Abstract
OBJECTIVES
To evaluate the effects of a brief educational program on beliefs, knowledge, and behaviors related to skin cancer control among internal medicine housestaff and attending physicians.
DESIGN
Randomized controlled trial.
SETTING
Urban academic general medicine practice.
PARTICIPANTS
Internal medicine housestaff and attending physicians with continuity clinics at the practice site.
INTERVENTION
Two 1-hour educational seminars on skin cancer control conducted jointly by a general internist and a dermatologist.
MEASUREMENTS AND MAIN RESULTS
Self-reported attitudes and beliefs about skin cancer control, ability to identify and make treatment decisions on 18 skin lesions, and knowledge of skin cancer risk factors were measured by a questionnaire before and after the teaching intervention. Exit surveys of patients at moderate to high risk of skin cancer were conducted 1 month before and 1 month after the intervention to measure physician skin cancer control practices reported by patients. Eighty-two physicians completed baseline questionnaires and were enrolled in the study, 46 in the intervention group and 36 in the control group. Twenty-five physicians attended both sessions, 11 attended one, and 10 attended neither. Postintervention, the percentage of physicians feeling adequately trained increased from 35% to 47% in the control group ( p = .34) and from 37% to 57% in the intervention group ( p = .06). Intervention physicians had an absolute mean improvement in their risk factor identification score of 6.7%, while control physicians’ mean score was unchanged ( p = .06). Intervention and control physicians had similar increases in their postintervention lesion identification and management scores. Postintervention, the mean proportion of patients per physician stating they were advised to watch their moles increased more among intervention physicians than control physicians (absolute difference of 19% vs −8%, p = .04). Other changes in behavior were not significant.
CONCLUSIONS
Although we observed a few modest intervention effects, overall this brief skin cancer education intervention did not significantly affect primary care physicians’ skin cancer control attitudes, beliefs, knowledge, or behaviors. A more intensive intervention with greater participation may be necessary to show a stronger impact on attitudes and knowledge about skin cancer control among primary care physicians.
Keywords: skin cancer, prevention, early detection, primary care, medical education
Skin cancer is a common diagnosis with significant associated morbidity and mortality. More than 800,000 new cases of skin cancer are diagnosed each year. Most of these diagnoses are of basal and squamous cell carcinomas, which are rarely fatal but can cause significant disfigurement.1 Melanoma, however, which accounts for less than 5% of all cases of skin cancer, is increasing in incidents and is expected to cause 7,300 deaths in 1997.2
Primary prevention through limiting exposure to solar radiation is likely to decrease the risk of both melanoma and nonmelanoma skin cancer given their strong association with sun exposure.3,4
The mortality and morbidity associated with skin cancer may also be mitigated with careful screening. Early detection of nonmelanoma may reduce morbidity, and early detection of melanoma may reduce mortality.5 Although no controlled studies have demonstrated that screening for melanoma by primary care physicians improves outcomes, a time series study of an educational campaign to encourage melanoma screening by primary care providers found a trend toward a reduction in mortality.6 In addition, regular screening of high-risk patients by dermatologists has been associated with decreased melanoma thickness, which may translate into decreased mortality.7,8
Despite the potential benefit of skin cancer prevention and early detection by the primary care physician, studies show that these physicians infrequently counsel patients on skin cancer and perform skin examinations.9–11 Potential barriers to skin cancer control practices by primary care physicians include lack of reimbursement for preventive care, distraction by other health care problems, and inadequate training in skin cancer counseling and performance of skin examinations.10,12 Several studies have shown that the ability of nondermatologists to identify and specifically name malignant or premalignant lesions is suboptimal when compared with that of dermatologists, although their ability to determine which of these lesions require biopsy may not be so disparate.9,13,14 Inadequate time for skin cancer control practices during an office visit is another important potential barrier as primary care physicians are presented with both an increasing number of competing demands and shorter office visits.10
In spite of the barriers, primary care physicians are in a key position to provide patients who are at risk of developing skin cancer with prevention counseling and early detection. Primary care physicians are more likely than dermatologists to have regular contact with patients before a diagnosis of melanoma.15 In a study by Geller et al., persons diagnosed with melanoma reported extensive contact with their primary care physician in the year before diagnosis.15 Twenty percent of these patients reported having regular contact with a dermatologist as compared with 87% who stated they saw a primary care physician on a regular basis.
As the managed care environment limits access to specialists, the role of primary care physicians in skin cancer control will continue to become more important. Given the time constraints of a busy primary care physician and the relatively low lifetime risk of melanoma of approximately 1%,16 targeting patients at risk of skin cancer for counseling and early detection efforts is a potentially more efficient, practical strategy than providing these practices to all patients regardless of individual risk. The purpose of this study was to test whether providing a brief educational program to general internal medicine housestaff and attending physicians could change their skin cancer control attitudes, improve their knowledge, and increase their counseling and examination performance among patients at moderate to high risk.
METHODS
Study participants were internal medicine housestaff and attending physicians with outpatient practices in the Division of General Internal Medicine of a midwestern urban university medical school. Physicians were stratified by training level (housestaff vs attending physicians) and randomized using a random number table into the intervention or control group.
Intervention
The intervention group physicians were invited to attend two 1-hour small group educational sessions on skin cancer control conducted jointly by a dermatologist and a general internist. The first session reviewed an approach for determining skin cancer risk and targeting individuals at moderate to high risk for counseling on skin cancer prevention strategies. The second session reviewed early detection of both nonmelanoma and melanoma skin cancers using photochromes from the personal collection of one of the investigators.
Physician Questionnaire
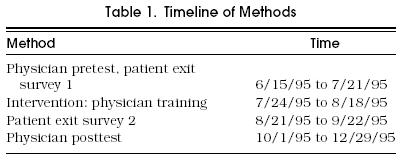
Before the intervention, a research assistant approached all of the physicians and asked them to fill out a baseline questionnaire on their skin cancer control attitudes, beliefs, knowledge, and clinical practices. The questionnaire was administered again approximately 1 month following the intervention (Table 1). The questionnaire consisted of questions on previous dermatology training, beliefs about skin cancer control, counseling, and examination practices; a section asking physicians to provide a list of skin cancer risk factors; and a section on lesion identification and management. A skin cancer risk score was computed as the percentage of 12 accepted risk factors that the physicians listed in the 12 blank spaces provided.17,18
Table 1.
Timeline of Methods
The questions in the section on lesion identification and management were based on 18 photographs of skin lesions with pathologically confirmed diagnoses obtained from the collection of one of the study investigators. The lesions consisted of four benign nevi, one seborrheic keratosis, one solar lentigo, three basal cell carcinomas, two squamous cell carcinomas, four melanomas, and two atypical nevi. Physicians were asked to name the lesion (fill in the blank) and choose a plan of action from four possible responses: (1) reassure patient, (2) schedule follow-up session, (3) perform cryotherapy, or (4) refer to dermatology for consultation or biopsy. For several questions, more than one response was accepted as correct. Blank responses were counted as incorrect. Lesion identification and management scores were determined for each physician as the percentage of 18 lesions correctly named and managed, respectively.
Patient Exit Interviews
One month before and one month after the intervention sessions, a sample of consecutive patients aged 18 to 50 years scheduled for new patient visits and general checkups at the study site were asked to complete a brief questionnaire on sun protection practices after their checkout process had been completed. The questionnaire consisted of eight questions used to categorize patients into skin cancer risk groups (low risk, moderate risk, or high risk)1,19; three questions on the patient’s behavior with regard to sun protection and deliberate tanning; and five questions on whether or not their current physician had, either that day or ever, counseled them on certain sun protection practices or performed a skin examination.
Statistical Analysis
The McNemar test was used to compare preintervention and postintervention responses to attitude and belief questions. For this analysis, responses to questions about who physicians believed should be targeted for skin cancer control practices were collapsed into two response categories, (1) those at increased risk of skin cancer and (2) all patients, as at most one physician responded that no patients should be targeted. The analysis was performed separately for intervention and control physicians. We used Fisher’s Exact Test to compare the absolute proportion of intervention and control physicians with the desired changes in each of the four attitudes or beliefs. Independent Student’s t tests were used to compare mean preintervention and postintervention differences in risk score, lesion identification score, and lesion management score between control and intervention physicians as well as between physicians who had attended both intervention sessions and those who did not attend either session.
The patient exit survey data were analyzed with the physician as the unit of analysis. Only data from patients classified as moderate to high risk, as determined by the sun sensitivity scale,17 were included for analysis of exit survey data. The proportion of moderate- to high-risk patients noting presence of each of the four skin cancer control practices was calculated for each physician. Mean proportions for each skin cancer control practice were then calculated for control and intervention physicians before and after the intervention. The mean differences in the paired preintervention and postintervention proportions for intervention and control physicians were compared by a weighted independent Student’s t test, where the weights were the total number of patients seen by a physician.
RESULTS
Eighty-two (86%) of 96 physicians completed the baseline questionnaire; 16 were attending physicians and 66 were housestaff physicians. There were 36 physicians in the control group and 46 in the intervention group. Of the 46 intervention physicians, 10 attended neither session, 11 attended only one session, and 25 attended both sessions.
Physician Self-Reported Attitudes
Control and intervention physicians responded similarly on the baseline questionnaire with regard to skin cancer control attitudes and beliefs (Table 2). Postintervention, the percentage of physicians feeling adequately trained increased from 35% to 47% in the control group ( p = .34) and from 37% to 57% in the intervention group ( p = .06). At baseline, more than 90% of all physicians surveyed believed that all patients should be counseled on sun protection behavior regardless of skin cancer risk, approximately two thirds believed that skin examinations should be performed on all asymptomatic patients regardless of risk; and 89% of control physicians and 78% of intervention physicians believed that they should advise all patients to perform skin self-examinations. Postintervention, there were trends toward an increase in the percentage of intervention physicians believing that skin examinations ( p = .18) and recommendation for skin self-examinations ( p = .18) should be practiced only among patients at increased risk of skin cancer.
Table 2.
Comparison of Skin Cancer Control Practices Among Intervention and Control Physicians
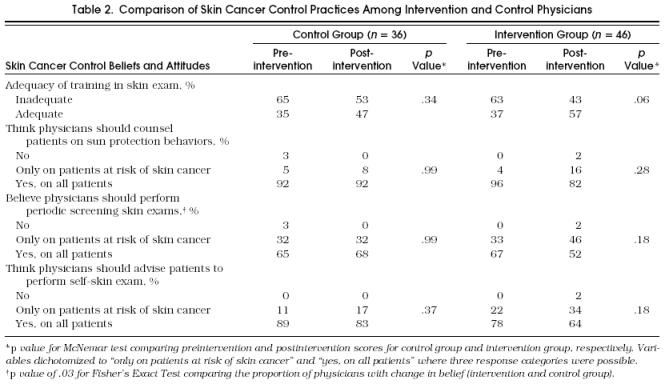
In a comparison of the absolute proportion of intervention and control physicians with the desired changes of the four attitudes or beliefs, the intervention physicians’ change in beliefs about performing periodic screening skin examinations only among at-risk patients ( p = .03) was the only significant difference noted.
Physician Knowledge Scores
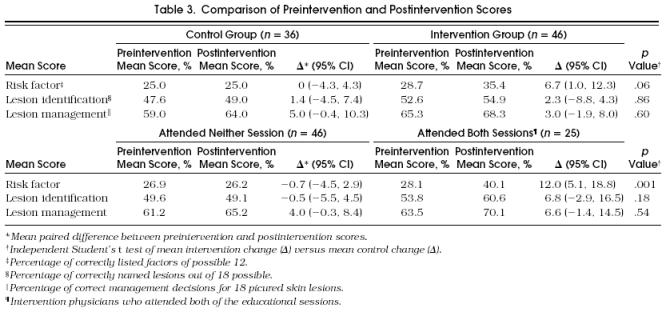
At baseline, there was a tendency for the intervention physicians to achieve higher mean scores than the control physicians, but these differences were not significant. Table 3 summarizes the preintervention and postintervention scores for risk factor identification, lesion identification, and lesion management among control and intervention physicians as well as for those physicians who attended both sessions compared with those who attended neither session. Intervention physicians had an absolute mean improvement in their risk factor identification score of 6.7% while control physicians’ mean score was unchanged ( p = .06). Intervention and control physicians had similar increases in their postintervention scores for lesion identification and management. When the data were reanalyzed comparing those who had attended both sessions compared with those who attended neither of the sessions, those physicians who attended both sessions had greater improvement in their risk identification score (absolute difference of 12.0% vs −0.7%, p = .004) and a greater, but nonsignificant improvement in their lesion identification score (absolute difference of 6.8% vs −0.5%, p = .18) and lesion management score (absolute difference of 6.6% vs 4.0%, p = .54).
Table 3.
Table Comparison of Preintervention and Postintervention Scores
Patient Exit Interviews
Table 4 summarizes the proportion of patients on whom physicians in the control and intervention groups performed skin cancer control practices as measured by patient exit survey. Preintervention and postintervention exit survey data were available for 12 control physicians and 18 intervention physicians. Of the control physicians in the analysis, 6 (50%) were housestaff compared with 11 (61%) of the intervention group. The groups were similar in their baseline beliefs about the adequacy of their previous training in skin examination. Of the 512 patients who completed the survey, patients of physicians with preintervention and postintervention exit survey data who were of moderate to high risk according to skin type were included in the analysis, leaving 82 preintervention patients and 113 postintervention patients.
Tabel 4.
Mean Proportion of Moderate- to High-Risk Patients per Physician Reporting Skin Cancer Control Practices
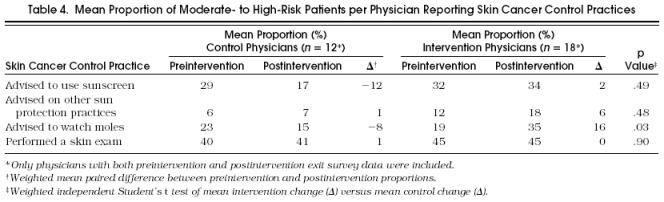
A skin examination was the most common practice noted by all patients with a preintervention mean per physician of 40% and 41%, respectively, reporting this practice. Postintervention changes in the mean proportion of patients per physician reporting that their physician advised them on sunscreen use or other sun protection strategies or performed a skin examination were similar in the two groups. The mean proportion of patients per physician stating they were advised to watch their moles increased more among intervention physicians than control physicians (absolute difference of 16% vs −8%, p = .03).
DISCUSSION
Although we observed a few modest intervention effects, overall this brief educational intervention did not significantly affect primary care physicians’ skin cancer control attitudes, beliefs, knowledge, or behaviors. Intervention physicians reported feeling more adequately trained in the skin examination after the intervention compared with baseline. When compared directly with control physicians, the only significant change in intervention physicians’ attitudes and beliefs was that they were more likely to shift their belief toward thinking that periodic skin examination should be performed only on patients at risk of skin cancer rather than on all patients. We view the shifting of skin cancer control practices to patients at moderate to high risk as a positive effect in that it enables primary care physicians to prioritize their preventive practices and acknowledges that lower-risk patients may derive greater benefit from receiving counseling on preventive issues more relevant to their individual risk panel. Intervention physicians also tended to have greater improvement in their ability to identify risk factors for skin cancer and were more likely than control physicians to advise moderate- to high-risk patients to watch their moles, but did not significantly improve in their performance of other skin cancer control practices or in their ability to name and make treatment decisions about skin lesions. There are several possible explanations for the absence of a stronger intervention effect on physicians’ skin cancer attitudes, beliefs, knowledge, and practices.
First, the number of physicians in each group may have been too small to detect significant differences in the main outcomes.
Second, only 56% of the intervention physicians actually attended both sessions, and only 78% attended at least one session. Although this low level of participation arguably simulates “real life” circumstances, it dilutes the potential differences in outcomes between the control and intervention physicians and decreases the likelihood of finding significant differences between the two groups. When the results were analyzed comparing physicians who had attended both sessions with those who had not attended either session, greater improvement was observed for all scores among those who attended both sessions. Although these results were not all significant, the consistency of this trend suggests that stronger positive results may have been found with greater physician participation. These groups were not based on random selection, however, and the findings may be biased, as the physicians who attended the sessions were likely to have been more interested and motivated to learn about skin cancer control.
Third, as the postintervention questionnaires were administered several months after the intervention, we may have missed an immediate, nonsustained difference between the two groups.
Fourth, as many of the patients who completed the exit interviews were new patients, we may have underestimated the proportions of patients per physician who might have received counseling or skin examinations if the physician had had more extended contact with the patient.
Lastly, a simple 2-hour educational intervention may not be sufficient to effect substantial change in attitudes, beliefs, and knowledge. Several educational sessions over a prolonged period of time, or providing physicians with ongoing practical experience in a dermatology practice, may be alternative methods of increasing knowledge and improving management skills and maintaining these changes over time.
In light of the lack of significant changes in attitudes and knowledge associated with the intervention, it is surprising that any difference at all was observed in physician skin cancer control behavior. Studies in other areas of preventive medicine have suggested that education alone is often not effective in improving performance of preventive services, and that sustained efforts may be required to modify established patterns of practice.20–23 Although the potential impact of the intervention on physician behavior is encouraging, some caution should be used when interpreting these results. The analysis was based on a small sample of physicians who were not randomly selected. Although these physicians displayed comparable baseline rates of behaviors and were similar with regard to perceptions of adequacy of previous skin examination training, it is possible that other baseline differences between the groups accounted for these findings or that these differences occurred by chance.
This brief educational intervention showed a trend toward some modest effects, but it did not significantly affect physicians’ attitudes, beliefs, knowledge, and behavior and would be unlikely to result in long-term change. Poor participation was an important limitation of the study and may suggest a lack of primary care physicians’ interest in this area. More intensive interventions may be necessary to show a stronger, long-term impact on skin cancer control attitudes and knowledge among primary care physicians. In addition to educational interventions, physician or patient reminders may be effective methods of changing physicians’ behavior with regard to skin cancer control practices. Further research into skin cancer control office systems and educational programs for primary care physicians will be necessary if primary care physicians are to effectively target skin cancer control services to appropriate patients.
References
- 1.Marks R. An overview of skin cancers: incidence and causation. Cancer. 1995;75(9):607–12. doi: 10.1002/1097-0142(19950115)75:2+<607::aid-cncr2820751402>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 2.Parker SL, Tong T, Bolden S, Wingo PA. Cancer statistics, 1997. CA Cancer J Clin. 1997;47(9):5–7. doi: 10.3322/canjclin.47.1.5. [DOI] [PubMed] [Google Scholar]
- 3.Glass AG, Hoover RN. The emerging epidemic of melanoma and squamous cell skin cancer. JAMA. 1989;262:2097–100. [PubMed] [Google Scholar]
- 4.Urback R. Incidence of nonmelanoma skin cancer. Dermatol Clin. 1991;9:751–5. [PubMed] [Google Scholar]
- 5.Koh HK. Cutaneous melanoma. N Engl J Med. 1991;325:171–82. doi: 10.1056/NEJM199107183250306. [DOI] [PubMed] [Google Scholar]
- 6.Mackie RM, Hole D. Audit of public education campaign to encourage earlier detection of melanoma. BMJ. 1992;304:1012–5. doi: 10.1136/bmj.304.6833.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rigel DS, Rivers JK, Kopf AW, et al. Dysplastic nevi: markers for increased risk for melanoma. Cancer. 1989;63:386–9. doi: 10.1002/1097-0142(19890115)63:2<386::aid-cncr2820630231>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 8.Tristen AD, Grin CM, Kopf AW, et al. Prospective follow-up for malignant melanoma in patients with atypical-mole syndrome. J Dermatol Surg Oncol. 1991;17:44–8. doi: 10.1111/j.1524-4725.1991.tb01592.x. [DOI] [PubMed] [Google Scholar]
- 9.Dolan NC, Martin GJ, Robinson JK, Rademaker AW. Skin cancer control practices among physicians in a university general medicine practice. J Gen Intern Med. 1995;10:515–9. doi: 10.1007/BF02602405. [DOI] [PubMed] [Google Scholar]
- 10.Wender RC. Barriers to effective skin cancer detection. Cancer. 1995;75S(9):691–8. doi: 10.1002/1097-0142(19950115)75:2+<691::aid-cncr2820751412>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 11.American Cancer Society. 1989 survey of physicians’ attitudes and practices in early cancer detection. CA Cancer J Clin. 1990;40(9):77–99. doi: 10.3322/canjclin.40.2.77. [DOI] [PubMed] [Google Scholar]
- 12.McCarthy GM, Lamb GC, Russel TJ, Young MJ. Primary care–based dermatology practice: internists need more training. J Gen Intern Med. 1991;6:52–6. doi: 10.1007/BF02599393. [DOI] [PubMed] [Google Scholar]
- 13.Cassileth BR, Clark WH, Lusk EJ, Frederick BE, Thompson CJ, Walsh WP. How well do physicians recognize melanoma and other problem lesions. J Am Acad Dermatol. 1986;14:555–60. doi: 10.1016/s0190-9622(86)70068-6. [DOI] [PubMed] [Google Scholar]
- 14.Ramsey DL, Fox AB. The ability of primary care physicians to recognize the common dermatoses. Arch Dermatol. 1981;117:620–2. [PubMed] [Google Scholar]
- 15.Geller AC, Koh HK, Miller DR, Clapp RW, Mercer MB, Lew RA. Use of health services before the diagnosis of melanoma. Implications for early detection and screening. J Gen Intern Med. 1992;7:154–7. doi: 10.1007/BF02598004. [DOI] [PubMed] [Google Scholar]
- 16.Ries LAG, Miller BA, Hankey BF, et al., editors. Bethesda, Md: National Cancer Institute; 1994. WEER Cancer Statistics Review, 1973–1991: Tables and Graphs. NIH publication no. 94-2789. [Google Scholar]
- 17.Rhodes AR, Weinstock MA, Fitzpatrick TB, Mihm MC, Sober AJ. Risk factors for cutaneous melanoma: a practical method of recognizing predisposed individuals. JAMA. 1987;258:3146–54. [PubMed] [Google Scholar]
- 18.Koh HK, Barnhill RL, Rogers Melanoma GS. Cutaneous Medicine and Surgery. In: Arndt K, LeBoit R, Robinson JK, Whitcomb R, editors. Philadelphia, Pa: WB Saunders Co.; 1996. pp. 1576–9. In: [Google Scholar]
- 19.Weinstock HA. Assessment of sun sensitivity by questionnaire: validity of items and formulation of a prediction rule. J Clin Epidemiol. 1992;45:547–52. doi: 10.1016/0895-4356(92)90104-u. [DOI] [PubMed] [Google Scholar]
- 20.Headrick LA, Speroff T, Pelecanos HI, Cebul RD. Efforts to improve compliance with the National Cholesterol Education Program guidelines. Results of a randomized controlled trial. Arch Intern Med. 1992;152:2490–6. [PubMed] [Google Scholar]
- 21.Browner WS, Baron RB, Solkowitz S, Adler LJ, Gullion DS. Physician management of hypercholesterolemia: a randomized trial of continuing medical education. West J Med. 1994;161(9):572–8. [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen SJ, Halvorson HW, Gosselink CA. Changing physician behavior to improve disease prevention. Prev Med. 1994;23(9):284–91. doi: 10.1006/pmed.1994.1040. [DOI] [PubMed] [Google Scholar]
- 23.McKinney WP, Barnas GP. Influenza immunization in the elderly: knowledge and attitudes do not explain physician behavior. Am J Public Health. 1989;79(9):1422–4. doi: 10.2105/ajph.79.10.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]


