in cultured cells: possible involvement of the rho signal cascade. Cell Struct Funct. 1996;21:317–326. doi: 10.1247/csf.21.317. [DOI] [PubMed] [Google Scholar]
- Euteneuer U, Schliwa M. Persistent, directional motility of cells and cytoplasmic fragments in the absence of microtubules. Nature. 1984;310:58–61. doi: 10.1038/310058a0. [DOI] [PubMed] [Google Scholar]
- Hangan D, Morris VL, Boeters L, von Ballestrem C, Uniyal S, Chan BM. An epitope on VLA-6 (α6β1) integrin involved in migration but not adhesion is required for extravasation of murine melanoma B16F1 cells in liver. Cancer Res. 1997;57:3812–3817. [PubMed] [Google Scholar]
- Hinz B, Alt W, Johnen C, Herzog V, Kaiser HW. Quantifying lamella dynamics of cultured cells by SACED, a new computer-assisted motion analysis. Exp Cell Res. 1999;251:234–243. doi: 10.1006/excr.1999.4541. [DOI] [PubMed] [Google Scholar]
- Kaverina I, Krylyshkina O, Small JV. Microtubule targeting of substrate contacts promotes their relaxation and dissociation. J Cell Biol. 1999;146:1033–1044. doi: 10.1083/jcb.146.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamoureux P, Steel VL, Regal C, Adgate L, Buxbaum RE, Heidemann SR. Extracellular matrix allows PC12 neurite elongation in the absence of microtubules. J Cell Biol. 1990;110:71–79. doi: 10.1083/jcb.110.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- Liao G, Nagasaki T, Gundersen GG. Low concentrations of nocodazole interfere with fibroblast locomotion without significantly affecting microtubule level: implications for the role of dynamic microtubules in cell locomotion. J Cell Sci. 1995;108:3473–3483. doi: 10.1242/jcs.108.11.3473. [DOI] [PubMed] [Google Scholar]
- Marsh L, Letourneau PC. Growth of neurites without filopodial or lamellipodial activity in the presence of cytochalasin B. J Cell Biol. 1984;99:2041–2047. doi: 10.1083/jcb.99.6.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrifield CJ, Moss SE, Ballestrem C, Imhof BA, Giese G, Wunderlich I, Almers W. Endocytic vesicles move at the tips of actin tails in cultured mast cells [In Process Citation] Nat Cell Biol. 1999;1:72–74. doi: 10.1038/9048. [DOI] [PubMed] [Google Scholar]
- Montell DJ. The genetics of cell migration in Drosophila melanogaster and Caenorhabditis elegans development. Development. 1999;126:3035–3046. doi: 10.1242/dev.126.14.3035. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J Cell Biol. 1999;144:1235–1244. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobes CD, Hawkins P, Stephens L, Hall A. Activation of the small GTP-binding proteins rho and rac by growth factor receptors. J Cell Sci. 1995;108:225–233. doi: 10.1242/jcs.108.1.225. [DOI] [PubMed] [Google Scholar]
- Reilein AR, Tint IS, Peunova NI, Enikolopov GN, Gelfand VI. Regulation of organelle movement in melanophores by protein kinase A (PKA), protein kinase C (PKC), and protein phosphatase 2A (PP2A) J Cell Biol. 1998;142:803–813. doi: 10.1083/jcb.142.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992a;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992b;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Rodionov VI, Gyoeva FK, Tanaka E, Bershadsky AD, Vasiliev JM, Gelfand VI. Microtubule-dependent control of cell shape and pseudopodial activity is inhibited by the antibody to kinesin motor domain. J Cell Biol. 1993;123:1811–1820. doi: 10.1083/jcb.123.6.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliwa M, Nakamura T, Porter KR, Euteneuer U. A tumor promoter induces rapid and coordinated reorganization of actin and vinculin in cultured cells. J Cell Biol. 1984;99:1045–1059. doi: 10.1083/jcb.99.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattil SJ, Ginsberg MH. Integrin signaling in vascular biology. J Clin Invest. 1997;100:S91–S95. [PubMed] [Google Scholar]
- Small JV, Rottner K, Kaverina I. Functional design in the actin cytoskeleton. Curr Opin Cell Biol. 1999;11:54–60. doi: 10.1016/s0955-0674(99)80007-6. [DOI] [PubMed] [Google Scholar]
- Sviderskaya EV, Wakeling WF, Bennett DC. A cloned, immortal line of murine melanoblasts inducible to differentiate to melanocytes. Development. 1995;121:1547–1557. doi: 10.1242/dev.121.5.1547. [DOI] [PubMed] [Google Scholar]
- Timokhina I, Kissel H, Stella G, Besmer P. Kit signaling through PI 3-kinase and Src kinase pathways: an essential role for Rac1 and JNK activation in mast cell proliferation. EMBO J. 1998;17:6250–6262. doi: 10.1093/emboj/17.21.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasiliev JM, Gelfand IM. Morphogenetic reactions and locomotory behavior of transformed cells in culture. In: Weiss L, editor. Fundamental Aspects of Metastasis. Amsterdam: North-Holland; 1976. pp. 71–98. [Google Scholar]
- Vasiliev JM, Gelfand IM, Domnina LV, Ivanova OY, Komm SG, Olshevskaja LV. Effect of colcemid on the locomotory behavior of fibroblasts. J Embryol Exp Morphol. 1970;24:625–640. [PubMed] [Google Scholar]
- von Ballestrem CG, Uniyal S, McCormick JI, Chau T, Singh B, Chan BM. VLA-β1 integrin subunit-specific monoclonal antibodies MB1.1 and MB1.2: binding to epitopes not dependent on thymocyte development or regulated by phorbol ester and divalent cations. Hybridoma. 1996;15:125–132. doi: 10.1089/hyb.1996.15.125. [DOI] [PubMed] [Google Scholar]
- Vosseller K, Stella G, Yee NS, Besmer P. c-kit receptor signaling through its phosphatidylinositide-3′-kinase-binding site and protein kinase C: role in mast cell enhancement of degranulation, adhesion, and membrane ruffling. Mol Biol Cell. 1997;8:909–922. doi: 10.1091/mbc.8.5.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadsworth P. Regional regulation of microtubule dynamics in polarized, motile cells. Cell Motil Cytoskeleton. 1999;42:48–59. doi: 10.1002/(SICI)1097-0169(1999)42:1<48::AID-CM5>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Waterman-Storer CM, Salmon E. Positive feedback interactions between microtubule and actin dynamics during cell motility. Curr Opin Cell Biol. 1999;11:61–67. doi: 10.1016/s0955-0674(99)80008-8. [DOI] [PubMed] [Google Scholar]
- Waterman-Storer CM, Salmon ED. Actomyosin-based retrograde flow of microtubules in the lamella of migrating epithelial cells influences microtubule dynamic instability and turnover and is associated with microtubule breakage and treadmilling. J Cell Biol. 1997;139:417–434. doi: 10.1083/jcb.139.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman-Storer CM, Worthylake RA, Liu BP, Burridge K, Salmon ED. Microtubule growth activates Rac1 to promote lamellipodial protrusion in fibroblasts [In Process Citation] Nat Cell Biol. 1999;1:45–50. doi: 10.1038/9018. [DOI] [PubMed] [Google Scholar]
- Zigmond SH, Levitsky HI, Kreel BJ. Cell polarity: an examination of its behavioral expression and its consequences for polymorphonuclear leukocyte chemotaxis. J Cell Biol. 1981;89:585–592. doi: 10.1083/jcb.89.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]