Abstract
PURPOSE
To summarize current knowledge of interventions that should improve the care of patients with type II diabetes mellitus. Interventions lie within the realms of prevention, screening, and treatment, all of which are focused on office practice.
METHODS
Review of the literature by a multidisciplinary team involved in the care of patients with diabetes, followed by synthesis of the literature into a clinical care guideline. Literature was identified through consultation with experts and a focused MEDLINE search.
MAIN RESULTS
An algorithm-based guideline for screening and treatment of the complications of diabetes was developed. The emphasis is on prevention of atherosclerotic disease, and prevention, screening, and early treatment of microvascular disease. Implementation of these practices has the potential to significantly improve quality of life and increase life expectancy in patients with type II diabetes mellitus.
Keywords: diabetes mellitus, non-insulin-dependent; guideline; prevention; complications; patient education
Type II diabetes is a common condition, affecting 2% to 3% of the adult population, and up to 20% to 25% of the elderly population.1 Type II diabetes typically occurs in patients who are over 30 years old and weigh more than 120% of ideal body weight, and accounts for 90% of all cases of diabetes mellitus diagnosed in the United States.1 Minorities have a prevalence of type II diabetes mellitus that is 2 to 6 times greater than that of white persons. The morbidity and mortality are higher for minorities than for white persons, and the rate is increasing.2 The reasons for this disparity remain unclear, but could include differences in disease severity, comorbidities, or access to care.
Diabetes has significant associated morbidity. Prevention and treatment of the complications of diabetes mellitus have the potential to improve quality of life and increase life expectancy.3 The rate of cardiovascular disease is markedly elevated among patients with type II diabetes, leading to an increased mortality rate compared with the general population.4,5 In addition, microvascular complications, which include retinopathy, nephropathy, and neuropathy, can progress to end-stage outcomes such as blindness, end-stage renal disease (ESRD), and amputation. Screening and early treatment for diabetic complications have been shown to be effective in reducing the incidence of end-stage disease6,7; despite this evidence, implementation rates of recommended interventions are low.8 This frequently leads to ineffective or delayed treatment of complications.9–11 Because optimal diabetes care is a complex process, we developed a clinical care guideline to help busy clinicians incorporate prevention, screening, and treatment recommendations into practice.
METHODS
A multidisciplinary team was assembled to develop an evidence-based guideline for the prevention and treatment of complications of type II diabetes. The multidisciplinary team included members from endocrinology, family practice, general internal medicine, obstetrics-gyncecology, nursing, and postgraduate medicine departments. Team members have an interest in diabetes care, are experts in diabetes care, or have experience with guideline development.
The consensus of the guideline development team was that preventive and screening measures should constitute the main focus of the guideline. There was uniform agreement that the two major complications of type II diabetes were macrovascular and microvascular disease. Preliminary evidence reviewed for guideline development included studies on type II diabetes felt to be important by the members of the guideline team. Consensus statements from expert panels on hypertension, lipids, and diabetes were examined, and the references from these statements were reviewed. Other literature was identified by means of a systematic MEDLINE search with the assistance of a reference librarian. Articles published between January 1976 and December 1996 were examined. The search began with the MeSH terms diabetes mellitus and non-insulin-dependent. The articles identified were then cross-referenced with each of the topics: retinopathy, nephropathy, neuropathy, hypertension, lipids, triglycerides, low-density lipoprotein (LDL), smoking, blood glucose, foot care, self-management, education, and preconception care. These articles were further cross-referenced with each of the topics: prevention, treatment, and control. Lastly, experimental and observational evidence was identified using the identifiers: clinical trial, controlled clinical trial, randomized controlled trial, cohort study, multicenter trial, and meta-analysis. This focused literature search identified more than 500 additional articles relevant to the review.
The abstracts of articles identified were reviewed independently by each of the authors. Criteria for full review of articles included applicability to the topic being evaluated, sample size over 30 patients, and duration of follow-up longer then 3 months. If several articles on the same topic were identified, randomized controlled trials were reviewed preferentially over observational or quasi-experimental study designs. Articles that met these criteria were critically reviewed, and any disagreements were settled by consensus opinion. Agreement between the authors was nearly universal. Bibliographies of relevant articles were scanned for other references; however, few additional articles were identified.
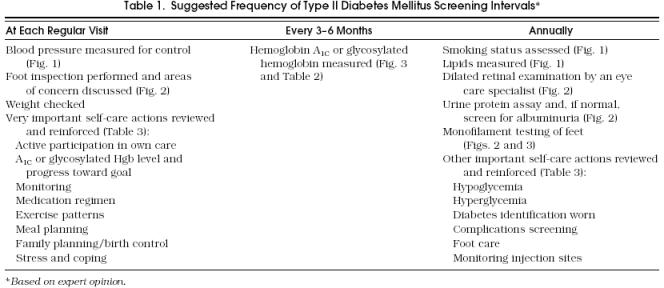
The articles identified for full review were evaluated by each of the authors, and recommendations for the care of diabetes were reached through consensus discussion of the available evidence. Recommendations are depicted in an algorithm format and accompanied by a discussion of the supporting evidence (Fig. 1–Fig. 3). Each recommendation is classified in terms of the level of evidence based on study design (randomized, controlled trials; controlled trials, no randomization; observational studies; and expert opinion). They are further classified by whether they are studies of patients with diabetes or are performed on the general or nondiabetic population. Few of the identified studies investigated appropriate screening intervals for each recommendation; however, suggested screening intervals, based on the consensus opinion of our guideline team, are depicted in Table 1.
Figure 1.
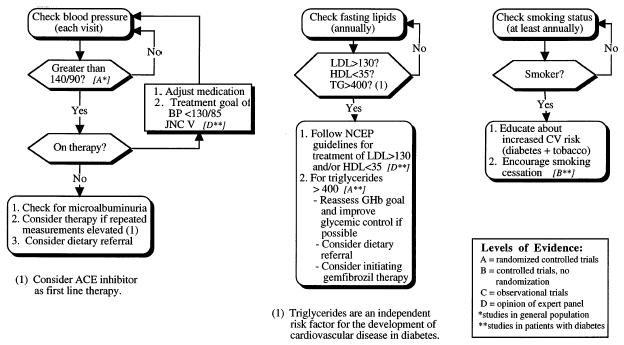
Screening, prevention, and treatment of cardiovascular risk factors in patients with type II diabetes mellitus: hypertension, hyperlipidemia, and smoking.
Figure 3.
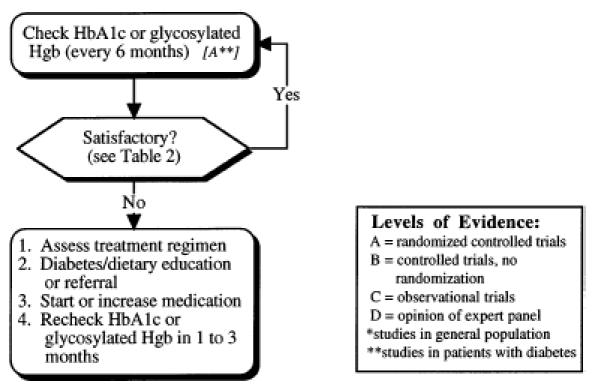
Monitoring glycemic control in patients with type II diabetes mellitus.
Tabel 1.
Suggested Frequency of Type II Diabetes Mellitus Screening Intervals
PREVENTION AND TREATMENT OF MACROVASCULAR DISEASE
Atherosclerotic disease, commonly referred to as “macrovascular” disease, is responsible for more than 50% of all mortality in type II diabetes mellitus.1 Type II diabetes is an independent risk factor for the development of atherosclerosis.12 The majority of atherosclerotic macrovascular complications are due to cardiovascular disease,4,5 with the remaining complications related to cerebral vascular or peripheral vascular disease. The pathogenesis of the increased rate of atherosclerosis in patients with type II diabetes is not completely defined; the relation between hyperinsulinemia, hyperglycemia, and atherosclerotic disease is an area of continuing controversy.13–20 The United Kingdom Prospective Diabetes Study (UKPDS), a large ongoing trial evaluating the efficacy of improved glycemic control in preventing macrovascular disease,21 may clarify the relation between insulin levels, glycemic control, and atherosclerosis. Factors such as hypertension, hyperlipidemia, and tobacco use clearly contribute to the risk of macrovascular disease in patients with type II diabetes. Therefore, the prophylactic use of aspirin, control of hypertension and hyperlipidemia, and cessation of smoking are particularly important in patients with type II diabetes mellitus.
Prophylactic Use of Aspirin
Meta-analysis and a large, randomized, controlled trial reveal that people with diabetes receive the same cardiovascular protection from aspirin as nondiabetic patients.22,23 Aspirin use in diabetic patients is not associated with an increased rate of adverse effects as compared with the general population.23 Although not all of the differences in outcomes measured in these studies achieve statistical significance, there is a trend among all aspirin-treated groups to have lower cardiovascular event rates and mortality. Therefore, we recommend preventive use of aspirin in patients with type II diabetes mellitus who are aged 50 years or older and/or have other cardiovascular risk factors,24 provided no contraindications are present. Although specific dosages have not been compared in clinical trials, the recommended dosage ranges between 81 mg (1 baby aspirin) and 325 mg/day.
Hypertension
Hypertension is a significant contributor to the development of atherosclerotic disease in patients with type II diabetes mellitus.25 Untreated hypertension can also lead to worsening of albuminuria and more rapid decline of the glomerular filtration rate.26 Therefore, screening and treatment of hypertension are important components of diabetes care.
Persons with type II diabetes develop hypertension at twice the rate of those without diabetes.27,28 There are racial differences in the prevalence of hypertension, with African Americans having the highest rates of hypertension, followed by whites, Hispanics, and Asians.29 Data from the National Health and Nutrition Examination Survey II (NHANES II) show that the prevalence of people with diabetes mellitus with hypertension increases with age; more than 60% of diabetic patients over 45 years of age are hypertensive.29 The majority of patients have essential hypertension or hypertension as the result of diabetic nephropathy.30 However, it is important to identify secondary causes of hypertension in patients who have clinical signs and syndromes suggestive of other diseases (e.g., renal artery stenosis, Cushing’s disease).
No randomized, controlled trials identifying optimal blood pressure (BP) levels for initiating treatment in patients with type II diabetes have been completed. A number of studies performed in the general population support treatment of a BP greater than 140/90.31,32 Large clinical trials assessing the effectiveness of antihypertensive medications often exclude persons with diabetes or fail to analyze the data separately. Although data regarding the efficacy of treatment for hypertension in patients with diabetes are limited, the Hypertension Detection and Follow-up Program Cooperative Group demonstrated that antihypertensive therapy in patients with diabetes affords a similar reduction in mortality (25%) to that in the overall study population.33 A recent subanalysis from the Systolic Hypertension in the Elderly Program revealed a 55% reduction in coronary heart disease end points for diabetic patients treated with low-dose diuretics when compared with placebo and antihypertensive drugs prescribed by the patient’s private physician.34
Although the optimal intervals for BP screening in patients with diabetes have not been determined in clinical studies, we recommend BP measurement at every regular visit. If a patient has repeated BP measurements of greater than 140/90 (Fig. 1), intervention should be considered.35 In patients with mild hypertension, initial nonpharmacologic measures including dietary modification, exercise, restriction of alcohol, and weight loss should be attempted36,37; in those who do not respond, or in patients with moderate to severe hypertension, pharmacologic therapy is indicated. Owing to a lack of evidence, controversy exists regarding the optimal target BP for patients with type II diabetes. Expert opinion from the Fifth Report of the Joint National Committee on Detection, Evaluation and Treatment of High Blood Pressure (JNC V) recommends treatment to achieve a BP of less than 130/85 based on the significantly elevated risk of cardiovascular disease in patients with type II diabetes.35 Aggressive treatment to a BP of less than 130/85 may be beneficial for patients with diabetic nephropathy.38,39
Angiotensin-converting enzyme (ACE) inhibitors are a reasonable first-line agent for patients with diabetes because of their potential benefits to renal function and lack of adverse effects on lipid and glucose metabolism.40–45 The angiotensin II receptor antagonists are effective antihypertensive drugs and do not cause cough.46 Studies are in progress to assess whether they exhibit the same renal protective effects as ACE inhibitors.
Low-dose diuretics do not appear to have significant adverse effects,47 and have been proven to reduce mortality in patients with diabetes.34 High-dose thiazide diuretics have been reported to have a variety of adverse effects including worsening of hyperlipidemia, deterioration of glycemic control, increased mortality, and impotence.48,49
The use of other antihypertensive agents should be based on the specific needs of the patient. Calcium channel blockers are effective,50 although caution should be exercised in using the dihydropyridine class as this group does not appear to have the same renal protective effects as other calcium channel blockers.51 As a class, calcium channel blockers have less protective effect on the kidneys than do ACE inhibitors.52–54
β-Blockers have an important role after myocardial infarction.55,56 Persons with diabetes have increased postinfarct mortality when compared with nondiabetic subjects, and diabetic patients experience greater cardioprotection with β-blockade. β-Blockers may obscure some of the symptoms of hypoglycemia (although this is rarely a problem in type II diabetics) and may decrease high-density lipoprotein (HDL) and increase triglyceride levels.57 α1-Adrenergic receptor blockers do not have adverse glycemic or lipid effects, but may aggravate postural hypotension in some persons with diabetes.58.
Studies reveal that 20% to 60% of patients are not sufficiently controlled on monotherapy.41 In these cases, combination therapy of an ACE inhibitor with a low-dose thiazide diuretic works well, and seems to ameliorate the metabolic problems seen with either treatment alone, at least in nondiabetic subjects. Some studies of the combination of an ACE inhibitor with a calcium channel blocker demonstrate greater benefits than either agent alone.59 The combination of β-blocker with a thiazide can lead to an increase in plasma glucose levels and should be used with caution.60,61
Lipids
Characteristically, persons with type II diabetes have elevated triglyceride and very-low-density lipoprotein ( VLDL) levels, while HDL levels are low. Low-density lipoprotein levels can also be elevated.62,63 Hyperlipidemia contributes significantly to the development of atherosclerotic disease in patients with type II diabetes.62 However, no trials identifying treatment target levels for HDL and LDL in patients with type II diabetes have been completed. In addition, optimal cholesterol screening intervals have not been determined for patients with diabetes. In high-risk, nondiabetic populations, randomized, controlled trials demonstrate that lowering LDL leads to a reduction in cardiovascular mortality.64,65 Because of the high prevalence of coronary artery disease (CAD) in diabetic patients, the National Cholesterol Education Panel (NCEP) recommends that diabetic patients be screened annually and treated like patients with known CAD, with treatment initiated at an LDL level above 130 mg/dL with an LDL goal of less than 100 mg/dL.66
Although 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA) reductase inhibitors are effective in lowering LDL levels,67,68 long-term benefit has not been evaluated in patients with type II diabetes. Clinical data are limited, but screening for hyperlipidemia and treatment of elevated lipid subfractions in patients with diabetes have the potential to similarly reduce cardiovascular morbidity and mortality. Thus, although studies have not been performed that specifically evaluate patients with diabetes, because of their markedly elevated cardiovascular risk, we recommend treating diabetic patients with elevated LDL levels according to the NCEP guidelines. The first line of therapy is dietary intervention, followed by the use of HMG CoA reductase inhibitors when necessary to achieve target LDL levels (Fig. 1). Nicotinic acid should be used with caution as it may worsen hyperglycemia.69
In patients with diabetes, triglycerides are an independent risk factor for the development of atherosclerotic disease.62,63 Initial therapy should consist of improving glycemic control with a combination of exercise, dietary changes, and increased intensity of oral hypoglycemics or insulin. If these changes are ineffective, then pharmacologic therapy with gemfibrozil should be considered if the triglyceride level remains greater than 400 mg/dL.62,66,70 Gemfibrozil has been shown to be effective in lowering the rate of cardiovascular, but not overall, mortality in the general population.71 Subgroup analysis suggests that patients with type II diabetes may receive greater benefit from treatment of hypertriglyceridemia; however, the number of patients studied was small and differences were not statistically significant.63 Until better evidence is available for individuals with type II diabetes mellitus, we recommend an unproven yet conservative approach of aggressive dietary intervention and glycemic control followed by gemfibrozil (when necessary) for hypertriglyceridemia.
Smoking
Longitudinal cohort studies have shown that smoking and diabetes are synergistic risk factors for the development of atherosclerotic disease.12,72 Patients with diabetes should be counseled regarding these risks, and all possible measures should be used to prevent and encourage discontinuation of tobacco use (Fig. 1). This includes enrollment in formal smoking cessation programs and use of alternative nicotine delivery systems when necessary. The use of nicotine patches is safe in patients with known cardiovascular disease.73
PREVENTION AND TREATMENT OF MICROVASCULAR DISEASE
Diabetes is the leading cause of new cases of blindness, renal failure, and nontraumatic amputation in the United States.1 These outcomes are frequently the result of progression of microvascular diabetic complications including retinopathy, nephropathy, and neuropathy. The risk of developing these complications can be significantly reduced by interventions that prevent the onset or slow the progression of early microvascular disease.
Retinopathy
In type I diabetes, randomized, controlled trials have demonstrated that improving glycemic control can decrease the incidence of diabetic retinopathy.74 Observational studies in patients with type II diabetes suggest a similar relation between level of glycemic control and rate of retinopathy.75–77 Primary prevention of diabetic retinopathy, therefore, consists of optimization of glycemic control. In addition, improving glycemic control slows the progression of retinopathy in patients who have already developed background retinopathy.74
Progression of diabetic eye disease to proliferative retinopathy and macular edema frequently leads to severe visual loss. Multicenter randomized controlled trials have shown that laser therapy of proliferative retinopathy and macular edema significantly reduces the incidence of visual loss in patients with diabetes.6,7 Despite the demonstrated effectiveness of laser therapy, it is estimated that only 35% to 60% of diabetic patients currently undergo annual retinal examination.8 While less frequent screening may be indicated for those considered to be at low risk of developing retinopathy,78 such as those without retinopathy at baseline and those with excellent glycemic control, formal risk-stratification based on these clinical indicators has not yet been widely implemented.
Studies that have evaluated the sensitivity and specificity of retinal screening show that primary care clinicians trained to perform dilated retinal examinations can detect proliferative retinopathy, but fail to consistently detect macular edema.78 Although the optimal frequency of screening has not been evaluated by randomized controlled trials, we suggest that all patients undergo annual dilated retinal examination by a trained specialist (Fig. 2). Laser therapy for proliferative retinopathy and macular edema should be performed by trained ophthalmologists.
Figure 2.
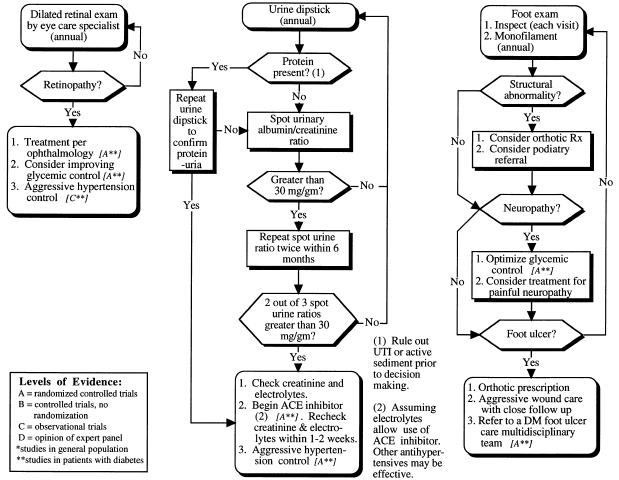
Screening, prevention, and treatment of microvascular complications in patients with type II diabetes mellitus: retinopathy, nephropathy, and neuropathy.
Nephropathy
As with retinopathy, optimization of glycemic control acts as primary prevention of diabetic nephropathy.74,77 Aggressive control of hypertension and ACE inhibitor therapy play a vital role in prevention of renal disease.
Microalbuminuria and proteinuria have been identified to be early signs of diabetic nephropathy.79 Although optimal screening intervals have not been determined, screening for early diabetic renal disease can allow interventions that lower the rate of progression to overt nephropathy and ESRD.44,45 The current recommended method of screening for early stages of diabetic nephropathy is outlined in Figure 2. This algorithm is based on the level of albumin excretion. Causes of elevated urinary albumin excretion in the absence of diabetic nephropathy include urinary tract infection, recent exercise, acute illness, hematuria, and congestive heart failure. It is recommended that urine be screened for hematuria, urinary tract infection, and overt diabetic nephropathy with a standard urine dipstick test prior to screening for early nephropathy.
Overt diabetic nephropathy is classified as a protein-positive (1+ or greater) urine dipstick test, which represents macroalbuminuria, or an albumin concentration of greater than 300 mg/g creatinine. This equates to approximately 300 mg of albumin excretion per day.80 Dipstick tests that are negative or show positive traces of protein should be followed with screening for early diabetic nephropathy, commonly referred to as microalbuminuria.
There are numerous methods of screening for microalbuminuria. The spot urinary albumin-creatinine ratio is a simple method that negates concerns about measurement errors inherent in 24-hour collections. Because of significant variation in urinary albumin concentration, it is recommended that, if the first test for albumin is positive (>30 mg/g creatinine), the test be repeated for confirmation. If the second test is negative, a third test should be performed. Two of the three tests should be positive, and other potential etiologies should be excluded before microalbuminuria is considered present (Fig. 2).
A clinical diagnosis of diabetic nephropathy may be made when an individual develops albuminuria and either has had diabetes for more than 5 years or has evidence of diabetic retinopathy.81 Because other complicating renal diseases may also cause albuminuria, a person who does not meet one of the above criteria or has factors suggestive of other renal diseases (such as active urinary sediment, nephrotic-range proteinuria, accelerated hypertension, or rapidly progressive renal insufficiency) will require further evaluation.
Adequate systemic blood pressure control38,39 and ACE inhibitors have been shown to independently reduce the rate of progression of early diabetic renal disease in randomized, controlled trials of persons with type II diabetes.44,45 Other antihypertensive agents (β-blockers, calcium channel blockers) can reduce the rate of progression of diabetic renal disease, but do not seem to have an effect independent of blood pressure control.49,50 Some members of the dihydropyridine class of calcium channel blockers may increase urinary albumin excretion, and should be avoided by patients with microalbuminuria and overt proteinuria.48 ACE inhibitors should be used as first-line therapy for all patients with diabetic nephropathy unless contraindications are present or side effects are intolerable.
Aggressive control of BP is of vital importance. Absolute target levels in patients with diabetic nephropathy have not been well delineated, but there is a clear association between BP and rate of progression of diabetic renal disease.38,39 In nonhypertensive patients with microalbuminuria, target dosages of ACE inhibitors or other antihypertensives are difficult to define; because of a lack of clear evidence, we recommend titrating medications upward to maximal doses or until side effects occur.
Dietary protein restriction appears to slow the progression of renal disease in patients with type I diabetes. In type II diabetes, however, protein restriction has not been consistently demonstrated to be effective in preventing the progression of early nephropathy.82
Neuropathy and Foot Care
Patients with diabetes are at increased risk of developing peripheral neuropathy and diabetic foot ulcers. Diabetic neuropathy is reported in more than 50% of patients who have had type II diabetes for more than 15 years.1 Improving glycemic control reduces the incidence of neu-ropathy in type I diabetes,74 and the epidemiologic literature suggests a similar association in type II diabetes.77,83 Evidence indicates that early detection of diabetic neuropathy results in fewer admissions for foot ulcers and amputations.84 As shown in Figure 4, sensory testing with nylon monofilament (10 g) should be done regularly to identify sensory loss at appropriate anatomic landmarks of the foot.85 The optimum interval between such examinations has not been determined, but many experts advise annual testing.
Figure 4.
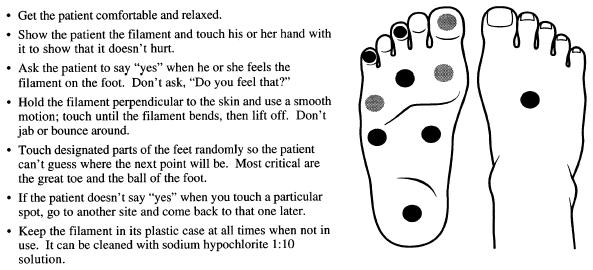
How to use the monofilament. (Source: The Gillis W. Long Hansen’s Disease Center, Carville, La., and Dr. Charles Patout, Jr. This is a public document. The center is no longer in existence.)
The combination of patient education regarding foot care and increased surveillance by physicians regarding foot-related risk factors for amputation has been examined in a randomized, controlled trial and a cohort trial.86,87 Each of the studies used a comprehensive program of diagnosis (including monofilament testing) and intervention. Both trials showed a significant reduction in serious foot lesions; however, the effectiveness of individual components of the comprehensive programs was not evaluated (Fig. 2).
We recommend, at each regular visit, that patients with diabetes have their feet inspected. The foot examination should also include identifying areas of callus formation, deformities, including prominent metatarsal heads (or other bony prominences) and other structural changes. Orthotic footwear should be prescribed to accommodate major foot deformities and cushion pressure areas; therapeutic footwear for diabetic patients is a Medicare benefit. For others with less deformity, athletic shoes with sufficient room for the toes and forefoot with cushioned socks are appropriate.
Patients with abnormal foot examination need education regarding optimal foot care, which includes daily inspection by the patient and appropriately fitting shoes.88 To minimize the risk of trauma, patients should be counseled to avoid walking barefoot, and those with neuropathy should avoid high-impact exercise and should test the temperature of hot water before use. A number of drugs are currently under investigation for the treatment of diabetic neuropathy, including aldose reductase inhibitors (ARIs), which block the conversion of glucose to sorbitol and nerve growth factors. Recent evidence indicates that the new, more potent ARIs promote nerve fiber regeneration and prevent slowing of nerve conduction velocity in diabetic neuropathy.89,90 These drugs are not yet available for use in the United States.
Painful diabetic neuropathy can be managed with low-dose tricyclic antidepressants, with the dose titrated as necessary. Careful attention should be paid to the etiology of painful lower extremities, as mechanical factors, rather than neuropathy, are often the cause, and may respond to medications such as nonsteroidal anti-inflammatory drugs.91
A diabetic foot ulcer is defined as any interruption of the integrity of the skin that extends through the entire dermis. Should a foot ulcer be found, early treatment should be undertaken with aggressive wound care, orthotic prescriptions or casting, pressure relief, and antibiotics as necessary.92 The indications for antibiotics treatment of diabetic foot ulcers have not been well defined. Studies have shown that patients with diabetic foot ulcers have the best outcomes if managed by a multidisciplinary team which specializes in diabetic foot care.93
Glycemic Control
The Diabetes Control and Complications Trial (DCCT ), a large randomized, controlled trial performed in patients with type I diabetes, demonstrated that improving glycemic control substantially reduces the development and progression of early microvascular complications.74 Observational studies in patients with type II diabetes mellitus have shown that level of glycemic control is associated with the development of microvascular diabetic complications.76,77 A single randomized, controlled trial of Japanese patients with type II diabetes has confirmed that the rate of microvascular complications can be reduced by improving levels of glycemic control as measured by hemoglobin A1c (or glycosylated hemoglobin), but these patients tended to be insulin sensitive, and may represent a different population from that typically seen in the United States.83 In summary, improving glycemic control appears to decrease the incidence of microvascular disease in both type I and type II diabetes; however, the experimental data are limited on patients with type II diabetes. The effect of glycemic control on cardiovascular disease remains uncertain, although studies evaluating this relation are in progress.21,94 The major risk of intensive control is hypoglycemia, which has been an infrequent occurrence (2% per year) in an ongoing trial of aggressive glycemic control in type II diabetes.21
Hemoglobin A1c, hemoglobin A1, and total glycosylated hemoglobin (GHb) are accurate measurements of long-term glycemic control.95–97 To facilitate glycemic management, we recommend that one of these indicators be checked every 6 months in the patient on a stable hypoglycemic regimen, and every 1 to 3 months if changes are being made. These recommendations are based on the half-life of GHb; studies examining the effect of GHb measurement on glycemic control are in progress. It should be emphasized that hemoglobin A1c, hemoglobin A1, and GHb have different normal ranges, and various laboratories use different measures; each laboratory should provide this information to clinicians.
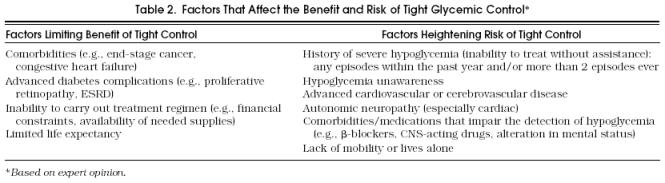
A GHb goal or target level should be discussed and agreed on by the patient and the primary care provider at the initial patient visit (Fig. 3 and Table 2). Several factors must be considered for each patient when selecting glycemic target levels. For young healthy patients, the possibility of eventually developing advanced complications (e.g. the possibility of eventually developing advanced complications (e.g., blindness, ESRD) should be of major concern ESRD) should be of major concern, and in general and in general, tight control should be advocated. However, in the presence of factors that affect the benefit and risk of glycemic control, it is less clear whether tight control is worth the associated risk and lifestyle modification. As depicted in Table 2, target glucose levels for patients with type II diabetes must be individually determined based on factors that affect the risk-benefit ratio of tight control. The actual target level of glycemic control selected for each person with type II diabetes mellitus must represent a balance among the patient’s self-determined diabetes care goals, the likelihood of benefit from attaining those goals, and the risks associated with the therapy required to achieve those goals. Once a glycemic target has been established, adjustments in diet and, if necessary, medications should be made until the target has been reached. Targets need to be reassessed on a regular basis, as the circumstances of each patient will change over time.
Table 2.
Factors That Affect the Benefit and Risk of Tight Glycemic Control
Glycemic Management
Diet and exercise are the cornerstones for the management of hyperglycemia in type II diabetes mellitus. Patients who do not achieve adequate glycemic control with an individualized meal plan and exercise program are candidates for pharmacologic therapy. Pharmacologic treatment options include oral administration of a sulfonylurea, a biguanide (metformin), α-glucosidase inhibitor (acarbose), a thiazolidinedione (troglitazone), and subcutaneous insulin. Even after pharmacologic therapy is instituted, careful attention must be paid to diet and activity.
Diet and Meal Plans
Meal planning for people with type II diabetes represents both first-line therapy and a cornerstone of their ongoing care. Although weight loss has traditionally been the primary focus of meal planning for people with type II diabetes, current dietary strategies and even very low calorie diets have not generally been effective in achieving long-term weight reduction.98 Therefore, it is recommended that medical nutrition therapy in type II diabetes emphasize achieving glucose, lipid, and BP goals.98
There is no one “diabetic” diet. The diet currently recommended by the American Diabetes Association is defined as a dietary prescription based on a nutrition assessment and designed to help the patient reach treatment goals.98 Ongoing medical nutrition therapy provided by a dietitian is effective for achieving metabolic and other clinical outcomes in type II diabetes.99 Referral to a dietitian is recommended at diagnosis and annually.100
Moderate weight loss by persons with type II diabetes can reduce hyperglycemia, dyslipidemia, and hypertension, even when desirable body weight is not achieved.101–103 It is currently recommended that providers emphasize reasonable rather than ideal or desirable body weights.104 Reasonable body weight is defined as the weight that is viewed by the individual with diabetes and the health care team as one that is achievable and maintainable both short and long-term. For some individuals, even those who are overweight, this may be current weight or a weight 5 to 10 kg less than the current weight.104
Other strategies such as spacing of meals throughout the day,105,106 regular exercise,107 and learning new behaviors108 can facilitate weight management and metabolic control. No one meal-planning method can be uniformly recommended for all people with type II diabetes. A nutritionally adequate meal plan focused on achieving metabolic and other goals can be provided using a healthy-food-choices approach, the exchange system, or carbohydrate counting.98
Sulfonylureas
Traditionally, sulfonylureas have been used as first-line therapy for patients with NIDDM in whom nonpharmacologic therapy has failed.109 Today the shorter-acting second-generation sulfonylureas are preferred over the earlier agents, such as chlonpropamide, which could cause hypoglycemia that was prolonged. Sulfonylureas act primarily by increasing pancreatic insulin secretion, but may also increase insulin receptor sensitivity.110 Patients may be treated with a sulfonylurea starting at a low dose that can be increased as necessary at weekly intervals until satisfactory glycemic control is achieved or the highest recommended dose is reached. If the patient has not achieved his or her glycemic goal at a maximal sulfonyl-urea dose, combination therapy with metformin, acarbose, or insulin should be considered.
Biguanides
Metformin, a biguanide, may also be selected as a first-line pharmacologic treatment for patients with NIDDM in whom nonpharmacologic therapy has failed.111,112 Metformin lowers blood glucose by increasing peripheral insulin sensitivity and decreasing hepatic glucose production.113 Metformin should be avoided in patients with renal, hepatic, or cardiac failure. Gastrointestinal side effects, including anorexia, nausea, diarrhea, and abdominal discomfort, are seen in up to 30% of patients. A beginning metformin dose of 500 mg per day will reduce these side effects and may be increased by 500 mg per week to a maximum dose of 2.5 g per day. If the patient has not achieved his or her glycemic goal at the maximum metformin dose, combination therapy or a change to insulin should be considered.
α-Glucosidase Inhibitor
α-Glucosidase inhibitors such as acarbose may also be used as monotherapy in conjunction with diet to lower blood glucose.114 Acarbose slows the digestion of ingested carbohydrates, delays glucose absorption into the bloodstream, and decreases postprandial blood glucose. The initial dose is 25 mg three times a day and should be taken with the first bite of each main meal. Gastrointestinal side effects including pain, flatulence, and diarrhea are common; although these effects usually diminish over time (4–8 weeks), they frequently lead to discontinuation of the drug. Some experts advocate starting at a lower dose (25 mg once a day) to minimize the initial side effects and increase compliance. The maintenance dose may be titrated to 50 to 100 mg three times per day.115
Thiazolidinediones
The thiazolidinediones are a new class of oral agents designed to enhance the actions of insulin.116 Troglitazone, the first drug in this class marketed in the United States, is currently approved for use in patients with type II diabetes on insulin therapy whose hyperglycemia is inadequately controlled despite multiple daily injections. To date, troglitazone has not been approved for use as a monotherapy or in combination with other oral agents, although studies are ongoing and it may be approved for broader indications in the near future.
Thiazolidinediones lower blood glucose levels by improving sensitivity to insulin in muscle and adipose tissue, and by inhibiting hepatic glucose production. Short-term trials in patients with type II diabetes have demonstrated reductions in fasting and postprandial glucose levels and insulin levels. Troglitazone has been associated with liver function abnormalities, slight reductions in hemoglobin and white cell counts, possibly related to dilutional effects, and resumption of ovulation in premenopausal anovulatory patients with insulin resistance (polycystic ovary syndrome). The latter patients may be at risk of pregnancy.116,117 Troglitazone is usually started at a dose of 200 mg/d, and over 2 to 4 weeks can be titrated to 400 mg/d. The maximal dose is 600 mg/d. Caution should be used when titrating as troglitazone, in combination with insulin, can produce hypoglycemia; the dosage of insulin should be reduced by 10% to 25% when the fasting blood glucose falls to less than 120 mg/dL.
Bedtime Insulin/Daytime Sulfonylurea Therapy
Bedtime insulin/daytime sulfonylurea (BIDS) therapy may be considered for patients who do not achieve glycemic goals despite maximum doses of oral agents.118 Metformin is discontinued, and the patient is continued on daytime sulfonylurea at a maximum dose. Self-monitoring of blood glucose is intensified and NPH insulin is added at bedtime. The usual starting dose of insulin is 0.3 units/kg of body weight with ongoing adjustment of therapy to achieve glycemic goals. In the Veterans Affairs Cooperative Study on Glycemic Control and Complications in Type II Diabetes, a mean insulin dose of 64 units was required to achieve glycemic goals in patients who were able to remain on BIDS therapy.20
Insulin
If combination therapy fails to achieve the patient’s glycemic goals, treatment can be changed to daily insulin injections. Therapy should be intensified as needed with twice-daily split/mixed insulin, three times daily insulin therapy, or multiple daily injections to achieve glycemic goals.20,119 Rapid-acting insulin is a new agent that is being used to achieve tight glycemic control in patients with type I diabetes; however, its role in type II diabetes is not yet defined.
Pregnancy and Preconception Counseling
Diabetes mellitus can significantly increase the risk of morbidity for the pregnant woman and the fetus or neonate.120 A significantly higher incidence of congenital anomalies occurs when maternal glycosylated hemoglobin in the first trimester is elevated.121 The effects of preconception care for diabetic mothers have been examined in nonrandomized, clinical trials, and observational studies in patients with type I diabetes. These studies demonstrate that preconception care for diabetic mothers can reduce the incidence of malformations from approximately 9% to 2%, a rate similar to that observed in infants of mothers without diabetes.120–127
Because of the increased risks associated with suboptimal preconception care, type II diabetic women who are of childbearing potential should receive preconception counseling. Preconception counseling and optimization of glycemic control in women with diabetes mellitus results in optimal maternal and fetal outcomes.128 However, less than 20% of women with type I and type II diabetes receive prepregnancy care.129 Women who are planning to become pregnant should be counseled regarding the increased risks of pregnancy, the genetics of diabetes, the changes in lifestyle necessary (i.e., a personal commitment to diabetes care by the woman and family), and the possibility of hospitalization during pregnancy. Women not currently planning pregnancy require general information regarding the risks of pregnancy and the need for prepregnancy planning. The importance of preventing pregnancy by establishing an acceptable method of birth control should be emphasized.
In pregnant women with diabetes, specific attention should be given to diet and exercise programs to allow adequate nutrition and optimal weight. As with all women contemplating pregnancy,130 folic acid (400 μg/d) should be prescribed before pregnancy. Cessation of tobacco, alcohol, illicit drug, and caffeine use should be emphasized. In addition to usual prenatal care, the management plan should include discontinuation of oral hypoglycemics (which cross the placenta and cause severe hypoglycemia)109 and ACE inhibitors (which can be teratogenic)131 and initiation of insulin therapy, with a plan of achieving blood glucose or GHb in the normal range.
Self-Management Education
At the time diabetes mellitus is diagnosed, the patient should be given extensive information about the disease and its management, including the importance of self-management. Meta-analyses and reviews of the diabetes literature have shown that diabetes self-management education is effective in improving knowledge, skill, self-care behaviors, psychosocial outcomes, and metabolic control.132–139 A recent epidemiologic study from Italy found that limited access to care and lack of an educational intervention led to an increased risk of developing the complications of diabetes, while self-management of insulin had a protective effect.140 Other studies have shown the benefits of diabetes foot care education in preventing amputations,87,141 and the efficacy of ongoing nutritional care.142
Self-management education is most effective when presented in collaboration with a provider who can reinforce this information.134,143,144 Therefore, an important part of the primary care provider’s role is to review and update the information the patient needs to manage the disease, ascertain the extent to which the patient is managing the disease appropriately, reinforce self-management behaviors, and refer to diabetes education programs or nutritionists.
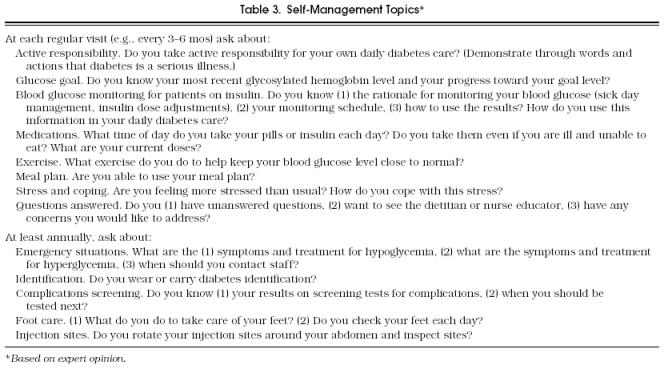
Table 3 lists important self-management topics and questions about issues that will help elicit the patient’s understanding of diabetes care. The entire list may be too long to go over at every visit, so the topics have been grouped to ensure that particularly important topics are checked frequently. However, any topic may be important at any visit, based on patient- and provider-identified needs. These topics and questions do not address all areas of diabetes self-management that have been identified as important, nor is there scientific evidence that these brief discussions will lead to improved outcomes. They do, however, represent content areas that have been identified as necessary to meet standards for diabetes education programs,100 that were based on an extensive review of the literature,135 and that are prudent and reasonable for a provider to address during a routine visit.
Table 3.
Self-Management Topics
CONCLUSIONS
Patients with type II diabetes mellitus are at significant risk of developing macrovascular disease and microvascular complications. Clinicians should focus on implementing interventions and providing education to prevent complications and encourage self-management in patients with type II diabetes mellitus. This evidenced-based clinical review incorporates “best evidence” into a clinical care guideline for busy clinicians. Implementing the screening, prevention, and treatment recommendations as outlined has the potential to improve quality of life and increase life expectancy in patients with type II diabetes mellitus.
Acknowledgements
The authors would like to recognize other members of the Type II Diabetes Mellitus Guideline Team: Douglas Greene, MD, and Catherine Martin, MS, RN, Division of Endocrinology and Metabolism; Evelyn Piehl, MS, RN, Department of Obstetrics and Gynecology; Barbara J. Ratliff, RN, Primary Care Nursing; Roland Hiss, MD, and R. Van Harrison, PhD, Postgraduate Medicine. A special thanks to Joel Howell, MD, and Rodney Hayward, MD for critically reviewing the article, and to Pat Barr, BS, for editing this manuscript.
References
- 1.National Diabetes Data Group. 2nd ed. Bethesda, Md: National Institutes of Health; 1995. Diabetes in America. [Google Scholar]
- 2.Carter JS, Pugh JA, Monterrosa A. Non-insulin-dependent diabetes mellitus in minorities in the United States. Ann Intern Med. 1996;125:221–32. doi: 10.7326/0003-4819-125-3-199608010-00011. [DOI] [PubMed] [Google Scholar]
- 3.The Diabetes Control and Complications Research Group. Lifetime benefits and costs of intensive therapy as practiced in the diabetes control and complications trial. JAMA. 1996;276:1409–15. [PubMed] [Google Scholar]
- 4.Krolewski AS, Czyczyk A, Janeczko D, Kopczynski J. Mortality from cardiovascular diseases among diabetics. Diabetologia. 1977;13:345–50. doi: 10.1007/BF01223277. [DOI] [PubMed] [Google Scholar]
- 5.Panzram G. Mortality and survival in type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1987;30:123–31. doi: 10.1007/BF00274216. Published erratum appears in Diabetologia. 1987;30:364. [DOI] [PubMed] [Google Scholar]
- 6.The Diabetic Retinopathy Study Research Group. Photocoagulation treatment of proliferative diabetic retinopathy: clinical application of diabetic retinopathy study findings. Ophthalmology. 1981;88(9):583–600. DRS Report Number 8. [PubMed] [Google Scholar]
- 7.Early Treatment Diabetic Retinopathy Study Research Group. Early photocoagulation for diabetic retinopathy. Ophthalmology. 1991;98(9):766–85. ETDRS Report Number 9. [PubMed] [Google Scholar]
- 8.Brechner RJ, Cowie CC, Howie LJ, Herman WH, Will JC, Harris MI. Ophthalmic examination among adults with diagnosed diabetes mellitus. JAMA. 1993;270:1714–18. [PubMed] [Google Scholar]
- 9.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin Epidemiologic Study of Diabetic Retinopathy, VI: retinal photocoagulation. Ophthalmology. 1987;94:747–53. doi: 10.1016/s0161-6420(87)33525-0. [DOI] [PubMed] [Google Scholar]
- 10.Witkin SR, Klein R. Ophthalmologic care for persons with diabetes. JAMA. 1984;251:2534–7. [PubMed] [Google Scholar]
- 11.Harris M. Medical care for patients with diabetes: epidemiologic aspects. Ann Intern Med. 1996;24(9):117–22. doi: 10.7326/0003-4819-124-1_part_2-199601011-00007. [DOI] [PubMed] [Google Scholar]
- 12.Kannel W, McGee DL. Diabetes and cardiovascular disease. The Framingham Study. JAMA. 1979;241:2035–8. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- 13.Ferrara A, Barrett-Connor EL, Edelstein SL. Hyperinsulinemia does not increase the risk of fatal cardiovascular disease in elderly men or women without diabetes: the Rancho Bernardo Study, 1984–1991. Am J Epidemiol. 1994;140:857–69. doi: 10.1093/oxfordjournals.aje.a117174. [DOI] [PubMed] [Google Scholar]
- 14.Ronnemaa T, Laakso M, Pyorala K, Kallio V, Puukka P. High fasting plasma insulin is an indicator of coronary heart disease in non-insulin-dependent diabetic patients and nondiabetic subjects. Arterioscler Thromb. 1991;11:80–90. doi: 10.1161/01.atv.11.1.80. [DOI] [PubMed] [Google Scholar]
- 15.Pyorala K, Uusitupa M, Laakso M, Siitonen B, Niskanen L, Ronnemaa T. Macrovascular complications in relation to hyperinsulinemia in non-insulin-dependent diabetes mellitus. Diabetes Metab. 1987;13:345–9. [PubMed] [Google Scholar]
- 16.Kuusisto J, Mykkanen L, Pyorala K, Laakso M. NIDDM and its metabolic control predict coronary heart disease in elderly subjects. Diabetes. 1994;43:960–7. doi: 10.2337/diab.43.8.960. [DOI] [PubMed] [Google Scholar]
- 17.Wingard DL, Barrett-Connor EL, Ferrara A. Is insulin really a heart disease risk factor. Diabetes Care. 1995;18:1299–1304. doi: 10.2337/diacare.18.9.1299. [DOI] [PubMed] [Google Scholar]
- 18.Uusitupa MI, Niskanen LK, Siitonen O, Voutilainen E, Pyorala K. Ten-year cardiovascular mortality in relation to risk factors and abnormalities in lipoprotein composition in type 2 (non-insulin-dependent) diabetic and non-diabetic subjects. Diabetologia. 1993;36:1175–84. doi: 10.1007/BF00401063. [DOI] [PubMed] [Google Scholar]
- 19.Despres JP, Lamarche B, Mauriege P, et al. Hyperinsulinemia as an independent risk factor for ischemic heart disease. N Engl J Med. 1996;334:952–7. doi: 10.1056/NEJM199604113341504. [DOI] [PubMed] [Google Scholar]
- 20.Abraira C, Colwell JA, Nuttall FQ, et al. Veterans Affairs cooperative study on glycemic control and complications in type II diabetes (VA CSDM). Results of the feasibility trial. Diabetes Care. 1995;18:1113–23. doi: 10.2337/diacare.18.8.1113. [DOI] [PubMed] [Google Scholar]
- 21.UK Prospective Diabetes Study Group. UK Prospective Diabetes Study, VIII: study design, progress, and performance. Diabetologia. 1991;34:877–90. [PubMed] [Google Scholar]
- 22.Antiplatelet Trialists Collaboration. Collaborative overview of randomized trials of antiplatelet therapy, I: prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ. 1994;308:81–106. [PMC free article] [PubMed] [Google Scholar]
- 23.ETDRS Investigators. Aspirin effects on mortality and morbidity in patients with diabetes mellitus: Early Treatment Diabetic Retinopathy Study Report 14. JAMA. 1992;268(9):1292–300. doi: 10.1001/jama.1992.03490100090033. [DOI] [PubMed] [Google Scholar]
- 24.Physicians’ Health Study Research Group. Final report on the aspirin component of the ongoing Physicians’ Health Study. N Engl J Med. 1989;321:129–35. doi: 10.1056/NEJM198907203210301. [DOI] [PubMed] [Google Scholar]
- 25.Hseuh WA, Anderson PW. Hypertension, the endothelial cell and the vascular complications of diabetes mellitus. Hypertension. 1992;20:253–63. doi: 10.1161/01.hyp.20.2.253. [DOI] [PubMed] [Google Scholar]
- 26.Mogensen CE. Long-term antihypertensive treatment inhibiting progression of diabetic nephropathy. BMJ. 1982;285:685–8. doi: 10.1136/bmj.285.6343.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fuller JH. Epidemiology of hypertension associated with diabetes mellitus. Hypertension. 1985;7(II):II-3–7. doi: 10.1161/01.hyp.7.6_pt_2.ii3. [DOI] [PubMed] [Google Scholar]
- 28.Sowers JR, Ebstein M. Diabetes mellitus and associated hypertension, vascular disease, and nephropathy. Hypertension. 1995;26(pt 1):869–79. doi: 10.1161/01.hyp.26.6.869. [DOI] [PubMed] [Google Scholar]
- 29. National Center for Health Statistics. Plan and operation of the Hispanic Health and Nutrition Examination Survey, 1982–1984. In: Vital Health Stat 1. Washington, DC: Department of Health and Human Services; 1985:19. DHHS publication PHS 85–1321. [PubMed]
- 30.National High Blood Pressure Education Program Working Group. Report on hypertension in diabetes. Hypertension. 1994;23:145–58. [PubMed] [Google Scholar]
- 31.Langer RD. General population studies of the benefits and epidemiology of hypertension. The epidemiology of hypertension control in populations. Clin Exp Hypertens. 1995;7(9):1127–44. doi: 10.3109/10641969509033658. [DOI] [PubMed] [Google Scholar]
- 32.Medical Research Council Working Party. MRC trial of treatment of mild hypertension: principal results. BMJ. 1985;291:97–104. doi: 10.1136/bmj.291.6488.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hypertension Detection and Follow-up Program Cooperative Group. Five-year findings of the Hypertension Detection and Follow-up Program, I: reduction in mortality in persons with high blood pressure, including mild hypertension. JAMA. 1979;242:2562–71. [PubMed] [Google Scholar]
- 34.Curb JD, Pressel SL, Cutler JA, et al. Effect of diuretic-based antihypertensive treatment on cardiovascular disease risk in older diabetic patients with isolated systolic hypertension. JAMA. 1996;276:1886–92. [PubMed] [Google Scholar]
- 35.The Fifth Report of the Joint National Committee on Detection, Evaluation and Treatment of High Blood Pressure (JNC V). Arch Intern Med. 1993;153:154–83. [PubMed] [Google Scholar]
- 36.Dodson PM, Beevers M, Hallworth R, Webberley MJ, Fletcher RF, Taylor KG. Sodium restriction and blood pressure in hypertensive type II diabetics: randomised blind controlled and crossover studies of moderate sodium restriction and sodium supplementation. BMJ. 1989;298:227–30. doi: 10.1136/bmj.298.6668.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dodson PM, Pacy PJ, Bal P, Kubicki AJ, Fletcher RF, Taylor KG. A controlled trial of a high fibre, low fat and low sodium diet for mild hypertension in type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1984;27:522–6. doi: 10.1007/BF00290388. [DOI] [PubMed] [Google Scholar]
- 38.Savage S, Nagel NJ, Estacio RO, Lukken N, Schrier RW. Clinical factors associated with urinary albumin excretion in type II diabetes. Am J Kidney Dis. 1995;25:836–44. doi: 10.1016/0272-6386(95)90565-0. [DOI] [PubMed] [Google Scholar]
- 39.Chuang LM, Jou TS, Wu HP, Tseng CH, Tai TY, Lin BJ. Effect of treatment of borderline hypertension on microalbuminuria in non-insulin-dependent diabetes mellitus. J Formos Med Assoc. 1991;90:531–5. [PubMed] [Google Scholar]
- 40.Lebovitz HE, Weigmann TB, Cnaan A, et al. Renal protective effects of enalapril in hypertensive NIDDM: role of baseline albuminuria. Kidney Int. 1994;45(9):S150–5. [PubMed] [Google Scholar]
- 41.Hypertension in Diabetes Study III. Prospective study of therapy of hypertension in type 2 diabetic patients: efficacy of ACE inhibition and β-blockade. Diabetes Med. 1994;11:773–82. [PubMed] [Google Scholar]
- 42.Neilson FS, Rossing P, Gall M, Skøtt P, Smidt UM, Parving HH. Impact of lisinopril and atenolol on kidney function in hypertensive NIDDM subjects with diabetic nephropathy. Diabetes. 1994;43:1108–13. doi: 10.2337/diab.43.9.1108. [DOI] [PubMed] [Google Scholar]
- 43.Lewis E, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting enzyme inhibition on diabetic nephropathy. N Engl J Med. 1993;329:1456–62. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 44.Ravid M, Savin H, Jutrin I, Bental T, Katz B, Lishner M. Long-term stabilizing effect of angiotensin-converting enzyme inhibition on plasma creatinine and on proteinuria in normotensive type II diabetic patients. Ann Intern Med. 1993;118:577–81. doi: 10.7326/0003-4819-118-8-199304150-00001. [DOI] [PubMed] [Google Scholar]
- 45.Ravid M, Lang R, Rachmani R, Lishner M. Long-term reno-protective effect of angiotensin-converting enzyme inhibition in non-insulin-dependent diabetes mellitus: a 7-year follow-up study. Arch Intern Med. 1996;156:286–9. [PubMed] [Google Scholar]
- 46.Goldberg AI, Dunlay MC, Sweet CS. Safety and tolerability of losartan potassium, an angiotensin II receptor antagonist, compared with hydrochlorothiazide, atenolol, felodipine ER, and angiotensin-converting enzyme inhibitors for the treatment of systemic hypertension. Am J Cardiol. 1995;75:793–5. doi: 10.1016/s0002-9149(99)80413-5. [DOI] [PubMed] [Google Scholar]
- 47.Ferrier C, Ferrari P, Weidman P, Keller U, Beretta-Piccoli C, Reisen WF. Antihypertensive therapy with Ca antagonist verapamil and/or ACE inhibitor enalapril in NIDDM patients. Diabetes Care. 1991;14:911–4. doi: 10.2337/diacare.14.10.911. [DOI] [PubMed] [Google Scholar]
- 48.Abbott K, Smith A, Bakris GL. Effects of dihydropyridine calcium antagonists on albuminuria in patients with diabetes. J Clin Pharmacol. 1996;36:274–9. doi: 10.1002/j.1552-4604.1996.tb04199.x. [DOI] [PubMed] [Google Scholar]
- 49.Hoelscher DD, Weir MR, Bakris GL. Hypertension in diabetic patients: an update of interventional studies to preserve renal function. J Clin Pharmacol. 1995;35:73–80. doi: 10.1002/j.1552-4604.1995.tb04748.x. [DOI] [PubMed] [Google Scholar]
- 50.Fogari R, Zoppi A, Pasotti C, et al. Comparative effects of ramipril and nitrendipine on albuminuria in hypertensive patients with non-insulin-dependent diabetes mellitus and impaired renal function. J Hum Hypertens. 1995;9:131–5. [PubMed] [Google Scholar]
- 51.Melbourne Diabetic Nephropathy Study Group. Comparison between perindopril and nifedipine in hypertensive and normotensive diabetic patients with microalbuminuria. BMJ. 1991;302:210–6. doi: 10.1136/bmj.302.6770.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Systolic Hypertension in the Elderly Program Cooperative Research Group. Implications of the Systolic Hypertension in the Elderly Program. Hypertension. 1993;21:335–43. [PubMed] [Google Scholar]
- 53.Warram JH, Laffel LM, Valsania P, et al. Excess mortality associated with diuretic therapy in diabetes mellitus. Arch Intern Med. 1991;151:1350–6. [PubMed] [Google Scholar]
- 54.Harper R, Ennis CN, Heaney AP, et al. A comparison of the effects of low and conventional-dose thiazide diuretics on insulin action in hypertensive patients with NIDDM. Diabetologia. 1995;38:853–9. doi: 10.1007/s001250050363. [DOI] [PubMed] [Google Scholar]
- 55.Olsoson G, Wikstrand J, Warnold I, et al. Metoprolol-induced reduction in postinfarction mortality: pooled results from five double blind randomized trials. Eur Heart J. 1992;13:28–32. doi: 10.1093/oxfordjournals.eurheartj.a060043. [DOI] [PubMed] [Google Scholar]
- 56.Gunderson T, Kuekshus JT. Timolol treatment after myocardial infarction in diabetic patients. Diabetes Care. 1983;6:285–90. doi: 10.2337/diacare.6.3.285. [DOI] [PubMed] [Google Scholar]
- 57.Roberts WC. Recent studies on the effects of beta-blockers on blood lipid levels. Am Heart J. 1989;117:709–14. doi: 10.1016/0002-8703(89)90757-6. [DOI] [PubMed] [Google Scholar]
- 58.Feher MD. Doxazosin therapy in the treatment of diabetic hypertension. Am Heart J. 1991;121:1291–301. doi: 10.1016/0002-8703(91)90436-l. [DOI] [PubMed] [Google Scholar]
- 59.Bakris G, Barnhill BW, Sadler R. Treatment of arterial hypertension in diabetic humans: importance of therapeutic selection. Kidney Int. 1992;41:912–9. doi: 10.1038/ki.1992.139. [DOI] [PubMed] [Google Scholar]
- 60.Schneider M, Lerch M, Papiri M, et al. Metabolic neutrality of combined verapamil-trandolapril treatment in contrast to beta-blocker–low-dose-chlortalidone treatment in hypertensive type 2 diabetics. J Hypertens. 1996;14:669–77. doi: 10.1097/00004872-199605000-00018. [DOI] [PubMed] [Google Scholar]
- 61.Dornhurst A, Powell SH, Pensky J. Aggravation by propranolol of hyperglycemic effect of hydrochlorothiazide in type II diabetics without alteration of insulin secretion. Lancet. 1985;1(9):123–6. doi: 10.1016/s0140-6736(85)91900-2. [DOI] [PubMed] [Google Scholar]
- 62.Fontbonne A, Eschwege E, Cambien F, et al. Hypertriglyceridemia as a risk factor of coronary heart disease mortality in subjects with impaired glucose tolerance or diabetes: results from the 11-year follow-up of the Paris Prospective Study. Diabetologia. 1989;32:300–4. doi: 10.1007/BF00265546. [DOI] [PubMed] [Google Scholar]
- 63.Koskinen P, Manttari M, Manninen V, Huttunen JK, Heinonen OP, Frick MH. Coronary heart disease incidence in NIDDM patients in the Helsinki Heart Study. Diabetes Care. 1992;15:820–5. doi: 10.2337/diacare.15.7.820. [DOI] [PubMed] [Google Scholar]
- 64.Scandinavian Simvastatin Survival Study Group. Randomized trial of cholesterol lowering in 4444 patients with coronary heart disease: The Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–9. [PubMed] [Google Scholar]
- 65.West of Scotland Coronary Prevention Study Group. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med. 1995;333:1301–7. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 66.Summary of the Second Report of the National Cholesterol Program (NCEP) Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel II). JAMA. 1993;269:3015–23. [PubMed] [Google Scholar]
- 67.Farrer M, Winocour PH, Evans K, et al. Simvastatin in non–insulin dependent diabetes mellitus: effect on serum lipids, lipoproteins and hemastatic measures. Diabetes Res Clin Pract. 1994;23:111–9. doi: 10.1016/0168-8227(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 68.Sweany A, Shapiro D, Tate A, Goldberg R, Stein E. Effects of simvastatin versus gemfibrozil on lipids and glucose control in patients with non–insulin dependent diabetes mellitus. Clin Ther. 1995;17:186–203. doi: 10.1016/0149-2918(95)80018-2. [DOI] [PubMed] [Google Scholar]
- 69.Garg A, Grundy S. Nicotinic acid as therapy for dyslipidemia in non-insulin-dependent diabetes mellitus. JAMA. 1990;264:723–6. [PubMed] [Google Scholar]
- 70.Shen D, Fuh MM, Shieh S, Ida Chen Y, Reaven GM. Effect of gemfibrozil treatment in sulfonylurea-treated patients with non insulin-dependent diabetes. J Clin Endocrinol Metab. 1991;73:503–10. doi: 10.1210/jcem-73-3-503. [DOI] [PubMed] [Google Scholar]
- 71.Huttunen JK, Heinonen OP, Manninen V, et al. The Helsinki Heart Study: an 8.5 year safety and mortality follow-up. J Intern Med. 1994;235:31–9. doi: 10.1111/j.1365-2796.1994.tb01029.x. [DOI] [PubMed] [Google Scholar]
- 72.Suarez L, Barrett-Conner E. Interaction between cigarette smoking and diabetes mellitus in the prediction of death attributed to cardiovascular disease. Am J Epidemiol. 1984;120:670–5. doi: 10.1093/oxfordjournals.aje.a113933. [DOI] [PubMed] [Google Scholar]
- 73.Joseph AM, Norman SM, Ferry LH, et al. The safety of transdermal nicotine as an aid to smoking cessation in patients with cardiac disease. N Engl J Med. 1996;335:1792–8. doi: 10.1056/NEJM199612123352402. [DOI] [PubMed] [Google Scholar]
- 74.The Diabetes Control and Complications Trials Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–83. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 75.Pirart J. Diabetes mellitus and its degenerative complications: a prospective study of 4400 patients observed between 1947 and 1973. Diabetes Care. 1978;1(pt 1):168–88. and 252–63(pt 2) [Google Scholar]
- 76.Klein R, Klein BEK, Moss SE. Glycosylated hemoglobin predicts the incidence and progression of diabetic retinopathy. JAMA. 1988;260(9):2864–71. [PubMed] [Google Scholar]
- 77.Klein R, Klein BEK, Moss SE. Relation of glycemic control to diabetic microvascular complications in diabetes mellitus. Ann Intern Med. 1996;124(9):90–6. doi: 10.7326/0003-4819-124-1_part_2-199601011-00003. [DOI] [PubMed] [Google Scholar]
- 78.Singer DE, Nathan DM, Fogel HA, Schachat AP. Screening for diabetic retinopathy. Ann Intern Med. 1992;116:660–71. doi: 10.7326/0003-4819-116-8-660. [DOI] [PubMed] [Google Scholar]
- 79.Mogensen CE. Microalbuminuria predicts clinical proteinuria and early mortality in maturity-onset diabetes. N Engl J Med. 1984;10(9):356–60. doi: 10.1056/NEJM198402093100605. [DOI] [PubMed] [Google Scholar]
- 80.Bennet PH, Haffner S, Kasiske BL, et al. Diabetic renal disease recommendations. Screening and management of microalbuminuria in patients with diabetes mellitus: recommendations to the Scientific Advisory Board of the National Kidney Foundation from an Ad Hoc Committee of the Council on Diabetes Mellitus of the National Kidney Foundation. Am J Kidney Dis. 1995;25:107–12. doi: 10.1016/0272-6386(95)90636-3. [DOI] [PubMed] [Google Scholar]
- 81.Delcourt C, Villatte-Cathelineau B, Vauzelle-Kervroedan F, Papoz L. Clinical correlates of advanced retinopathy in type II diabetic patients: implications for screening. J Clin Epidemiol. 1996;49(9):679–85. doi: 10.1016/0895-4356(95)00582-x. [DOI] [PubMed] [Google Scholar]
- 82.Pedrini MT, Levey AS, Lau J, Chalmers TC, Wang PH. The effect of dietary protein restriction on the progression of diabetic and non-diabetic renal diseases: a meta-analysis. Ann Intern Med. 1996;124:627–32. doi: 10.7326/0003-4819-124-7-199604010-00002. [DOI] [PubMed] [Google Scholar]
- 83.Ohkubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28:103–17. doi: 10.1016/0168-8227(95)01064-k. [DOI] [PubMed] [Google Scholar]
- 84.Bild DE, Selby JV, Sinnock P, Browner WS, Braveman P, Showstack JA. Lower extremity amputation in people with diabetes: epidemiology and prevention. Diabetes Care. 1989;12:24–31. doi: 10.2337/diacare.12.1.24. [DOI] [PubMed] [Google Scholar]
- 85.Sonsenko JM, Kato M, Soto R, Bild DE. Comparison of quantitative sensory-threshold measures for their association with foot ulceration in diabetic patients. Diabetes Care. 1990;13:1057–61. doi: 10.2337/diacare.13.10.1057. [DOI] [PubMed] [Google Scholar]
- 86.Rith-Najarian SJ, Stolusky T, Gohdes DM. Identifying patients at risk for lower extremity amputation in a primary health care setting: a prospective evaluation of simple screening criteria. Diabetes Care. 1992;15:1386–9. doi: 10.2337/diacare.15.10.1386. [DOI] [PubMed] [Google Scholar]
- 87.Litzelman D, Slemenda C, Langefield C, et al. Reduction in lower extremity clinical abnormalities in patients with non-insulin-dependent diabetes. Ann Intern Med. 1993;119:36–41. doi: 10.7326/0003-4819-119-1-199307010-00006. [DOI] [PubMed] [Google Scholar]
- 88.Uccioli L, Faglia E, Monticone G, et al. Manufactured shoes in the prevention of diabetic foot ulcers. Diabetes Care. 1995;10:1376–8. doi: 10.2337/diacare.18.10.1376. [DOI] [PubMed] [Google Scholar]
- 89.Sima AAF, Bril V, Nathaniel V, et al. Regeneration and repair of myelinated fibers in sural nerve biopsies from patients with diabetic neuropathy treated with an ARI. N Engl J Med. 1988;319:548–55. doi: 10.1056/NEJM198809013190905. [DOI] [PubMed] [Google Scholar]
- 90.Greene D, Arezzo J, Brown M, the Zenarestat Study Group Dose-related effects of the aldose reductase inhibitor Zenarestat on nerve sorbitol levels, nerve conduction velocity and nerve fiber density in human diabetic neuropathy. Diabetes. 1996;45:A9. Abstract. [Google Scholar]
- 91.Feldman EL, Stevens MJ, Greene DA. Treatment of diabetic neuropathy. In: Mazzaferri EL, Bar RS, editors. Advances in Endocrinology and Metabolism, Vol 5. St. Louis, Mo: Mosby Year Book Inc.; 1994. pp. 393–428. 5. [Google Scholar]
- 92.Caputo GM, Cavanagh PR, Ulbrecht JS, Gibbons GW, Karchmer AW. Assessment and management of foot disease in patients with diabetes. N Engl J Med. 1994;331:854–60. doi: 10.1056/NEJM199409293311307. [DOI] [PubMed] [Google Scholar]
- 93.Edmonds NE, Blundell MP, Morris ME, et al. Improved survival of the diabetic foot: the role of a specialized foot clinic. QJM. 1986;6:763–71. [PubMed] [Google Scholar]
- 94.UK Prospective Diabetes Study. Overview of 6 years’ therapy of type II diabetes: a progressive disease. U.K. Prospective Diabetes Study Group. Diabetes. 1995;4:1249–58. [PubMed] [Google Scholar]
- 95.McCance DR, Hanson RL, Charles M, et al. Comparison of tests for glycated haemoglobin and fasting and two hour plasma glucose concentrations as diagnostic methods for diabetes. BMJ. 1994;308:1323–8. doi: 10.1136/bmj.308.6940.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gabbay KH, Hasty K, Breslow JL, et al. Glycosylated hemoglobins and long-term blood glucose control in diabetes mellitus. J Clin Endocrinol Metab. 1977;44:859–64. doi: 10.1210/jcem-44-5-859. [DOI] [PubMed] [Google Scholar]
- 97.Bunn HF. Evaluation of glycosylated hemoglobin in diabetic patients. Diabetes. 1981;30:613–7. doi: 10.2337/diab.30.7.613. [DOI] [PubMed] [Google Scholar]
- 98.American Diabetes Association. Nutrition recommendations and principles for people with diabetes mellitus. Diabetes Care. 1997;20(9):S14–7. [PubMed] [Google Scholar]
- 99.Franz MJ, Monk A, Barry B, et al. Effectiveness of medical nutrition therapy provided by dietitians in the management of non-insulin-dependent diabetes mellitus: a randomized, controlled clinical trial. J Am Diet Assoc. 1995;95:1009–17. doi: 10.1016/S0002-8223(95)00276-6. [DOI] [PubMed] [Google Scholar]
- 100.American Diabetes Association. National standards for diabetes self-management education programs. Diabetes Care. 1995;18:141–4. doi: 10.2337/diacare.18.5.737. [DOI] [PubMed] [Google Scholar]
- 101.Wing RR, Koeske R, Epstein LH, Nowalk MP, Gooding W, Becker D. Long-term effects of modest weight loss in type II diabetic patients. Arch Intern Med. 1987;147:1749–53. [PubMed] [Google Scholar]
- 102.Reaven GM. Beneficial effect of moderate weight loss in older patients with non-insulin-dependent diabetes mellitus poorly controlled with insulin. J Am Geriatr Soc. 1985;33:93–5. doi: 10.1111/j.1532-5415.1985.tb02272.x. [DOI] [PubMed] [Google Scholar]
- 103.Watts NB, Spanheimer RG, DiGirolamo M, et al. Prediction of glucose response to weight loss in patients with non-insulin-dependent diabetes mellitus. Arch Intern Med. 1990;150:803–6. [PubMed] [Google Scholar]
- 104.Franz MJ, Horton ED, Sr, Bantle JP, et al. Nutrition principles for the management of diabetes and related complications. Diabetes Care. 1994;17:490–518. doi: 10.2337/diacare.17.5.490. [DOI] [PubMed] [Google Scholar]
- 105.Jenkins DJA, Ocana A, Jenkins A, et al. Metabolic advantages of spreading the nutrient load: effects of increased meal frequency in non-insulin-dependent diabetes. Am J Clin Nutr. 1992;55:461–7. doi: 10.1093/ajcn/55.2.461. [DOI] [PubMed] [Google Scholar]
- 106.Bertelsen J, Christiansen C, Thomsen C, et al. Effect of meal frequency on blood glucose, insulin and free fatty acids in NIDDM subjects. Diabetes Care. 1993;16:3–7. doi: 10.2337/diacare.16.1.4. [DOI] [PubMed] [Google Scholar]
- 107.Pavlou K, Krey NS, Steffee WP. Exercise as an adjunct to weight loss and maintenance in moderately obese subjects. Am J Clin Nutr. 1989;49:1115–23. doi: 10.1093/ajcn/49.5.1115. [DOI] [PubMed] [Google Scholar]
- 108.Wing RR. Behavioral treatment of obesity: its application to type II diabetes. Diabetes Care. 1993;16:193–9. doi: 10.2337/diacare.16.1.193. [DOI] [PubMed] [Google Scholar]
- 109.Gerich JE. Oral hypoglycemic agents. N Engl J Med. 1989;321:1231–45. doi: 10.1056/NEJM198911023211805. [DOI] [PubMed] [Google Scholar]
- 110.Lebovitz HE. Sulfonylurea drugs. In: Lebovitz HE, editor. Therapy for Diabetes and Related Disorders. 2nd ed. Alexandria, Va: American Diabetes Association; 1995. pp. 116–23. [Google Scholar]
- 111.DeFronzo RA, Goodman AM the Multicenter Metformin Study Group. Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. N Engl J Med. 1995;333:541–9. doi: 10.1056/NEJM199508313330902. [DOI] [PubMed] [Google Scholar]
- 112.Bailey CJ, Turner RC. Metformin. N Engl J Med. 1996;334:574–9. doi: 10.1056/NEJM199602293340906. [DOI] [PubMed] [Google Scholar]
- 113.Stumvoll M, Nurjhan N, Periello G, Dailery G, Derich JE. Metabolic effects of metformin in non–insulin dependent diabetes mellitus. N Engl J Med. 1995;333:550–5. doi: 10.1056/NEJM199508313330903. [DOI] [PubMed] [Google Scholar]
- 114.Chiasson JL, Josse RG, Hunt JA, et al. The efficacy of acarbose in the treatment of patients with non-insulin-dependent diabetes mellitus: a multicenter controlled clinical trial. Ann Intern Med. 1994;1221:928–35. doi: 10.7326/0003-4819-121-12-199412150-00004. [DOI] [PubMed] [Google Scholar]
- 115.Lebovitz HE. A new oral therapy for diabetes management: alpha-glucosidase inhibition with acarbose. Clin Diabetes. 1995:99–103. Nov/Dec. [Google Scholar]
- 116.Saltiel AR, Olefsky JM. Thiazolidinediones in the treatment of insulin resistance and type II diabetes. Diabetes. 1996;45:1661–9. doi: 10.2337/diab.45.12.1661. [DOI] [PubMed] [Google Scholar]
- 117.Kumar S, Boulton AJM, Beck-Nielson H, et al. Troglitazone, an insulin action enhancer, improves metabolic control in NIDDM patients. Diabetologia. 1996;39:701–9. doi: 10.1007/BF00418542. [DOI] [PubMed] [Google Scholar]
- 118.Soneru IL, Agrawal L, Murphy J, Lawrence AM, Abraira C. Comparison of morning and bedtime insulin with and without glyburide in secondary sulfonylurea failure. Diabetes Care. 1993;16:896–901. doi: 10.2337/diacare.16.6.896. [DOI] [PubMed] [Google Scholar]
- 119.Yki-Jarvinen H, Kauppila M, Kujansuu E, et al. Insulin regimens in non-insulin-dependent diabetes mellitus. N Engl J Med. 1992;327:1426–33. doi: 10.1056/NEJM199211123272005. [DOI] [PubMed] [Google Scholar]
- 120.Mills JL, Simpson JL, Driscoll SG, et al. Incidence of spontaneous abortion among normal women and women with insulin dependent diabetes whose pregnancies are identified within 21 days of conception. Diabetes in Early Pregnancy Study. N Engl J Med. 1988;319:1617–23. doi: 10.1056/NEJM198812223192501. [DOI] [PubMed] [Google Scholar]
- 121.Greene M, Hare JW, Cloherty JP, et al. First trimester HbA1c and the risk for major malformation and spontaneous abortion. Tera-tology. 1989;39:225–31. doi: 10.1002/tera.1420390303. [DOI] [PubMed] [Google Scholar]
- 122.Fuhrmann K, Reiher H, Semmler K, Fischer F, Fischer M, Glockner E. Prevention of congenital malformations in infants of insulin-dependent diabetic mothers. Diabetes Care. 1983;6:219–23. doi: 10.2337/diacare.6.3.219. [DOI] [PubMed] [Google Scholar]
- 123.Fuhrmann K, Reiher H, Semmler K, Glockner E. The effect of intensified conventional insulin therapy before and during pregnancy on the malformation rate in offspring of diabetic mothers. Exp Clin Endocrinol. 1984;83:173–7. doi: 10.1055/s-0029-1210327. [DOI] [PubMed] [Google Scholar]
- 124.Goldman JA, Dickerd D, Feldberg D. Pregnancy outcome in patients with insulin-dependent diabetes mellitus with pre-conceptual diabetic control: a comparative study. Am J Obstet Gynecol. 1986;155:193–7. doi: 10.1016/0002-9378(86)90812-4. [DOI] [PubMed] [Google Scholar]
- 125.Mills JL, Knopp RH, Simpson JL, et al. Lack of relation of increased malformation rates in infants of diabetic mothers to glycemic control during organogenesis. N Engl J Med. 1988;318:671–6. doi: 10.1056/NEJM198803173181104. [DOI] [PubMed] [Google Scholar]
- 126.Steel JM, Jonstone FD, Hepburn DA, Smith A. Can prepregnancy care of diabetic women reduce the risk of abnormal babies? BMJ. 1990;301:1070–4. doi: 10.1136/bmj.301.6760.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kitzmiller JL, Gavin LA, Gin GD, Jovanovic L, Main EK, Zigrang WD. Preconception care of diabetes: glycemic control prevents congenital anomalies. JAMA. 1991;265:73l–6. [PubMed] [Google Scholar]
- 128.Pregnancy outcomes in the Diabetes Control and Complications Trial. Am J Obstet Gynecol. 1996;174(4):1343–53. doi: 10.1016/s0002-9378(96)70683-x. [DOI] [PubMed] [Google Scholar]
- 129.Janz NK, Herman WH, Becker MP, et al. Diabetes and pregnancy: factors associated with seeking pre-conception care. Diabetes Care. 1995;18:157–65. doi: 10.2337/diacare.18.2.157. [DOI] [PubMed] [Google Scholar]
- 130.Kitzmiller JL, Buchanan TA, Kjos S, Combs CA, Ratner RE. Pre-conception care of diabetes, congenital malformations, and spontaneous abortions: a technical review. Diabetes Care. 1996;19:514–41. doi: 10.2337/diacare.19.5.514. [DOI] [PubMed] [Google Scholar]
- 131.Shotan A, Widerhorn J, Hurst A, Elkayam U. Risks of angiotensin-converting enzyme inhibition during pregnancy; experimental and clinical evidence, potential mechanisms, and recommendations for use. Am J Med. 1994;96:451–6. doi: 10.1016/0002-9343(94)90172-4. [DOI] [PubMed] [Google Scholar]
- 132.Brown SA. Effects of educational interventions and outcomes in diabetic adults: a meta–analysis revisited. Patient Educ Couns. 1990;16:189–215. doi: 10.1016/0738-3991(90)90070-2. [DOI] [PubMed] [Google Scholar]
- 133.Brown SA. Studies of educational interventions in diabetes care: a meta-analysis of findings. Nurs Res. 1988;37:223–30. [PubMed] [Google Scholar]
- 134.Clement S. Diabetes self-management education: a technical review. Diabetes Care. 1995;18:1204–14. doi: 10.2337/diacare.18.8.1204. [DOI] [PubMed] [Google Scholar]
- 135.Funnell MM, Haas LB. National standards for diabetes self-management education programs: a technical review. Diabetes Care. 1995;18:100–16. doi: 10.2337/diacare.18.1.100. [DOI] [PubMed] [Google Scholar]
- 136.Goodall TA, Halford WK. Self-management of diabetes mellitus: a critical review. Health Psychol. 1991;10:1–8. doi: 10.1037//0278-6133.10.1.1. [DOI] [PubMed] [Google Scholar]
- 137.Gruesser M, Bott U, Ellermann P, Kronsbein P, Joergens V. Evaluation of a structured treatment and teaching program for non–insulin treated type II diabetic outpatients in Germany after the nationwide introduction of reimbursement policy for physicians. Diabetes Care. 1993;16:1268–75. doi: 10.2337/diacare.16.9.1268. [DOI] [PubMed] [Google Scholar]
- 138.Padgett D, Mumford E, Hynes M, Carter R. Meta-analysis of the effects of educational and psychosocial interventions on management of diabetes mellitus. J Clin Epidemiol. 1988;41:1007–30. doi: 10.1016/0895-4356(88)90040-6. [DOI] [PubMed] [Google Scholar]
- 139.Rubin RR, Peyrot M. Psychosocial problems and interventions in diabetes: a review of the literature. Diabetes Care. 1992;15:1640–57. doi: 10.2337/diacare.15.11.1640. [DOI] [PubMed] [Google Scholar]
- 140.Nicolucci A, Cavaliere D, Scorpiglione N, et al. A comprehensive assessment of the avoidability of long-term complications of diabetes. Diabetes Care. 1996;19:927–33. doi: 10.2337/diacare.19.9.927. [DOI] [PubMed] [Google Scholar]
- 141.Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation: basis for prevention. Diabetes Care. 1990;13:513–21. doi: 10.2337/diacare.13.5.513. [DOI] [PubMed] [Google Scholar]
- 142.Franz MJ, Monk A, Barry B, et al. Effectiveness of medical nutrition therapy provided by dietitians in the management of non insulin-dependent diabetes mellitus: a randomized, controlled clinical trial. J Am Diet Assoc. 1995;95:1009–17. doi: 10.1016/S0002-8223(95)00276-6. [DOI] [PubMed] [Google Scholar]
- 143.Raz I, Soskolne V, Stein P. Influence of small-group education sessions on glucose homeostasis in NIDDM. Diabetes Care. 1990;13:513–21. doi: 10.2337/diacare.11.1.67. [DOI] [PubMed] [Google Scholar]
- 144.Muhlhauser I, Berger J. Diabetes education and insulin therapy. When will they ever learn? J Intern Med. 1993;233:321–6. doi: 10.1111/j.1365-2796.1993.tb00679.x. [DOI] [PubMed] [Google Scholar]


