Abstract
OBJECTIVE
Determine the relation of race and gender to outcome from bleeding peptic ulcer.
DESIGN
Retrospective cohort study.
SETTING
All acute care hospitals in the United States.
PATIENTS
A 100% sample of hospitalized Medicare beneficiaries older than 64 years (n = 82,868) with a primary discharge diagnosis of peptic ulcer with hemorrhage.
MEASUREMENTS AND MAIN RESULTS
Surgical treatment was performed in 6.9% of patients, 30-day mortality was 8.5%, and average length of stay was 9.4 days. Surgery was somewhat more common in men than women (7.3% vs 6.5%, p < .001), and in whites than African Americans (6.9% vs 6.3%, p < .001), but neither race nor gender was associated with surgery in multivariable analysis adjusting for potentially confounding factors. Mortality rates were similar in African Americans and whites (8.5%), and somewhat higher in men than women (10.7% vs 9.3%, p < .001). In multivariable analysis, there was no difference in mortality across gender and racial groups. Although unadjusted and adjusted lengths of stay were longer for African Americans and shorter for men, the differences were modest (i.e., 16% increase and 6% decrease in multivariable analysis, respectively, p < .0001).
CONCLUSIONS
In this national sample, there is no significant gender or racial difference in therapy and outcome for patients with hemorrhagic peptic ulcer. The findings raise the possibility that studies that have shown race and gender differences in management of coronary artery disease and cancer may not be generalizable to other common diagnoses.
Keywords: peptic ulcer, African Americans, women, Medicare
In the last several years, numerous studies have examined the relation of gender and race to the treatment and outcome of both acute and chronic diseases, particularly cardiovascular conditions. The studies that have utilized administrative data have generally documented lower utilization of selected angiographic and surgical procedures in African–American patients1–4 and female patients,5,6 as well as poorer survival following diagnosis.6,7 Moreover, in analyses that have been limited to patients with equal access to a health care system, racial differences in therapy have also been reported,2,3,8 suggesting that factors other than access to care may be involved in treatment decisions.
Because the majority of reports have been limited to diseases in which treatment decisions are either made electively or selected among several alternatives, the etiology of the racial and gender disparity may be due in part to systematic differences in physician discretion or patient preferences. Moreover, lower reported rates of a given procedure may be offset by higher utilization of an equally effective strategy. In contrast, selection of treatment alternatives by both patients and physicians may be more limited in diseases such as bleeding peptic ulcer because the presentation is generally acute, hospitalization is nearly universally required, management is standardized,9,10 and criteria for surgical intervention, which is often performed as an emergency procedure, are relatively explicit.10,11 Also, any observed outcome difference between gender and racial groups may be attributable in part to clinical factors that are independently associated with treatment and outcome.
We therefore performed a retrospective cohort study of hospitalized Medicare beneficiaries with bleeding peptic ulcer to examine both the relation of race and gender to therapy and outcome, as well as the impact of other measurable clinical factors on any differences that are observed. Our goal was to determine if previous studies of racial and gender disparities in patients with coronary artery disease were generalizable to patients with hemorrhagic peptic ulcer disease.
METHODS
Patients
A cohort of all hospitalized patients in 1991 with a primary discharge diagnosis of peptic ulcer disease with hemorrhage was identified from the Medicare Provider Analysis and Review (MEDPAR) files, which contain information from hospitalizations recorded on the standard billing form (UB-82 and UB-92). Each record on the MEDPAR files contains up to five discharge diagnoses and three procedures, coded according to the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM). The records also include hospital and patient identifiers, age, gender, and race. Survival data from the Social Security Administration linked to the MEDPAR files were used to determine vital status for all patients in the postdischarge period.
We included patients with one of the following primary discharge diagnoses: gastric ulcer with hemorrhage (ICD-9 codes 531.00, 531.01, 531.20, 531.21, 531.40, 531.41, 531.60, 531.61); duodenal ulcer with hemorrhage (532.00, 532.01, 532.20, 532.21, 532.40, 532.41, 532.60, 532.61); peptic ulcer, site unspecified, with hemorrhage (533.00, 533.01, 533.20, 533.21, 533.40, 533.41, 533.60, 533.61); and gastrojejunal ulcer with hemorrhage (534.00, 534.01, 534.20, 534.21, 534.40, 534.41). Patients who were less than 65 years of age and enrolled in Medicare because of end-stage renal disease or chronic disability, and patients for whom race was listed as “other” or not specified were excluded.
Measures
Patients were considered to have undergone surgical treatment of the ulcer if one of the following procedural codes was included: partial gastrectomy (ICD-9 codes 43.50– 43.89), total gastrectomy (43.90 – 43.99), vagotomy (44.00 – 44.03), pyloroplasty (44.21, 44.29), and suture of ulcer (44.40 – 44.42).
Major comorbid illnesses were also determined using secondary ICD-9 diagnostic codes, and included acute isch-emic heart disease (410 – 410.99), chronic ischemic heart disease (412– 414.99), congestive heart failure (402.01, 402.11, 402.91, 428 – 428.99), other chronic heart diseases (393–398, 415 – 416.99, 420 – 427.99), chronic pulmonary diseases (480 – 493.99, 496 – 496.9), chronic renal failure (582–583.99, 585 – 587.99), cirrhosis (571.2, 571.5, 571.6, 572.2–572.4), other chronic liver disease (571–571.1, 571.3, 571.49, 571.8 –571.9, 572.8), cerebrovascular disease (430 – 438.99), cancer (140 –199), and malnutrition (263.0, 263.9).
Hospitals were categorized as public, private nonprofit, private for-profit, and osteopathic on the basis of data from the 1988 American Hospital Association files. Allopathic hospitals were also divided into teaching and nonteaching status on the basis of membership in the Council of Teaching Hospitals.
The 50 states and the District of Columbia were divided into nine geographic regions: New England (Maine, New Hampshire, Vermont, Massachusetts, Rhode Island, Connecticut); Mid Atlantic (New York, New Jersey, Pennsylvania); East North Central (Ohio, Indiana, Illinois, Michigan, Wisconsin); West North Central (Minnesota, Iowa, Missouri, North Dakota, South Dakota, Nebraska, Kansas); South Atlantic (Delaware, Maryland, District of Columbia, Virginia, West Virginia, North Carolina, South Carolina, Georgia, Florida); East South Central (Kentucky, Tennessee, Alabama, Mississippi); West South Central (Arkansas, Louisiana, Oklahoma, Texas); Mountain (Montana, Idaho, Wyoming, Colorado, New Mexico, Arizona, Utah, Nevada); and Pacific (Washington, Oregon, California, Alaska, Hawaii).
Analysis
Three outcome measures were considered: mortality in the 30-day interval following admission, length of stay, and performance of surgery. Unadjusted mortality and surgical rates were compared between patient, geographic, and hospital subgroups with χ2 testing, and length of stay was compared with the Wilcoxon Signed-Rank or Kruskal-Wallis tests. Characteristics were also compared between men and women and between African–American and white patients with χ2 testing.
To determine the independent association of gender and race with patient outcome after adjusting for differences in potentially confounding factors, multivariable regression analysis (logistic for mortality and surgery, linear for length of stay) was used. Variables included in each model were age, race, gender, individual comorbidities, location of ulcer, geographic region, and hospital type. In analyses of mortality and length of stay, performance of surgery was also included as a covariate. Because of skewness in length of stay, the natural logarithm of length of stay was used as the dependent variable in these analyses.
RESULTS
In 1991, a total of 87,566 Medicare beneficiaries older than 64 years were discharged with diagnoses consistent with peptic ulcer disease complicated by hemorrhage. Among this cohort, 2,777 were excluded because race was listed as “unknown,” and 1,921 because race was coded as “other.” The remaining 82,868 patients were the subject of this study. The mean age of this cohort was 77.2 ± 7.5 years, 52.2% were female, and 91.5% were classified as white. Major comorbidities included other chronic heart diseases (16.9% of patients), chronic pulmonary disease (16.7%), congestive heart failure (12%), chronic ischemic heart disease (12%), cancer (8.6%), cerebrovascular disease (3.9%), acute ischemic heart disease (2.4%), chronic renal failure (2.1%), cirrhosis (1.8%), malnutrition (1.7%), and other chronic liver disease (0.3%). More patients (p < .001) had gastric ulcers (53.6%) than duodenal ulcers (36.6%) or ulcers of other locations (9.9%). Nearly two thirds (63.7%) of the study sample were hospitalized in nonprofit nonteaching hospitals, 12.3% in publicly owned nonteaching hospitals, 10.9% in privately owned teaching hospitals, 10.2% in for-profit nonteaching hospitals, 1.7% in osteopathic hospitals, and 1.3% in publicly owned teaching hospitals.
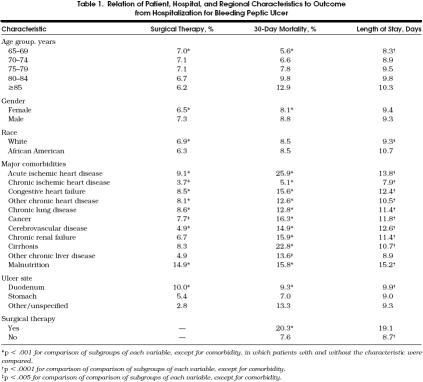
A procedural code for surgical intervention of bleeding ulcers was documented in 5,997 patients (6.9%). Although surgery was somewhat more frequently performed in male patients and white patients, the differences were relatively modest in magnitude (Table 1). Surgery was also utilized more often for patients with certain comorbidities, patients with bleeding duodenal ulcers (Table 1), and patients admitted to publicly owned teaching hospitals (9.8%).
Table 1.
Relation of Patient, Hospital, and Regional Characteristics to Outcome from Hospitalization for Bleeding Peptic Ulcer
The 30-day mortality for the cohort was 8.5% (7,405 patients). Mortality rates were similar in African Americans and whites, but somewhat higher in male patients than females (Table 1). Short-term mortality was also higher in older patients, patients with most major comorbidities, patients with hemorrhage sources other than duodenal or gastric ulcers, and patients who underwent surgical intervention (Table 1). There was also variability in mortality among hospital types (range 8.1% in non-profit nonteaching hospitals to 11.9% in publicly owned teaching hospitals), but little difference in mortality among geographic regions.
The average length of stay was 9.4 ± 8.4 days. Length of stay was similar for men and women, and was longer for African American patients (Table 1). Average length of stay was also longer for older patients, patients with major comorbidities, patients with bleeding duodenal ulcers, and patients who underwent surgical therapy (Table 1). Variability in average length of stay was also observed between hospital types (range 8.3 days in publicly owned nonteaching hospitals to 11.5 days in publicly owned teaching hospitals) and geographic regions (range 7.4 days in Mountain states to 11.6 days in Mid Atlantic states).
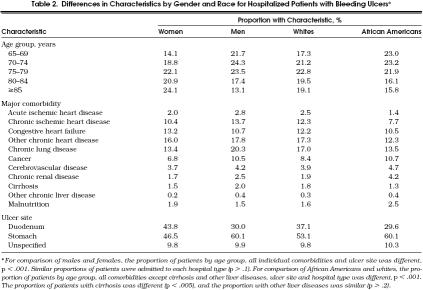
Compared with the female patients in the cohort, the male patients were somewhat younger (76.0 ± 7.1 vs 78.6 ± 7.7 years; p < .0001), were more likely to have most major comorbidities documented, and more often had a duodenal ulcer as the source of hemorrhage (Table 2). African–American patients were also somewhat younger (76.2 ± 7.6 vs 77.4 ± 7.6 years; p < .0001), more frequently had comorbidities of cancer, chronic renal disease, cerebrovascular disease, and malnutrition, and had the other comorbidities less frequently (Table 2). They were also more likely to have a gastric ulcer (Table 2), and were more frequently admitted to hospitals that were teaching or publicly owned or both (i.e., 17.8% of African–American vs 10.3% of white patients were admitted to privately owned teaching hospitals and 5.7% of African–American vs 1.0% of white patients were admitted to publicly owned teaching hospitals; p < .001).
Table 2.
Differences in Characteristics by Gender and Race for Hospitalized Patients with Bleeding Ulcers*
Because of differences in clinical characteristics between men and women and between African–American and white patients, multivariable techniques were used to determine the independent association of race and gender with outcome, after adjusting for potentially confounding factors (age, comorbidity, ulcer site, geographic region, hospital type). In these analyses, neither patient gender nor race was associated with 30-day mortality, with multivariable odds ratios (ORs) for death of 1.05 (95% confidence interval [CI] 0.98, 1.11) for men, and 1.08 (95% CI 0.98, 1.19) for African–American patients. Similarly, neither gender nor race was independently associated with performance of surgery, after adjustment for potentially confounding factors, with ORs, of 1.01 (95% CI 0.96, 1.07) for men and 0.95 (95% CI 0.85, 1.06) for African Americans. In a multivariable analysis, adjusted length of stay was somewhat shorter for men than women, and somewhat longer for African–American patients. However, although statistically significant, the differences were modest, with a 6% decrease for men (95% CI 7%, 5%; p < .0001), and a 16% increase for African Americans (95% CI 14%, 17%; p < .0001).
DISCUSSION
In this retrospective cohort study of a 100% sample of Medicare beneficiaries hospitalized for hemorrhagic peptic ulcer disease, we examined the relation of gender and race to treatment and short-term outcome. Three findings are emphasized. First, in unadjusted analyses, we did not find major differences in either the frequency of surgical intervention or 30-day mortality rates between men and women, or between African Americans and whites. Second, although differences in clinical characteristics were observed between gender and racial groups, after adjustment for these factors, no significant differences in the use of surgery or mortality were present. Third, although length of stay was somewhat longer for men and African Americans, the differences were small in magnitude. Thus, these data were not consistent with systematic differences in treatment and outcome between men and women, or between African–American and white patients.
Our findings contrast with studies of other diseases that have lower rates of procedures and higher mortality reported for both African Americans and women. In several studies that used administrative data, as well as medical record review, African–American patients were found to have lower utilization of diagnostic and therapeutic procedures. Whereas most analyses have been limited to cardiac catheterization, coronary angioplasty, and coronary bypass grafting,1–4,6,7 others have reported less frequent surgical intervention for African–American patients with colorectal cancer,12 breast cancer,13,14 and bladder cancer,15 as well as lower rates of outpatient preventive services.16,17 Although the concept of race is somewhat tenuous and may be a surrogate for socioeconomic status,18 two reports from the Veterans Administration have demonstrated lower rates of cardiovascular procedures in African Americans,2,3 suggesting that social and clinical factors are also important.
In some studies, mortality rates following hospitalization have been higher in African Americans, which may be in part due to the lower rates of treatment.13–15 In contrast, others have not demonstrated racial disparity in survival despite differential treatment of cardiac procedures,3 and seriously ill patients.19,20 Moreover, a recent study that included a greater level of clinical detail, as well as disease-specific severity adjustment, reported similar or even lower mortality rates for African–American patients with six common medical diagnoses.21
A series of studies has also reported lower use of invasive procedures and higher mortality for women with cardiovascular disease.4–7 For example, women were 30% to 70% less likely to undergo cardiac catheterization and coronary bypass surgery after myocardial infarction,4 as well as half as likely to undergo peripheral vascular surgery.5 Similarly, mortality rates in women following acute myocardial infarction7 and coronary bypass grafting 6 have been reported to be, respectively, 54% and 120% higher than in men. The etiology of the gender differences is not clear, but may be due in part to anatomic factors (i.e., coronary artery diameter),6 or differences in results of noninvasive testing,22 as well as possible physician or patient biases.
The results of the present study, in which no racial or gender differences in surgical intervention or short-term mortality were observed, differ from those of previous reports. There are several potential explanations for the disparity in findings. In contrast to diseases with a long duration of symptoms prior to invasive treatment, or for which surgical intervention is usually an elective decision, surgical treatment for bleeding ulcers is frequently performed as an emergency procedure in the setting of active bleeding, after conservative therapy has failed. Treatment alternatives are also more limited for bleeding ulcers than for coronary artery disease, and mainly include pharmacotherapy as well as diagnostic and therapeutic endoscopy.10 Surgical intervention is less commonly performed than for other diseases, and criteria for surgery are fairly stringent.10,11 Finally, although there may not be an absolute correlation with ultimate treatment selection, physician discretion, or the degree of controversy surrounding the indication for the procedure,23 it would be expected to be lower for ulcer surgery than for other interventions.
We recognize several important limitations of the study. First, Medicare data are not designed for measurement of ulcer characteristics such as stigmata of recent hemorrhage or size, or other prognostic factors such as comorbidity. Second, the accuracy of diagnostic and procedure coding has been questioned. In a report that compared approximately 2,500 medical record audits with the corresponding MEDPAR files,24 inaccuracies (principally undercoding) were found in 15% of comorbidities, accounted for mostly by coronary atherosclerosis, old myocardial infarction, and atrial fibrillation. Diagnostic coding for peptic ulcer appears to be more accurate, with a sensitivity (proportion of cases with the diagnosis present on chart review that had the code on the MEDPAR file) of 92% and a positive predictive value (proportion of cases on the MEDPAR file that had the diagnosis present on chart review) of 89%.25 The accuracy of coding for surgical procedures approaches 100%.25 Third, because MEDPAR data include only inpatient treatment of ulcers, it is possible that there were gender and racial differences in outpatient management or propensity for hospitalization. However, because older age is viewed as a prognostic factor in upper gastrointestinal hemorrhage,10 it would be expected that the vast majority of such patients would be hospitalized.
Given the lack of data on the accuracy of procedural coding for endoscopy, we did not measure its use, nor differentiate between diagnostic and therapeutic applications of this procedure. Thus, there may have been gender and racial differences in the use of endoscopic procedures. If endoscopy was required to diagnose a bleeding ulcer and was used less often in women or African–American patients, then they may have been underrepresented in the sample. Similarly, if the accuracy of coding varied across racial or gender groups, the sample may have been biased in favor of white or male patients. Finally, we were unable to directly measure process of care.26 Therefore, the implication of our findings on quality of care is uncertain, and we suggest that future studies include record review for direct measurement of quality indicators.
In summary, our findings in a national sample of hospitalized patients with complicated peptic ulcers suggest that there is no significant gender and racial difference in therapy and outcome. The findings raise the possibility that the phenomenon of gender and racial disparity in treatment, well described for patients with a few diagnoses, may not be generalizable to patients with other known diagnoses. These findings are noteworthy at a time when the significance of previous studies that reported racial variation in access to care and therapy received is being actively investigated.
References
- 1.Goldberg KC, Hartz AJ, Jacobsen SJ, Krakauer H, Rimm AA. Racial and community factors influencing coronary artery bypass graft surgery rates for all 1986 Medicare patients. JAMA. 1992;267:1473–7. [PubMed] [Google Scholar]
- 2.Whittle J, Conigliaro J, Good CB, Lofgren RP. Racial differences in the use of invasive cardiovascular procedures in the Department of Veterans Affairs medical system. N Engl J Med. 1993;329:621–7. doi: 10.1056/NEJM199308263290907. [DOI] [PubMed] [Google Scholar]
- 3.Peterson ED, Wright SM, Daley J, Thibault GE. Racial variation in cardiac procedure use and survival following acute myocardial infarction in the Department of Veterans Affairs. JAMA. 1994;271:1175–80. [PubMed] [Google Scholar]
- 4.Giles WH, Anda RF, Casper ML, Escobedo LG, Taylor HA. Race and sex differences in rates of invasive cardiac procedures in U.S. hospitals. Arch Intern Med. 1995;155:318–24. [PubMed] [Google Scholar]
- 5.Feinglass J, McDermott MM, Foroohar M, Pearce WH. Gender differences in interventional management of peripheral vascular disease: evidence from a blood flow laboratory population. Ann Vasc Surg. 1994;8:343–9. doi: 10.1007/BF02132995. [DOI] [PubMed] [Google Scholar]
- 6.O’Connor GT, Morton JR, Diehl MJ, et al. Differences between men and women in hospital mortality associated with coronary artery bypass graft surgery. Circulation. 1993;88:2104–10. doi: 10.1161/01.cir.88.5.2104. [DOI] [PubMed] [Google Scholar]
- 7.Becker RC, Terrin M, Ross R, et al. Comparison of clinical outcomes for women and men after myocardial infarction. Ann Intern Med. 1994;120:638–45. doi: 10.7326/0003-4819-120-8-199404150-00003. [DOI] [PubMed] [Google Scholar]
- 8.Katz SJ, Hofer TP. Socioeconomic disparities in preventive care persist despite universal coverage. JAMA. 1994;272:530–4. [PubMed] [Google Scholar]
- 9.NIH Consensus Conference. Therapeutic endoscopy and bleeding ulcers. JAMA. 1989;262:1369–72. [PubMed] [Google Scholar]
- 10.Laine L, Peterson WL. N Engl J Med. Vol. 331. 1994. Bleeding peptic ulcer; pp. 717–27. [DOI] [PubMed] [Google Scholar]
- 11.Debas HT, Orloff SL. Surgery for peptic ulcer disease. In: Yamada T, editor. Textbook of Gastroenterology. Philadelphia, Pa: JB Lippincott; 1991. pp. 1379–98. [Google Scholar]
- 12.Cooper GS, Yuan Z, Landefeld CS, Rimm AA. Surgery for colorectal cancer: race-related differences in rates and survival among Medicare beneficiaries. Am J Public Health. 1996;86:852–6. doi: 10.2105/ajph.86.4.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eley JW, Hill HA, Chen VW, et al. Racial differences in survival from breast cancer. JAMA. 1994;272:947–54. doi: 10.1001/jama.272.12.947. [DOI] [PubMed] [Google Scholar]
- 14.Natarajan N, Nemoto T, Mettlin C, Murphy GP. Race related differences in breast cancer patients. Cancer. 1985;56:1704–9. doi: 10.1002/1097-0142(19851001)56:7<1704::aid-cncr2820560740>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 15.Mayer WJ, McWhorter WP. Black/white differences in non-treatment of bladder cancer patients and implications for survival. Am J Public Health. 1989;79:772–4. doi: 10.2105/ajph.79.6.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gornick ME, Eggers PW, Reilly TW, et al. Effects of race and income on mortality and use of services in Medicare beneficiaries. N Engl J Med. 1996;335:791–9. doi: 10.1056/NEJM199609123351106. [DOI] [PubMed] [Google Scholar]
- 17.Jepson C, Kessler LG, Portnoy B, Gibbs T. Black-white differences in cancer prevention knowledge and behavior. Am J Public Health. 1991;81:501–4. doi: 10.2105/ajph.81.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caldwell SH, Popenoe R. Perceptions and misperceptions of skin color. Ann Intern Med. 1995;122:614–7. doi: 10.7326/0003-4819-122-8-199504150-00010. [DOI] [PubMed] [Google Scholar]
- 19.Williams JF, Zimmerman JE, Wagner DP, Hawkins M, Knaus WA. African-American and white patients admitted to the intensive care unit: Is there a difference in therapy and outcome? Crit Care Med. 1995;23:626–36. doi: 10.1097/00003246-199504000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Phillips RS, Hamel MB, Teno JM, et al. Race, resource use, and survival in seriously ill hospitalized adults. J Gen Intern Med. 1996;11:387–96. doi: 10.1007/BF02600183. [DOI] [PubMed] [Google Scholar]
- 21.Gordon HS, Harper DL, Rosenthal GE. Racial variation in predicted and observed in-hospital death: a regional analysis. JAMA. 1996;276:1639–44. [PubMed] [Google Scholar]
- 22.Mark DB, Shaw LK, DeLong ER, Califf RM, Pryor DB. Absence of sex bias in the referral of patients for cardiac catheterization. N Engl J Med. 1994;330:1101–6. doi: 10.1056/NEJM199404213301601. [DOI] [PubMed] [Google Scholar]
- 23.Mort EA, Weissman JS, Epstein AM. Physician discretion and racial variation in the use of surgical procedures. Arch Intern Med. 1994;154:761–7. [PubMed] [Google Scholar]
- 24.Green J, Wintfeld N. How accurate are hospital discharge data for evaluating effectiveness of care? Med Care. 1993;31:719–31. doi: 10.1097/00005650-199308000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Fisher ES, Whaley FS, Krushat WM, et al. The accuracy of Medicare’s hospital claims data: progress has been made but problems remain. Am J Public Health. 1992;82:243–8. doi: 10.2105/ajph.82.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kahn KL, Pearson ML, Harrison ER, et al. Health care for black and poor hospitalized Medicare patients. JAMA. 1994;271:1169–74. [PubMed] [Google Scholar]