Abstract
OBJECTIVE
Hypothyroidism often remains undetected because of the difficulty associating symptoms with disease. To determine the relation between symptoms and biochemical disease, we assessed symptoms and serum thyroid function tests, concurrently, for patients with and without hypothyroidism.
DESIGN
Cross-sectional study.
SETTING/ PATIENTS
Seventy-six newly diagnosed case patients with overt hypothyroidism and 147 matched control patients identified through outpatient laboratories in Michigan and Colorado.
MEASUREMENTS AND MAIN RESULTS
Patient symptoms were assessed by questionnaire. Case patients reported a higher proportion of hypothyroid symptoms than did control patients (30.2% vs 16.5%, p < .0001). Univariate analysis identified three significant predictors of an elevated level of thyroid-stimulating hormone (TSH) ( p < .05), and 13 symptoms which, when they had changed in the past year, were reported more often by case patients with hypothyroidism than by control patients ( p < .005). Individuals reporting changes in 7 or more symptoms were significantly more likely to have hypothyroidism (likelihood ratio [LR] = 8.7, 95% confidence interval [CI] 3.8, 20.2); those reporting changes in 2 or fewer symptoms were less likely to have hypothyroidism (LR = 0.5, 95% CI 0.4 , 0.7).
CONCLUSIONS
In this sample, the number of hypothyroid symptoms reported was directly related to the level of TSH. The association was stronger when more symptoms were reported. Symptoms that had changed in the past year were more powerful than symptoms reported present at the time of testing. This suggests that traditional symptoms are valuable when deciding which patients to test for hypothyroidism.
Keywords: hypothyroidism, symptoms, diagnosis
Traditional symptoms of hypothyroidism have been well known to medical practitioners since the clinical syndrome was first described by Gull in 1874.1–4 Yet hypothyroidism often remains undetected because of the difficulty with ascribing symptoms to disease. The pathophysiologic changes generally require months or years to manifest as clinical signs and symptoms.2 Furthermore, the onset of hypothyroidism is so insidious that even classic symptomatology may go unnoticed or undiagnosed.5 Although most hypothyroid patients do have some signs and symptoms indicative of disease,6 it may be difficult to identify a “classic” clinical picture because symptoms may be nonspecific and thus confused with other health problems.2,5,7
The relation between symptoms and physiologic disease is so complex that clinicians turn to biochemical measures of thyroid dysfunction for diagnosis. However, it is unclear who should be tested. Many investigators,8–12 and several organizations, such as the American Thyroid Association, the American College of Physicians, the American Academy of Family Physicians, and the American Association of Clinical Endocrinologists recommend testing persons who have a greater likelihood of being hypothyroid., 13–16 The recommendations of the American College of Physicians, for example, include testing women over age 50 “who have general symptoms that could be caused by thyroid disease.”14 The U.S. Preventive Services Task Force does not recommend for or against screening in high-risk patients, such as older women. However, it does state that, “Clinicians should remain alert for subtle or nonspecific symptoms of thyroid dysfunction when examining such patients, and maintain a low threshold for diagnostic evaluation of thyroid function.”17 Evidence is lacking as to which symptom or symptoms increase the likelihood of biochemical hypothyroidism.
This study’s hypothesis is that symptoms correlate with biochemical hypothyroidism. In addition, symptoms that are reported to have developed recently may better distinguish between euthyroid and hypothyroid individuals than symptoms that are reported as simply present or absent. Therefore, symptoms may identify persons who are more likely to have the disease. Helfand and Crapo reported that, “in retrospect, patients detected by laboratory screening have clinical findings that suggest a thyroid disorder, but these findings did not lead clinicians to suspect the problem.”12 Currently, symptoms are not used systematically to decide whom to test for hypothyroidism. Previous research has shown that combinations of hypothyroid signs and symptoms can be used to select patients with a higher likelihood of disease.18–20 Identifying symptoms that increase disease likelihood could indicate who should have serum thyroid function tests. To determine whether traditional hypothyroid symptoms characterize biochemically defined disease, we compared self-reported symptoms with laboratory test results in a population of patients receiving blood tests at outpatient clinical laboratories.
METHODS
Patient Selection
Case patients with hypothyroidism and matched euthyroid control patients were identified through the laboratories of a university hospital, private teaching hospital, Veterans Administration hospital, or HMO laboratory in Denver, Colorado, between July 1991 and March 1993, and the laboratory of a 525-bed university-affiliated community teaching hospital in Grand Rapids, Michigan, between October 1992 and December 1995. Each facility’s institutional review board approved the study protocol.
Consecutive elevated levels of thyroid-stimulating hormone (TSH) were logged by laboratory personnel at each site. New adult hypothyroid case patients identified through these laboratories were eligible to participate in the study if their serum TSH level was above 20 μU/mL and a free or total thyroxine (T4) level was decreased. These criteria were chosen to recruit patients with overt hypothyroidism and not patients with mild or subclinical hypothyroidism. If patients had only the T SH test ordered by their physician, a free or total T4 test was performed on their previously drawn blood sample. Patients were excluded who were previously diagnosed with hypothyroidism or depression, were pregnant, had been hospitalized within the previous month, or were on β-adrenergic blocker medication at the time of contact. Patients who were unable to complete the questionnaire because of language barriers, retardation, or dementia were also excluded. It was possible for laboratory personnel to exclude many subjects, such as for known normal T4 level.
Case patients who did not have any exclusion criteria known to laboratory personnel were contacted by one of the authors (GJC) or a research assistant. If the subject could not be reached by telephone, a letter was mailed. Each patient was approached for enrollment using words similar to the following: “You are being invited to participate in a study that looks at various symptoms which you may or may not have, in order to relate the symptoms which patients report to the blood tests that doctors order.” Though efforts were made to identify potential subjects before they were diagnosed by their physician, some patients were inevitably suspicious of a diagnosis of thyroid disease. Those who agreed to participate were mailed, in most cases, a self-administered symptoms questionnaire and a consent form. A few individuals completed the questionnaire and consent form at the participating institution, and in one case, the patient completed a faxed copy of the questionnaire and consent form while in the waiting room of his primary care physician’s office before his scheduled follow-up appointment. Anyone completing less than 75% of the symptom questions was excluded from further analysis.
Controls were selected from all outpatients who had blood drawn for reasons other than thyroid function testing at the participating laboratories in Colorado and Michigan. The intended ratio of controls to cases was 2:1 to increase statistical power. For most case patients there were two control patients; however, a second control was not available for each case. Only serum samples that did not have any orders for thyroid function tests were included. Thyroid-stimulating hormone and T4 levels were obtained from the remaining serum according to study protocol. Control patients were matched by gender and age to the hypothyroid case patients. Specifically, each case patient was matched to a control patient who was the case patient’s age plus or minus 5 years. The control patients who carried a diagnosis of thyroid disease or depression, were pregnant, had been hospitalized within the previous month, or were taking β-adrenergic blockers were excluded to maintain comparability to cases.
The control group was chosen in the above manner to create distinct study groups, with and without disease. This was done to determine if symptoms could distinguish obvious cases of disease from euthyroid individuals. Symptoms that could not differentiate between these two groups would be unlikely to distinguish patients seen in clinical practice with other conditions that have overlapping symptoms.21 Control patients were approached for enrollment in the same manner as case patients. As with the case patients, those not completing at least 75% of the questionnaire and those who were unable to finish the questionnaire for logistic reasons were excluded. The remaining patients served as euthyroid controls. There were no exclusions with regard to race or gender among either case or control patients.
Symptom Assessment
Classic symptoms of hypothyroidism were assessed by questionnaire. Symptoms were selected for study by first compiling a list of 29 symptoms of hypothyroidism from the literature and the work of previous investigators.3,4,7,18,19,22 General internists and endocrinologists were surveyed to determine which symptoms were most suggestive of hypothyroidism in their clinical experience. The final questionnaire included the symptoms reported most often in the survey. Questions asked both about the presence or absence of the symptom, and whether or not the symptom had changed within the previous year. This permitted comparison of the presence of symptoms versus the recent onset of symptoms in the characterization of disease. (The questionnaire is available from the authors on request.)
The final questionnaire included 17 thyroid symptoms. Three other “thyroid-neutral” questions, unrelated to hypothyroidism, were included to avoid patient bias in reporting symptoms specific to one disease. No questions were taken from published questionnaires. The symptoms questionnaire responses were directional as written, so that the range of each symptom response was 1 through 5, with 3 being a neutral response and each extreme representing either complete absence of the hypothyroid symptom or marked severity. (The direction varied from question to question to avoid bias in reporting symptoms, but responses were recoded so that those numbered 4 or 5 consistently represented symptoms of clinical disease and those numbered 1, 2, or 3 were negative for disease.) The symptoms questionnaire underwent extensive pilot testing in a primary care practice. Readability was determined by Walch’s Readability Rater for Printed Materials, and tested to a third grade level in its final form.
Statistical Analysis
The number and percentage of positive symptom responses were determined for each case and control patient. The mean percentage, rather than the number of reported symptoms, was compared between case and control patients because the number of questions a participant could answer varied by gender and menstrual status. The symptom severity (whether the symptom was reported at a severity level of 4 or at a severity level of 5) was used only when comparing the percentage of symptoms between the two groups. For all other analyses, symptoms were dichotomized: symptoms reported as level 4 or 5 were considered positive symptoms, and responses 1, 2, and 3 were considered negative. Among cases, Spearman’s correlation coefficient was calculated between TSH level and the percentage of reported hypothyroid symptoms, to relate symptoms to progressively worsening thyroid function.
The χ2 test or Student’s t test was used for univariate comparison of symptoms and disease. The proportion of hypothyroid individuals and euthyroid individuals reporting each symptom was calculated. Likelihood ratios (LRs) were calculated as the ratio of the likelihood of a positive symptom in a patient with disease versus the likelihood of a positive symptom in a patient without disease. Though the LRs derived from this study population may not be applicable to the general population, they may suggest the relative strength of individual symptoms to enhance posttest odds. For example, if a patient reports a symptom with LR = 5, the likelihood of being hypothyroid would rise from a pretest probability of approximately 5% to approximately 20%.23,24 Certainly, a patient reporting symptoms or groups of symptoms with greater LRs would have a higher likelihood of disease, perhaps crossing the physician’s threshold for laboratory testing. Confidence intervals were calculated using Epi Info version 6.02.
The subset of univariately significant symptoms was used to figure the number and percentage of hypothyroid and euthyroid subjects who reported increasing numbers of symptoms. Likelihood ratios were calculated for strata of increasing numbers of reported symptoms.
Conditional stepwise logistic regression was applied to determine which symptoms were independent predictors of disease state while controlling for other symptoms, (The SAS System, PHR EG Procedure). Exploratory analysis among menstruating women found that the two questions regarding menstrual symptoms were not statistically significant (p < .05). Therefore, the regression analysis evaluated the remaining symptoms in the entire study population. Conditional regression analysis was used to account for matching, and was based on 69 case and 132 control patients because of missing data. Receiver-operating characteristic (ROC) areas were calculated using ROC Curve Analyzer, version 6 (Robert M. Centor and Jerry Keightley).
RESULTS
Between July 1991 and December 1995, 76 case patients with newly diagnosed hypothyroidism and 147 control patients were enrolled. One case patient was enrolled into the study for approximately every 10 potential case patients recruited in Colorado, though this varied by institution. In Michigan, one case patient was enrolled for about every 14 potential case patients, also identified on the basis of having had thyroid function tests ordered. The exact number excluded for each criterion is unknown because exclusions occurred both through the laboratory and through the investigators. The above estimates of enrollment were made according to the mean number of all elevated T SH levels reported by each laboratory. Many thyroid function tests were ordered on patients for reasons other than suspected new hypothyroidism, accounting for our low “enrollment rate.” When subsequent interview or chart review excluded potential enrollees, this was most often because thyroid function tests had been ordered to monitor thyroid hormone replacement in known hypothyroid patients.
Among the 76 case patients, 36 were enrolled from Colorado and 40 from Michigan. Fifty-four (71.0%) were women. Age ranged from 20 to 85 years, and the mean age was 44.4 years. Of the 71 respondents to the question of family history, 30 (42.2%) reported at least one family member with thyroid illness (Table 1). Among the 147 control patients enrolled proportionately from the Colorado and Michigan hospitals, 104 (70.7%) were women. The mean age of the control group was 45.8 years (range 19–86 years), and 24 (16.8%) of 143 respondents reported a family history of thyroid disease. Case and control patients differed demographically in the reported family history and, as would be expected, in the mean TSH level (Table 1). The T4 levels were not compared statistically, as approximately half of the samples were tested by total T4 assay, while the other half were tested by free T4 according to the policies of the individual laboratories.
Table 1.
Demographics
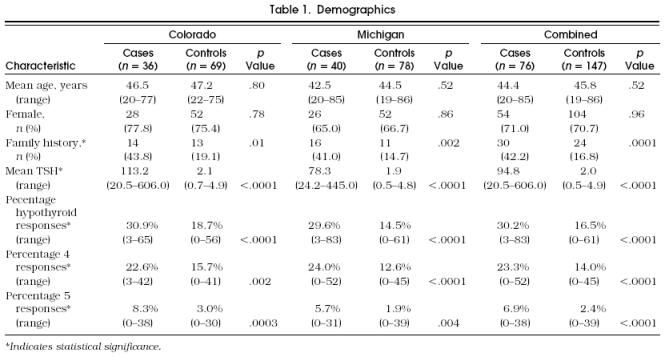
Case patients reported higher percentages of total positive hypothyroid responses than did controls (30.2% vs 16.5%, p < .0001). When positive symptoms were split according to severity, the difference between case and control patients in the percentage of reported symptoms remained significant regardless of level 4 or level 5 severity (Table 1). The number of symptoms correlated weakly with TSH (r = .35, p < .0001), showing increasing numbers of reported symptoms with decreasing thyroid function.
Univariate analysis showed that three “current” symptoms differed significantly between case and control subjects: hoarse voice, dry skin, and muscle cramps (Table 2). Among the “changed” symptoms, a hoarser voice, deeper voice, drier skin, feeling colder, feeling more tired, having puffier eyes, more muscle cramps, weaker muscles, more constipation, feeling more depressed, slower thinking, poorer memory, and having more difficulty with math were significant (p < .05) (Table 3). In general, the proportion of hypothyroid patients reporting individual symptoms was low. However, some symptoms were reported in very low percentages among the euthyroid group, such as having a hoarse voice.
Table 2.
Current Symptoms of Hypothyroidism
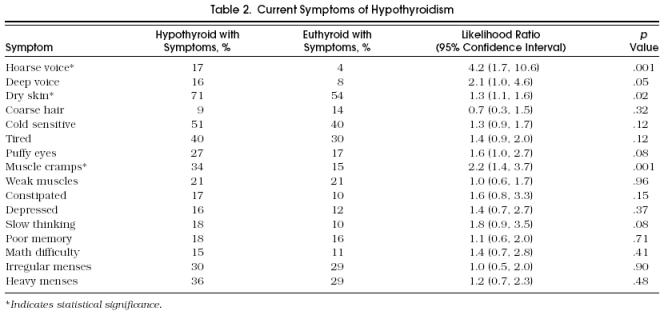
Table 3.
Changed Symptoms of Hypothyroidism
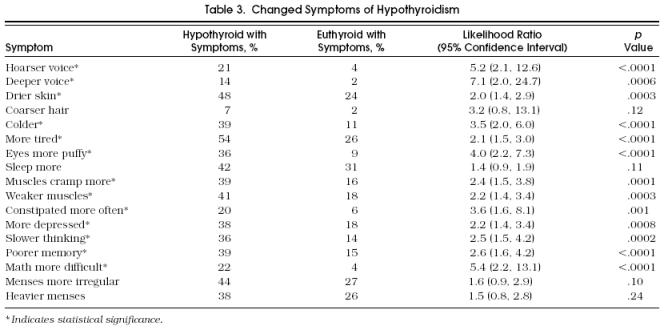
Symptoms were entered into conditional stepwise logistic regression analysis. Two current symptoms, hoarse voice and muscle cramps, were significant in both univariate and multivariate analysis. Of the 13 changed symptoms, only 2 remained significant in multivariate analysis: puffier eyes and being constipated more often.
In ROC analysis the area under the curve was equal to 0.72 when using the 13 changed symptoms, and 0.66 when using only the three current symptoms. When the numbers of changed symptoms were grouped, control patients reported zero, one, or two symptoms significantly more often, while case patients reported seven or more symptoms more often (Fig. 1).
Figure 2.
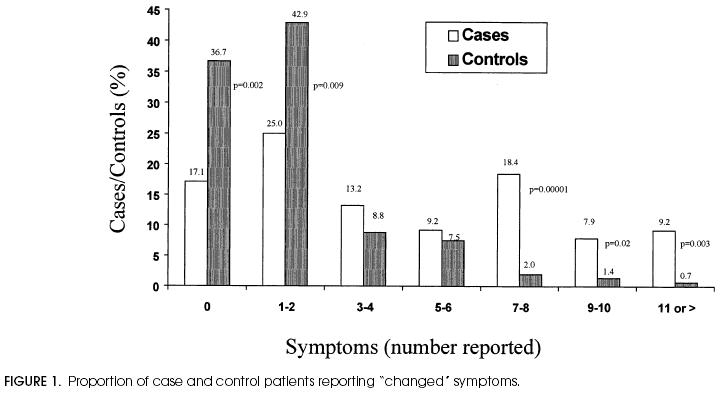
Proportion of case and control patients reporting “changed” symptoms.
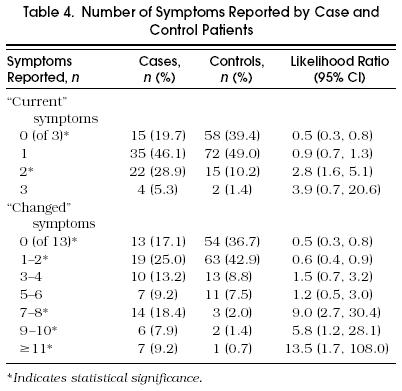
Likelihood ratios were reported for all individual symptoms (Table 2 and Table 3), and increasing numbers of univariately significant symptoms (Table 4" and increasing numbers of univariately significant symptoms (Table 4). The LRs were small for many individual symptoms, corresponding to small changes in disease likelihood. When the number of symptoms was evaluated, the LR associated with no reported symptoms was 0.5, suggesting a somewhat greater likelihood of being euthyroid. Likelihood ratios had greater impact when patients reported more symptoms. Specifically, patients reporting seven or more changed symptoms were much more likely to be hypothyroid (LR = 8.7; 95% CI 3.8, 20.2).
Table 4.
Number of Symptoms Reported by Case and Control Patients
DISCUSSION
This study compared patients with elevated TSH and euthyroid control patients, and found that symptoms associated with hypothyroidism were predictive of abnormal serum T SH in this population. The strength of association was proportional to the number of symptoms reported, particularly symptoms that had changed in the previous year. Although symptoms do not replace serum thyroid function tests in the diagnosis of disease, symptoms may help identify patients who should be tested for hypothyroidism.
The total percentage of self-reported symptoms was significantly higher in hypothyroid than in euthyroid subjects, and this was true regardless of symptom severity. A positive correlation, albeit modest, was found between the serum TSH concentration and the percentage of positive hypothyroid symptoms reported. This reflected a small increase in total symptoms with progressive deterioration of thyroid function.
Supporting the association of symptoms and disease was the observation that the likelihood of hypothyroidism increased in a graded manner as the number of reported symptoms increased. Other researchers have found that combinations of signs and symptoms increase the likelihood of hypothyroidism.18–20 Billewicz’ group is perhaps best known for investigating the value of symptoms in characterizing hypothyroidism. Both Billewicz’ and Seshadri’s groups combined symptoms to develop a symptoms score18,19; higher scores were more suggestive of hypothyroidism. White and Walmsley found that the proportion of patients requiring treatment for hypothyroidism was highest among those reporting five or more signs or symptoms.20 A similar observation is reported here: the likelihood of disease rose with increasing numbers of reported symptoms, and the LRs were greater when more symptoms were reported.
Previous studies have not distinguished between chronic and acute symptoms of hypothyroidism. In this study, only three current symptoms were significant by χ2 test, whereas 13 changed symptoms were significant. These findings suggest that a recent change in symptomatology is better able to distinguish the hypothyroid from the euthyroid state. Likelihood ratios were also generally higher when symptoms were reported as changed than when symptoms were reported as currently present or absent.
Few symptoms remained as independent predictors of disease in multivariate analysis. This suggests a high degree of intercorrelation among hypothyroid symptoms. Although multiple regression was somewhat limited by the number of cases relative to the number of symptoms under study, the results support the idea that the number of symptoms reported may be a stronger indicator of disease than a prediction rule based on multivariate modeling. The area under the ROC curve for changed symptoms was 0.72, indicating that in this population the reported changed symptoms will correctly distinguish subjects with and without an elevated T SH 72% of the time.
Despite this association, it is evident that symptoms cannot identify hypothyroidism as well as biochemical thyroid function tests. Symptoms of hypothyroidism have historically been regarded as nonspecific (that is, they are reported by a high proportion of individuals without hypothyroidism). In this study, it is evident that the proportion of hypothyroid subjects reporting any individual symptom is low. The presence of symptoms is suggestive of disease, but their absence fails to exclude disease. Although patients who report no symptoms are about twice as likely to be euthyroid as hypothyroid (Table 4), a patient who reports no changed symptoms still has a 19% chance of being hypothyroid. Greater precision is obviously attained by sensitive serum TSH measurements.
The results obtained in this study may not be directly applicable to other populations. The number of cases enrolled is relatively small. Also, the control group was selected to create distinct groups with and without disease for the reasons stated above. The LRs observed in this study may overestimate the value of symptoms in patients who have conditions with substantial symptom overlap with hypothyroidism.
In conclusion, traditional hypothyroid symptoms will not replace the supersensitive serum TSH assay in the diagnosis of disease. However, patients who report more symptoms, and more recently developed symptoms, are more likely to have hypothyroidism. This being the case, patients who report more symptoms, particularly symptoms of new onset, should be tested with serum thyroid function tests.
Acknowledgments
Many thanks go to Dr. Larry Baer for his assistance with analysis; to Fred Ullrich for aid in multivariable methods; to Drs. Robert Wigton and Thomas Tape for their thoughtful review of an earlier draft of this manuscript; to Shirley Yeich, Kathy McCormack, and Darlene Mayhak for manuscript preparation; and to Dr. Albert G. Canaris for his always excellent advice.
References
- 1.Evered DC, Ormston BJ, Smith PA, Bird T. Grades of hypothyroidism. BMJ. 1973;1:657–62. doi: 10.1136/bmj.1.5854.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larsen PR, Ingbar SH. The thyroid gland. In: Wilson JD, Foster DW, editors. Williams Textbook of Endocrinology. Philadelphia, Pa: WB Saunders Co; 1992. pp. 357–487. In. eds. [Google Scholar]
- 3.Wartofsky L, Ingbar SH. Diseases of the thyroid. In: Wilson JD, Braunwald E, Isselbacher KJ, et al., editors. Harrison’s Principles of Internal Medicine. 12th ed. New York, NY: McGraw-Hill; 1991. pp. 1692–712. In. eds. [Google Scholar]
- 4.Golding DN. Hypothyroidism presenting with musculoskeletal symptoms. Ann Rheum Dis. 1970;29:10–4. doi: 10.1136/ard.29.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gavin LA. The diagnostic dilemmas of hyperthyroxinemia and hypothyroxinemia. Adv Intern Med. 1988;33:185–204. [PubMed] [Google Scholar]
- 6.Tachman ML, Guthrie GP. Hypothyroidism: diversity of presentation. Endocr Rev. 1984;5:456–65. doi: 10.1210/edrv-5-3-456. [DOI] [PubMed] [Google Scholar]
- 7.Schectman JM, Kallenberg GA, Shumacher RJ, Hirsch RP. Yield of hypothyroidism in symptomatic primary care patients. Arch Intern Med. 1989;149:861–4. [PubMed] [Google Scholar]
- 8.Bahemuka M, Hodkinson HM. Screening for hypothyroidism in elderly inpatients. BMJ. 1975;2:601–3. doi: 10.1136/bmj.2.5971.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sawin CT, Chopra D, Azizi F, Mannix JE, Bacharach P. The aging thyroid. Increased prevalence of elevated serum thyrotropin levels in the elderly. JAMA. 1979;242:247–50. doi: 10.1001/jama.242.3.247. [DOI] [PubMed] [Google Scholar]
- 10.Eggertsen R, Petersen K, Lundberg PA, Hystrom E, Lindstedt G. Screening for thyroid disease in a primary care unit with a thyroid stimulating hormone assay with a low detection limit. BMJ. 1988;297:1586–92. doi: 10.1136/bmj.297.6663.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danese MD, Powe NR, Sawin CT, Ladenson PW. Screening for mild thyroid failure at the periodic health examination. A decision and cost-effectiveness analysis. JAMA. 1996;276:285–92. [PubMed] [Google Scholar]
- 12.Helfand M, Crapo LM. Screening for thyroid disease. Ann Intern Med. 1990;112:840–9. doi: 10.7326/0003-4819-112-11-840. [DOI] [PubMed] [Google Scholar]
- 13.Singer PA, Cooper DS, Levy EG, et al. Treatment guidelines for patients with hyperthyroidism and hypothyroidism. JAMA. 1995;273:808–12. [PubMed] [Google Scholar]
- 14.Eddy DM. Philadelphia, Pa: American College of Physicians; 1991. Common Screening Tests. [Google Scholar]
- 15.American Academy of Family Physicians . Kansas City, Mo: American Academy of Family Physicians; 1994. Age Charts for Periodic Health Examination. [Google Scholar]
- 16.American Association of Clinical Endocrinologists . Jacksonville, Fla: American Association of Clinical Endocrinologists and American College of Endocrinology; 1995. Clinical Practice Guidelines for the Evaluation of Hyperthyroidism and Hypothyroidism. [Google Scholar]
- 17.U.S. Preventive Services Task Force . 2nd ed. Baltimore, Md: Williams and Wilkins; 1996. Screening for thyroid disease. Guide to Clinical Preventive Services; pp. 209–18. In: [Google Scholar]
- 18.Billewicz WZ, Chapman RS, Crooks J, et al. Statistical methods applied to the diagnosis of hypothyroidism. QJM. 1969;38:255–66. [PubMed] [Google Scholar]
- 19.Seshadri MS, Samuel BU, Kanagasabapathy AS, Cherian AM. Clinical scoring system for hypothyroidism: is it useful? J Gen Intern Med. 1989;4:490–2. doi: 10.1007/BF02599546. [DOI] [PubMed] [Google Scholar]
- 20.White GH, Walmsley RN. Can the initial clinical assessment of thyroid function be improved? Lancet. 1978;2:933–5. doi: 10.1016/s0140-6736(78)91645-8. [DOI] [PubMed] [Google Scholar]
- 21.Nierenberg AA, Feinstein AR. How to evaluate a diagnostic marker test. Lessons from the rise and fall of dexamethasone suppression test. JAMA. 1988;259:1699–702. [PubMed] [Google Scholar]
- 22.Cooper DS, Halpern R, Wood LC, Levin AA, Ridgway EC. L-Thyroxine therapy in subclinical hypothyroidism. Ann Intern Med. 1984;101:18–24. doi: 10.7326/0003-4819-101-1-18. [DOI] [PubMed] [Google Scholar]
- 23.Sackett DL, Haynes RB, Guyatt GH, Tugwell P. 2nd ed. Boston, Mass: Little, Brown and Co.; 1991. Clinical Epidemiology: A Basic Science for Clinical Medicine. [Google Scholar]
- 24.Jaeschke R, Guyatt GH, Sackett DL. Users’ guides to the medical literature, III: how to use an article about a diagnostic test, B: what are the results and will they help me in caring for my patients? JAMA. 1994;271:703–7. doi: 10.1001/jama.271.9.703. [DOI] [PubMed] [Google Scholar]


