Abstract
Candida glabrata has emerged in recent years as a significant cause of systemic fungal infection. We have previously reported on the first three patients receiving radiation for head and neck cancer to develop oropharyngeal candidiasis due to C. glabrata. The goal of this study was to track the development of increased fluconazole resistance in C. glabrata isolates and to evaluate previously described genetic mechanisms associated with this resistance from one of these three patients. The patient was a 52-year-old man with squamous cell carcinoma treated with radiation. At week 7 of his radiation, he developed oropharyngeal candidiasis, which was treated with 200 mg of fluconazole daily for 2 weeks. Serial cultures from this and three subsequent time points yielded C. glabrata. Isolates from these cultures were subjected to antifungal susceptibility testing, DNA karyotyping, and evaluation of the expression of genes previously associated with C. glabrata resistance to fluconazole, CgCDR1, CgCDR2, and CgERG11. Two strains (A and B) of C. glabrata were identified and found to display different patterns of resistance development and gene expression. Strain A developed resistance over a 2-week period and showed no overexpression of these genes. In contrast, strain B first showed resistance 6 weeks after fluconazole therapy was discontinued but showed overexpression of all three genes. In conclusion, development of resistance to fluconazole by C. glabrata is a highly varied process involving multiple molecular mechanisms.
Candida glabrata has emerged in recent years as a significant cause of fungal infections (17). The role of C. glabrata in oropharyngeal candidiasis (OPC) is somewhat controversial. When cultured from patients with OPC, this organism is most often detected along with C. albicans (17). C. glabrata comprises between 5 and 10% of all oral isolates recovered from human immunodeficiency virus (HIV) patients with OPC. In the past, most investigators felt that C. glabrata was simply a commensal organism and did not contribute to infection (7). However, OPC infections with mixed C. albicans and C. glabrata in HIV patients tend to be more clinically severe and require larger doses of fluconazole for clinical cure than infections with C. albicans alone (16).
OPC infections due solely to C. glabrata have been described. Hoegl et al. reported two such OPC infections in a female HIV-positive drug abuser over a 6-year period (9). Canuto et al. evaluated 179 HIV-positive patients in two Spanish hospitals for risk factors associated with the isolation of fluconazole-resistant oral Candida and found that 14% of all the OPC infections were caused by C. glabrata (2). C. glabrata may be emerging as a potential pathogen in elderly populations. Lockhart et al. reported that 29% of patients older than 80 years were colonized orally with C. glabrata. If these patients wore dentures, the colonization rate increased to 58%. Studies are being done to investigate the rates of OPC due to C. glabrata in the elderly (9).
We have previously reported on the epidemiology and clinical course of OPC in patients receiving radiation therapy for head and neck cancer. While C. albicans was the primary pathogen, C. glabrata was found to be a relatively common colonizing organism in these patients (15). Recently, we described the first three patients to develop OPC due to C. glabrata. In one of these patients the C. glabrata isolates developed increased microbiological resistance after short-term exposure to fluconazole (18).
Several mechanisms for the development of azole resistance in C. albicans have been described. Increased efflux of azole medications from fungal cells has been correlated with the upregulation of multidrug efflux transporter genes, the ATP binding cassette transporters CDR1 and CDR2, and the major facilitator MDR1 (19, 20). ERG11, the gene that codes for the target enzyme of azole medications, lanosterol demethylase, is upregulated along with the development of azole resistance (10). CDR1, MDR1, and ERG11 upregulation has also been demonstrated when C. dubliniensis develops resistance to fluconazole (14). More recently resistance mechanisms have been investigated for C. glabrata. Sanglard et al. have shown upregulation of CDR1 and CDR2 when fluconazole MICs rise (21, 22). Marichal et al. have shown an eightfold increase in ERG11 expression in an azole-resistant C. glabrata strain (12).
The goal of this study was to track the epidemiology, using DNA typing, of the development of fluconazole-resistant C. glabrata and to evaluate the previously described genetic mechanisms associated with this resistance on isolates from a patient who developed OPC while receiving radiation to treat head and neck cancer.
MATERIALS AND METHODS
The patient was receiving ionizing radiation for head and neck cancer and was participating in a clinical study where patients were given preemptive fluconazole therapy (100 mg/day orally for the duration of radiation) if they had oral culture positive for colonization by C. albicans at any time during their radiation therapy. Oral specimens were obtained from the patient and cultured every week for the duration of his radiation treatment. OPC was verified by clinical presentation of white plaques, positive KOH slide, and positive culture. Specimens were collected by using an oral swab and a swish sample of 10 ml of normal saline instilled in the mouth for 10 s and then collected in a sterile container. These samples were plated on blood agar, RPMI medium, and CHROMagar Candida (CHROMagar Co., Paris, France) chromogenic medium. The colony color on chromogenic medium was recorded. Yeasts were identified using standard techniques including analysis of germ tube formation, growth at 37 and 42°C, and identification by API-20C (bioMériux, Marcy-1'Etoile, France). For all cultures, three to five yeast colonies from primary plates were selected and stored on Sabouraud dextrose slants for antifungal susceptibility testing and DNA typing.
Broth microdilution antifungal susceptibility testing to fluconazole was performed by the Fungus Testing Laboratory, University of Texas Health Science Center at San Antonio. The NCCLS-approved method for fungal drug susceptibility, involving a broth microdilution method with RPMI 1640 medium (Angus Buffers, Niagara Falls, N.Y.) buffered to pH 7.0 with MOPS (3-[N-morpholino]propanesulfonic acid) with an inoculum of 0.5 × 103 to 2.5 × 103 cells per ml, was used (13). Specifically, five colonies of each isolate were selected, placed in medium with MOPS, and diluted to the desired concentration using spectrophotometric techniques. Serial dilutions of drugs were made from 0.03 to 128 μg/ml, the yeast cell inoculum was added, and the mixture was incubated at 35°C for 48 h. Following incubation, the growth in each well was scored as follows: 0, optically clear; 1+, slightly hazy; 2+, prominent reduction in turbidity compared with that of the drug-free control (80% inhibition end point); 3+, slight reduction in turbidity compared with that of the drug-free control; 4+, no reduction in turbidity compared with that of the drug-free control. As recommended by Espinel-Ingroff et al., the MIC of azoles is defined as the lowest concentration in which the growth score was 2+ (80% inhibition) or less following 48 h of incubation (6). The intralaboratory reproducibility of this method has been shown to be >95% within a fourfold concentration range. Thus, significant changes in the MICs are considered to occur when the MIC increases twofold or more for serial isolates tested in parallel.
Strain identity was established by electrophoretic karyotyping (EK). Chromosomal DNA from each isolate was prepared in agarose plugs and separated by pulsed-field gel electrophoresis with a CHEF-DRIII instrument (Bio-Rad, Hercules, Calif.). Briefly, yeast DNA in 0.75% agarose plugs was resolved on a 1% agarose gel by contour-clamped homogenous electric field (CHEF) gel electrophoresis in 0.5× Tris-borate-EDTA buffer at 14°C. The running conditions for EK were as follows: block I, 120 s 4.5 V/cm 22 h; block II, 300 s 4.5 V/cm 5 h; block III, 300 s 3.4 V/cm 23 h. Gels were stained with ethidium bromide (1 μg/ml) and photographed (5). Fingerprints were considered highly similar when all visible bands showed the same migration distance for each isolate. Variations in the intensity and shape of bands among isolates were not considered differences. The presence or absence of more than one distinct band was considered a difference (11).
The expression patterns of the known C. glabrata resistance-associated genes Cg CDR1, Cg CDR2, and Cg ERG11 were determined by Northern blot analyses. Each of the clinical isolates was mechanically disrupted in TRI reagent (Molecular Research Center, Cincinnati, Ohio) using a mini-beadbeater (Biospec Products, Bartlesville, Okla.), and total RNA was precipitated from the resulting supernatant with isopropyl alcohol. Approximately equal amounts of RNA obtained from each isolate were resolved on formaldehyde-agarose gels and subsequently transferred to Nytran supercharged membranes by using a Turboblotter (Schleicher & Schuell, Keene, N.H.) as specified by the manufacturer.
DNA probes for the C. glabrata genes Cg CDR1, Cg CDR2, and Cg ERG11 were PCR amplified from genomic DNA prepared from one of the strain A isolates using primers designed from their GenBank database entries (accession numbers AF109723, AF251023 and LF40389, respectively). Hybridizations were performed by the method of Church and Gilbert (3), with all blots washed to high stringency (40 mM Na2HPO4-0.1% sodium dodecyl sulfate at 65°C) and exposed to X-ray film at room temperature overnight.
RESULTS
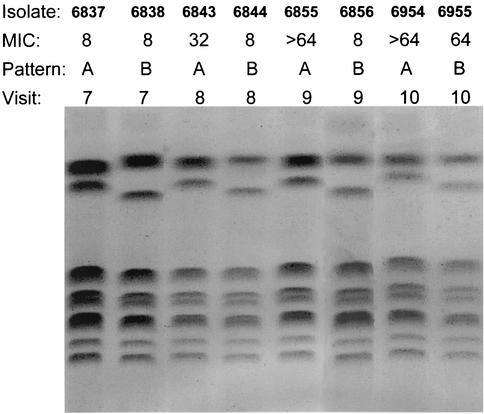
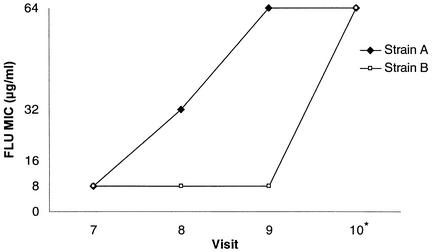
The patient was a 52-year-old man diagnosed with squamous cell carcinoma of the floor of the mouth, stage T4N2bM0, treated with 5,910 cGy of radiation over a 9-week period. At visits 3 through 6, his oral cultures grew C. glabrata and/or C. krusei but he exhibited no clinical disease. He was not given fluconazole because he had not been culture positive for C. albicans. At visit 7 he presented with white plaques on his oral mucosa that were KOH positive, and swab and swish cultures were plated on CHROMagar Candida to help with identification. The predominant growth on his swab plate and the only growth on his swish plate were lavender colonies consistent with C. glabrata. There were a few beige colonies on the swab culture consistent with C. krusei. The predominant organism was confirmed to be C. glabrata. The patient was given 200 mg of fluconazole per day based on screening results from CHROMagar Candida containing various concentrations of fluconazole. CHEF karyotypes showed two C. glabrata strains (A and B), both of which had 48-h fluconazole MICs of 8.0 μg/ml (Fig. 1). At visit 8, there were no clinical signs of OPC but colonization cultures were again positive primarily for C. glabrata with a few colonies of C. krusei. The fluconazole MICs for C. glabrata strains A and B were now 32.0 and 8.0 μg/ml, respectively. Fluconazole therapy was continued. Visit 9 was made at the conclusion of radiation therapy. At that visit, there was no sign of OPC and the fluconazole therapy was discontinued. Colonization cultures showed the same pattern of C. glabrata and C. krusei as seen at visit 8. Fluconazole MICs for strains A and B were now 64.0 and 8.0 μg/ml, respectively. Visit 10 was a 6-week follow-up after radiation therapy, and the fluconazole therapy had been discontinued. The colonization culture grew only C. glabrata. Fluconazole MICs for strains A and B were both 64.0 μg/ml (Fig. 2).
FIG. 1.
EK patterns and MICs of fluconazole for strains A and B from visits 7 through 10.
FIG. 2.
Graph of the increase in the fluconazole MICs for strains A and B from visits 7 through 10. *, visit occurred 6 weeks after cessation of treatment.
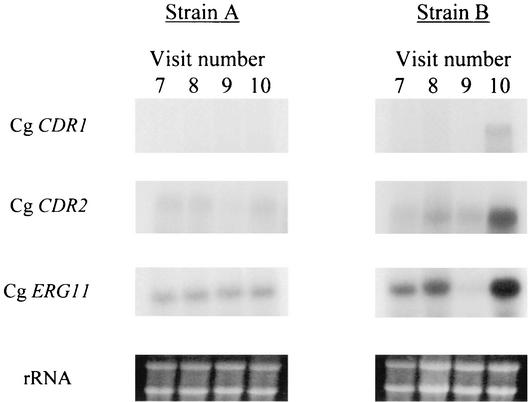
As with antifungal susceptibility, strains A and B showed very different expression patterns of Cg CDR1, Cg CDR2, and Cg ERG11. Expression did not change in the serial isolates of strain A even after the fluconazole MICs rose from 8.0 to 64 μg/ml. Strain B showed no change in gene expression of Cg CDR or Cg CDR2 from visits 7 to 9, when the fluconazole MIC remained at 8.0 μg/ml. Expression of Cg ERG11 appeared to drop at visit 9. However, at visit 10 strain B showed overexpression of all three genes tested, corresponding to the increased fluconazole resistance from 8 to 64 μg/ml (Fig. 3).
FIG. 3.
Expression patterns of known C. glabrata resistance-associated genes in the serial isolates of strains A and B. Total RNA was prepared from each isolate and probed with PCR-amplified fragments of the C. glabrata Cg CDR1, Cg CDR2, and Cg ERG11 genes by Northern blot analysis. The rRNA bands of the blotted gels are shown to demonstrate the roughly equal loading of the samples.
DISCUSSION
C. glabrata has recently emerged as a significant systemic pathogen, and there are increasing numbers of reports that it causes oral disease. It has been shown typically to have increased fluconazole MICs compared with C. albicans, and these MICs can rise rapidly when the organism is exposed to fluconazole (17, 18).
The purpose of this study was to evaluate the molecular mechanisms involved in the development of resistance by C. glabrata after fluconazole treatment of an oral infection in a patient receiving radiation for head and neck cancer. This patient could be said to have had a mixed infection since a few colonies of C. krusei grew on the swab culture. However, C. glabrata predominated on the swab culture and was present alone on the swish culture. Also, this patient responded to a dose of fluconazole (200 mg/day) that is well suited to treat an organism generally considered to be susceptible or dose-dependent susceptible to fluconazole, as is the case for C. glabrata, but not well suited to treat an inherently fluconazole-resistant organism such as C. krusei. Therefore, we feel that this infection was caused by C. glabrata and the patient was colonized by C. krusei. However, we cannot rule out the possibility of a mixed infection.
Matched sets of susceptible and resistant isolates are required to evaluate molecular mechanisms of resistance. EK was used to show the strain relatedness of susceptible and resistant isolates in this patient since it is the most reliable technique to type C. glabrata (1, 4). Two isogenic strains were cultured that persisted over time and became resistant after exposure to fluconazole.
The two strains displayed increased microbiological resistance after short-term exposure to fluconazole, but in two very different patterns. After 2 weeks of fluconazole treatment, the MIC for strain A increased from 8.0 to 64.0 μg/ml but the MIC for strain B remained constant at 8.0 μg/ml. At a 6-week follow-up, the resistance of strain A remained stable (MIC, 64.0 μg/ml) but the MIC for strain B was now also at 64.0 μg/ml. Interestingly, even with these rises in MIC, the patient responded to 200 mg of fluconazole per day and did not relapse with the increase in MIC. NCCLS document M27-A has proposed interpretive breakpoints for Candida tested against fluconazole, with MICs below 16 μg/ml indicating susceptibility and MICs above 32.0 μg/ml indicating resistance (13). It is possible that strain B was the predominant strain in the clinical infection and remained susceptible while the infection was being treated, showing a rise in the fluconazole MIC only a long time after the infection had responded to therapy. The risk of developing OPC falls after radiation therapy has been completed. Also, most of the patient data evaluated for the NCCLS breakpoints were obtained from patients with HIV or other immunocompromising conditions. Patients receiving head and neck radiation alone are not generally immunosuppressed and may be better able to clear OPC at modestly increased doses of fluconazole.
Resistance to fluconazole can be induced by the following mechanisms: (i) accumulation of the drug in the cell can be impaired; (ii) the ERG11 content of the cell can be elevated; (iii) point mutations in ERG11 may decrease the affinity for fluconazole; and (iv) the ergosterol biosynthetic pathway may be altered (23). We evaluated three genes that have been previously described to be upregulated in resistant isolates of C. glabrata, Cg CDR1, Cg CDR2, and Cg ERG11. Like the MIC patterns, the patterns of gene expression were very different between the two strains. Surprisingly, strain A showed no upregulation of Cg CDR1, Cg CDR2, and Cg ERG11 when the fluconazole MIC rose to 64 μg/ml. Other mechanisms apparently are in operation for this strain. However, we specifically limited our examination to these three genes, since they are, so far, the only ones to have been definitively associated with the development of fluconazole resistance in C. glabrata. Another factor is that while MDR1 is a major player in the development of fluconazole resistance in C. albicans, to our knowledge neither it nor any other member of the major facilitator superfamily has yet been described in C. glabrata (as indicated by current entries in the GenBank database). That such homologues exist and may be involved in acquired fluconazole resistance in some instances in C. glabrata is not in doubt. However, this is also likely to be true for many of the “drug” efflux pumps which have been characterized in C. albicans but remain to be discovered in C. glabrata. We feel that identifying the C. glabrata homologue of every one of these genes and examining their expression profile is beyond the scope of this study and still may not reveal the nature of the fluconazole resistance which developed in strain A. Other resistance mechanisms may include ERG11 point mutations and ergosterol pathway alterations. We hope to evaluate strain A for these potential other mechanisms in the future. In contrast, strain B was much more consistent with patterns for C. glabrata found by other investigators, since overexpression of all three genes in this strain closely matched the rise in MIC. Interestingly, expression of Cg ERG11 appeared to drop after the 2-week treatment with fluconazole but then the gene became overexpressed after 6 weeks of no fluconazole exposure.
It is difficult to determine the clinical significance of the development of resistance in these C. glabrata isolates. Infection was controlled with a fluconazole dose of 200 mg/day, which was consistent with the baseline MIC. If, as mentioned above, strain B was the pathogenic strain, the genes upregulated when this strain developed resistance appeared to be involved in the clinically important mechanisms in this patient.
In conclusion, we have evaluated the epidemiology of the development of resistance to fluconazole by two different strains of C. glabrata in a patient with OPC who was receiving radiation for head and neck cancer. We have also investigated the expression of three genes previously described as important for the development of this resistance, Cg CRD1, Cg CDR2, and Cg ERG11. Our results showed highly varied patterns since one strain showed no overexpression of the three genes tested with the development of resistance whereas the other strain showed overexpression of all three. This patient is only one patient infected with two strains of C. glabrata, and it is premature to speculate about the relative importance of our findings to the understanding of the resistance mechanisms operant in this organism. It is clear that development of resistance to fluconazole by C. glabrata is a highly varied process involving multiple molecular mechanisms, some of which remain to be determined.
Acknowledgments
This work was supported by a grant from Pfizer, Inc., Public Health Service grant 5-R01-DE11381-6 from the National Institutes of Health, and the Dental Oncology Education Program.
REFERENCES
- 1.Barchiesi, F., L. Di Francesco, D. Arzeni, F. Caselli, D. Gallo, and G. Scalise. 1999. Electrophoretic karyotyping and triazole susceptibility of Candida glabrata clinical isolates. Eur. J. Microbiol. Infect. Dis. 18:184-187. [DOI] [PubMed] [Google Scholar]
- 2.Canuto, M. M., F. G. Rodero, V. O. Ducasse, I. H. Aguado, C. M. Gonzalez, A. S. Sevillano, and A. M. Hildalgo. 2000. Determinants for the development of oropharyngeal colonization or infection by fluconazole-resistant Candida strains in HIV-infected patients. Eur. J. Clin. Microbiol. Infect. Dis. 19:593-601. [DOI] [PubMed] [Google Scholar]
- 3.Church, G. M., and W. Gilbert. 1984. Genomic sequencing. Proc. Natl. Acad. Sci. USA 81:1991-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Francesco, L., F. Barchiesi, F. Caselli, O. Cirioni, and G. Scalise. 1999. Comparison of four methods for DNA typing of clinical isolates of Candida glabrata. J. Med. Microbiol. 48:955-963. [DOI] [PubMed] [Google Scholar]
- 5.Doebbling, B. N., R. F. Lehmenn, R. J. Hollis, L. C. Wu, A. F. Widmer, A. Voss, and M. A. Pfaller. 1993. Comparison of pulsed-field gel electrophoresis with isoenzyme profiles as a typing system for Candida tropicalis. Clin. Infect. Dis. 16:377-383. [DOI] [PubMed] [Google Scholar]
- 6.Espinel-Ingroff A., C. W. Kish, Jr., T. M. Kerkering, R. A. Fromtling, K. Bartizal, and J. N. Galgiani. 1992. Collaborative comparison of broth macrodilution and microdilution antifungal susceptibility tests. J. Clin. Microbiol. 30:3138-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fidel, P. L., J. A. Vazquez, and J. D. Sobel. 1999. Candida glabrata: review of epidemiology, pathogenesis, and clinical disease with comparison to C. albicans. Clin. Microbiol. Rev. 12:80-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoegl, L., E. Thoma-Greber, M. Röcken, and H. Korting. 1998. Persistent oral candidosis by non-albicans Candida strains including Candida glabrata in a human immunodeficiency virus-infected patient observed over a period of 6 years. Mycoses 41:335-338. [DOI] [PubMed] [Google Scholar]
- 9.Lockhart, S. R., S. Joly, K. Vargas, J. Swails-Wenger, and D. R. Soll. 1999. Natural defenses against Candida colonization breakdown in the oral cavities of the elderly. J. Dent. Res. 78:857-868. [DOI] [PubMed] [Google Scholar]
- 10.Lopez-Ribot, J. L., R. K. McAtee, L. Lee, W. R. Kirkpatrick,. T. C. White, D. Sanglard, and T. F. Patterson. 1998. Distinct patterns of gene expression associated with the development of fluconazole resistance in serial Candida albicans isolates from HIV infected patients with oropharyngeal candidiasis. Antimicrob. Agents Chemother. 42:2932-2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Magee, P. T., L. Bowdin, and J. Staudinger. 1992. Comparison of molecular typing methods for Candida albicans. J. Clin. Microbiol. 30:2674-2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marichal, P., H. Vanden Bossche, F. C. Odds, G. Nobels, D. W. Warnock, V. Timmerman, C. Vanbroeckhoven, S. Fay, and P. Mosel-Larsen. 1997. Molecular characterization of an azole-resistant Candida glabrata isolate. Antimicrob. Agents Chemother. 41:2229-2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard. NCCLS document M27-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 14.Perea, S., J. L. Lopez-Ribot, B. L. Wickes, W. R. Kirkpatrick, O. P. Dib, S. P. Bachmann, S. M. Keller, M. Martinez, and T. F. Patterson. 2002. Molecular mechanisms of fluconazole resistance in Candida dubliniensis isolates from human immunodeficiency virus infected patients with oropharyngeal candidiasis. Antimicrob. Agents Chemother. 46:1695-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Redding, S. W., R. C. Zellars, W. R. Kirkpatrick, R. K. McAtee, M. A. Caceres, A. W. Fothergill, J. L. Lopez-Ribot, C. W. Bailey, M. G. Rinaldi, and T. F. Patterson. 1999. Epidemiology of oropharyngeal Candida colonization and infection in patients receiving radiation for head and neck cancer. J. Clin. Microbiol. 37:3896-3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Redding, S. W., W. R. Kirkpatrick, O. P. Dib, A. W. Fothergill, M. G. Rinaldi, and T. F. Patterson. 2000. The epidemiology of non-albicans Candida in oropharyngeal candidiasis in HIV patients. Spec. Care Dent. 20:178-181. [DOI] [PubMed] [Google Scholar]
- 17.Redding, S. W. 2001. The role of yeasts other than Candida albicans in oropharyngeal candidiasis. Curr. Opin. Infect. Dis. 14:673-677. [DOI] [PubMed] [Google Scholar]
- 18.Redding, S. W., W. R. Kirpatrick, B. J. Coco, L. Sadkowski, A. W. Fothergill, M. G. Rinaldi, T. Y. Eng, and T. F. Patterson. 2002. Candida glabrata oropharyngeal candidiasis in patients receiving radiation treatment for head and neck cancer. J. Clin. Microbiol. 40:1879-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanglard, D., K. Kuchler, F. Ischer, J. L. Pagani, M. Monod, and J. Bille. 1995. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob. Agents Chemother. 39:2378-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanglard, D., F. Ischer, L. Koymans, and J. Bille. 1997. Cloning of Candida albicans genes conferring resistance to antifungal agents: characterization of CDR2, a new multidrug ABC-transporter gene. Microbiology 143:405-416. [DOI] [PubMed] [Google Scholar]
- 21.Sanglard, D., F. Ischer, D. Calabrese, P. Majcherczyk, and J. Bille. 1999. The ATP binding cassette transporter gene Cg CDR1 from Candida glabrata is involved in the resistance of clinical isolates to azole antifungal agents. Antimicrob. Agents Chemother. 43:2753-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanglard, D., F. Ischer, and J. Bille. 2001. Role of ATP-binding-cassette transporter genes in high-frequency acquisition of resistance to azole antifungals in Candida glabrata. Antimicrob. Agents Chemother. 45:1174-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanglard, D., and J. Bille. 2002. Action of and resistance to antifungal agents, p. 349-383. In R. A. Calderone (ed.), Candida and candidiasis. ASM Press, Washington, D.C.