Abstract
OBJECTIVE
To determine whether persons living with HIV find a disease-specific advance directive more acceptable than a generic directive.
DESIGN
Randomized clinical trial.
SETTING
HIV consumer organization and hospital-based HIV clinic.
PARTICIPANTS
Volunteer sample of persons with HIV.
INTERVENTIONS
The disease-specific HIV Living Will, the generic Centre for Bioethics Living Will, or both.
MEASUREMENTS AND MAIN RESULTS
Of 101 participants who received both advance directives, 78 (77.2%) preferred the disease-specific HIV Living Will and 23 (22.8%) preferred the generic Centre for Bioethics Living Will (p < .001). Most participants who preferred the HIV Living Will did so because it was more specific or relevant to their situation.
CONCLUSIONS
Persons living with HIV prefer a disease-specific to a generic advance directive. They should be offered a disease-specific advance directive. Our findings should also encourage investigators to develop and evaluate disease-specific advance directives in other clinical settings.
Keywords: ethics, end of life, advance directives, living wills, human immunodeficiency virus
Advance directives indicate who a person would want to make treatment decisions on his or her behalf and what treatments a person would or would not want in various situations.1,2 Completed by a person who can understand and appreciate the consequences of treatment decisions (i.e., when he or she is competent or capable), an advance directive is used at a time when the person has become incompetent or incapable.
Current advance directive documents—for example, the Medical Directive,3 Let Me Decide directive,4 University of Toronto Centre for Bioethics Living Will,5 Values History,6,7 or the forms prepared by lawyers and governments—are all generic. Intended for the general public, generic advance directives can be criticized because they contain hypothetical, often irrelevant choices and inadequate prognostic information.
Compared with generic advance directives, advanced directives designed specifically for people who have a particular disease have several potential advantages.8 First, they present patients with choices that are more relevant than those contained in generic advance directives. Second, because the group of patients completing the advance directive document is more homogenous, more specific prognostic information can be presented. Finally, because the patient already has experience of the illness that may lead to those choices, the choices themselves are less hypothetical. Whether these theorized advantages of disease-specific advance directives are borne out in practice is currently unknown.
The purpose of this study was to determine whether persons living with HIV find a disease-specific HIV advance directive more acceptable than a generic advance directive.
METHODS
Study Design
In this randomized trial, participants were randomized to one of three groups: a group receiving the generic Centre for Bioethics Living Will alone, a group receiving the disease-specific HIV Living Will alone, or a group receiving both of these advance directives. Participants were followed over two visits. At the first visit, participants were screened for eligibility, received information about the study, provided consent to participate in the research, viewed a 17-minute educational video about advance directives developed specifically for persons with HIV, and received the generic Centre for Bioethics Living Will alone, the disease-specific HIV Living Will alone, or both advance directives to review at home. At the second visit (2 weeks later), participants completed the advance directive they had received (those subjects randomized to receive both advance directives completed the one they preferred), and rated the acceptability of the advance directive (those randomized to receive both advance directives rated both). Participants were enrolled between November 1, 1994, and June 15, 1995.
Participants
A volunteer sample of persons with HIV responded to the study advertisements or posters distributed in the community by the AIDS Committee of Toronto and placed in the waiting rooms of the Toronto Hospital Immunodeficiency Clinic. In addition, patients attending the Immunodeficiency Clinic were given information about the study and a telephone number to call if they were interested in participating. During the study period, the charts of all patients at the Immunodeficiency Clinic had study labels on which to note if a patient had been given the study information, chose not to participate, or had been excluded. Participants were excluded if they were less than 16 years old, were not fluent in English, could not read, were incapable of completing an advance directive (as measured by a Standardized Mini-Mental Status Evaluation [SMMSE] test score < 23), would experience undue emotional distress from completing an advance directive (as measured by self-report), resided outside Metropolitan Toronto, or refused participation in the research. A log of rejected participants documenting reasons for exclusion and refusals was kept.
At the Toronto Hospital Immunodeficiency Clinic, there were 753 patients being followed during the study period. Of these, 268 did not attend the clinic during the study period, 200 were not approached (reason not identified), and 70 were excluded by clinic staff (41 were too ill, 20 were unable to read or understand English well, 3 were mentally incapable, and 6 were excluded by clinic staff for unidentified reasons). The remaining 215 patients were approached by the clinic staff or the study coordinator and given information about the study. Of these, 41 were satisfied with a previously completed advance directive and chose not to participate, 9 lived outside the study area, 12 had entered the study through the AIDS Committee of Toronto, 1 scored below 22 on the SMMSE, 6 refused, and 40 did not contact the study coordinator. The remaining 106 patients participated in the study, but 2 withdrew, leaving a total of 104 participants who completed the study through this site. The AIDS Committee of Toronto received 114 referrals. Of these, 5 decided not to participate, 5 withdrew, 3 were too ill to participate, 1 did not read or understand English, 1 was unable to read, and 99 completed the study. Therefore, at both sites, a total of 203 participants completed the study.
Interventions
Two advance directives were used in this study: the Centre for Bioethics Living Will and the HIV Living Will. The Centre for Bioethics Living Will, a generic advance directive, was previously found to be more acceptable than the Medical Directive.9 It was also evaluated for face or content validity by an interdisciplinary group of experts (lawyers, doctors, nurses and philosophers): of 15 respondents, 14 (93.3%) indicated the Centre for Bioethics Living Will fulfills its purpose of “document[ing] the wishes of competent persons regarding the use of life-sustaining treatments and regarding a proxy decision maker for situations of future incompetence.”
The HIV Living Will is a modification of the Centre for Bioethics Living Will that incorporates prognostic information from the published literature and was developed in consultation with physicians, nurses, social workers, clergy, lawyers, and representatives of organizations that provide services to persons living with HIV. Development of the HIV disease-specific advance directive began with a review of the literature on prognosis in patients with HIV or AIDS. Next we drafted a version of the HIV Living Will, modified from the generic Centre for Bioethics Living Will, which incorporated the prognostic information from the literature searches. Then we conducted informal focus group discussions and consultations with physicians, nurses, social workers, clergy, lawyers, and consumers with expertise in HIV and AIDS. On the basis of these comments, we revised the HIV Living Will. The face or content validity of the HIV Living Will was reviewed by a group of AIDS service providers: 8 (89%) of 9 respondents agreed that the HIV Living Will fulfilled its purpose.
Both advance directives are booklets of approximately 30 pages with chapters on questions and answers about advance directives, the legal status of advance directives, information about health care decisions, information about personal care decisions, a chapter containing the advance directive form itself and an identification card. The primary difference between the two advance directives is the chapter about information about health care decisions and the instruction directive part of the advance directive form. The different categories of health states contained in the two advance directives are shown in Figures 1 and 2. Throughout the description of health states and treatments, the HIV Living Will refers specifically to issues in the care of persons with HIV, while the Centre for Bioethics Living Will is generic. For instance, the description of acute or potentially reversible illness in the HIV Living Will mentions Pneumocystis cariniipneumonia, central nervous system toxoplasmosis, and cryptococcal meningitis. By contrast, the current health section in the Centre for Bioethics Living Will mentions cardiac arrest and pneumonia. Both living wills are posted on the University of Toronto Joint Centre for Bioethics Web site (URL:www.utoronto.ca/jcb).
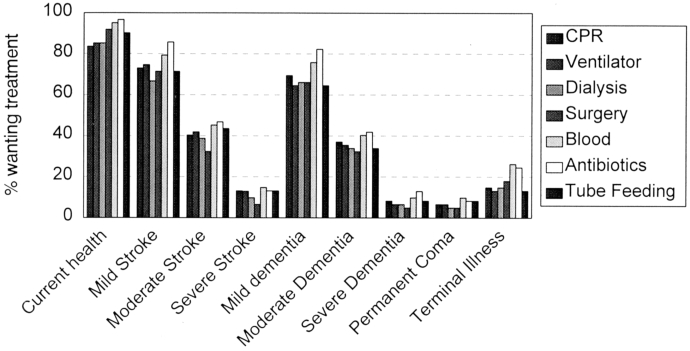
Treatment preferences on the Centre for Bioethics Living Will. The height of each bar represents the proportion of participants at the second interview who wanted the specified treatment in the specified health situation. The number of participants answering for each situation-treatment choice ranges from 61 to 63. The health states are indicated along the x axis; within each health state, the bars (from left to right) represent cardiopulmonary resuscitation, ventilator, dialysis, surgery, blood transfusion, antibiotics, and tube feeding.
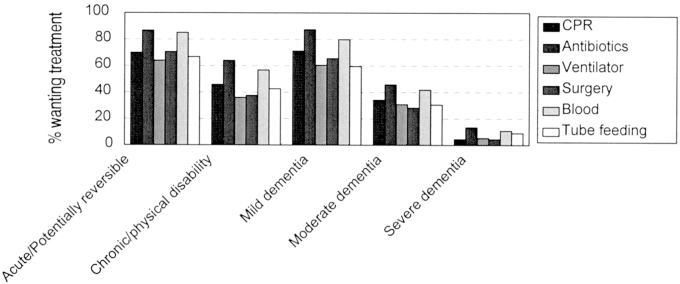
Treatment preferences on the HIV Living Will. The height of each bar represents the proportion of participants at the second interview who wanted the specified treatment in the specified health situation. The number of participants answering each situation-treatment choice ranges from 108 to 114. The health states are indicated along the x axis; within each health state, the bars (from left to right) represent cardiopulmonary resuscitation, antibiotics, ventilator, surgery, blood transfusion, and tube feeding.
Outcome Measures
Acceptability of the advance directives used in the study was measured using the Advance Directive Choice Questionnaire (ADCQ) and Advance Directive Acceptability Questionnaire (ADAQ). The ADCQ asks respondents, “We are interested in which of the two living wills you liked best. If you had to choose one of these two living wills, which one would you choose to complete?” It also elicits the reasons for their choice. The ADAQ contains 14 items rated on a 5-point ordinal scale from excellent to poor. The ADAQ was initially used in a previous study and subsequently modified on the basis of results of factor analysis and open-ended responses by participants.10 It has been evaluated for face or content validity by an interdisciplinary panel of experts: of 22 respondents, 19 (86%) said the ADAQ fulfilled its stated purpose and 3 (14%) said it did not; the current version of the ADAQ incorporates the suggestions of these experts. In a previous study, internal consistency reliability of the ADAQ measured using Cronbach's α was 0.93.
Data Analysis
The proportion of participants who preferred each advance directive was compared using the χ2 test. The total score on the ADAQ was calculated as the sum of the individual items and reported as a percentage of the highest achievable score. These scores were analyzed using repeated measures data analysis by the Proc Mixed procedure of SAS 6.11. Initially, an analysis to evaluate the potential confounding effect of receiving one advance directive or two on the main effect of advance directive type (i.e., Centre for Bioethics Living Will vs HIV Living Will) was conducted. As this factor or its interaction with the main effect of advance directive type did not prove to be a significant confounder, the analysis was simplified to model only the main effect of advance directive type on total ADAQ score. As well, we recorded and described, using content analysis, participants' open-ended responses regarding why they preferred one or the other advance directive.
RESULTS
Participant Characteristics
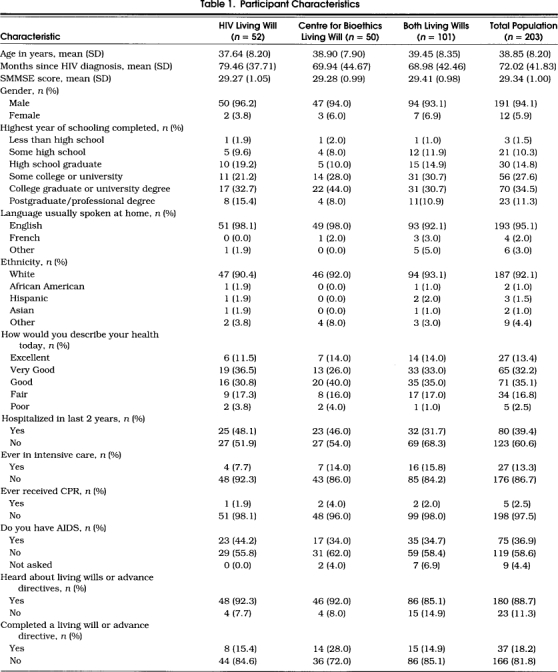
The characteristics of the 203 participants are shown in Table 1. There were no significant differences at baseline among the three groups.
Table 1.
Participant Characteristics
Treatment Preferences
Treatment preferences recorded in the Centre for Bioethics Living Will and the HIV Living Will by the end of the second interview are shown in Figures 1 and 2. As shown in Figures 1 and 2, health states and severity of illness have a greater influence on preferences than do treatments. For instance, preferences across health states in the Centre for Bioethics Living Will vary widely from 84% of respondents wanting cardiopulmonary resuscitation (CPR) in their current health state to 7% wanting CPR in permanent coma. Moreover, 69% of respondents would want CPR in mild dementia, 37% in moderate dementia, and 8% in severe dementia. By contrast, in current health, the range of preferences is from 84% of respondents wanting CPR to 97% wanting antibiotics.
Acceptability of the Advance Directives
Of the 203 participants, 50 received the generic Centre for Bioethics Living Will alone, 52 received the disease-specific HIV Living Will alone, and 101 received both advance directives. Of the 101 participants who received both when asked, “If you had to choose one of these two living wills, which one would you choose to complete?,” 78 (77.2%) preferred the HIV Living Will and 23 (22.8%) preferred the generic Centre for Bioethics Living Will (p < .001; reject null hypothesis that p = q = .5). Of the 78 participants who chose the disease-specific HIV Living Will, 72 did so because it was more specific or relevant to their situation (for instance, participants said the HIV Living Will “related to my situation without irrelevant verbiage,” “seems more appropriate to my care,” “zeros in on specific treatments relating to AIDS/HIV,” “pertains to the possible problems that might arise”); 5 said the HIV Living Will was more specific but they would add “stroke and other chronic conditions”; and 1 wanted “more control.” Of the 23 participants who chose the generic Centre for Bioethics Living Will, 20 did so because it was more detailed or comprehensive (for instance, one participant said, “HIV-positive people also get strokes, car accidents, etc.”); one participant did not want his family to know he had HIV; one was concerned about the potential for discrimination against people with HIV; and one said it was the “lesser of two evils.”
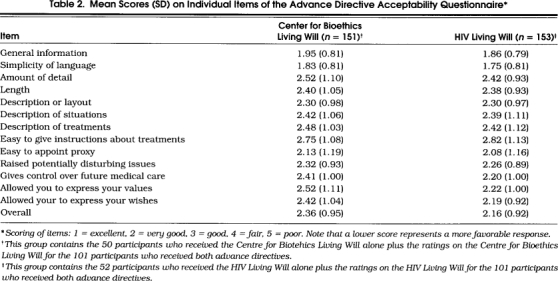
The mean ADAQ score was 68.5% for the HIV Living Will and 66.2% for the Centre for Bioethics Living Will (F= 3.9, p= .0510). The acceptability ratings for the individual item scores for the two advance directives are shown in Table 2. Most of the ratings were in the very good to good range.
Table 2.
Mean Scores (SD) on Individual Items of the Advance Directive Acceptability Questionnaire*
DISCUSSION
The major finding of this study is that persons with HIV preferred a disease-specific advance directive over a generic one. This preference was shown in two ways—based on a choice of the disease-specific advance directive when the two advance directives were reviewed together, and based on a trend toward higher ADAQ scores for the disease-specific advance directive. The disease-specific advance directive was preferred because it was more specific and relevant to persons with HIV. This study confirms that the theorized advantages of disease-specific advance directives 8 are borne out in practice, at least for patients with HIV.
Persons with HIV who wish to complete an advance directive should be offered a disease-specific HIV advance directive. This recommendation is consistent with previous studies, which have shown that people prefer detailed advance directives,11 that general instructions are not helpful in communicating patient wishes regarding specific life-sustaining procedures,12 and that more specific advance directives result in more uniform interpretation by physicians.13 Although some might argue that acceptability is a weak foundation on which to base such a recommendation, we believe that the most important effects of advance directives and advance care planning are not on those clinical and financial outcomes that have been measured to date,14 but rather on psychosocial outcomes related to people preparing for death and dying.15 Our findings should also encourage investigators to develop and evaluate specific advance directives for other diseases such as cancer and Alzheimer disease.
The pattern of treatment preferences has implications for the design of generic advance directives and physician-patient discussions. As shown in Figures 1 and 2, health states and severity of illness have a greater influence on preferences than do treatments. The same pattern of treatment preferences was previously documented in patients undergoing hemodialysis.9 To elicit a full set of preferences, advance directives should focus on descriptions of a spectrum of health states, and the descriptions of these health states should be at least as comprehensive as the description of treatments. In discussions with patients about future treatment choices, such as discussions about “Do not resuscitate” orders, physicians should focus on the resultant health states as well as the treatments proposed. Moreover, an advance directive or physician-patient discussion that does not probe for differences in illness severity within health states will miss major variations in preferences. Our study did not explicitly examine how the duration of life-sustaining treatment, a factor that is probably also important, influences the pattern of treatment preferences.
Disease-specific advance directives have one major disadvantage compared with generic advance directives. Although disease-specific advance directives focus on the most likely future situations for patients with a particular disease, unlikely events sometimes occur. Persons living with HIV may still have a stroke or automobile accident, as was pointed out by our participants. In addition, an unanticipated and unintended problem with disease-specific advance directives in the context of HIV is the issue of confidentiality. Of those who preferred the generic Centre for Bioethics Living Will, one participant did so because of confidentiality concerns. Two other participants mentioned this as well, although it was not the primary reason they chose the generic advance directive. The issue of confidentiality could be addressed, in part, by not writing “HIV” on the cover of the living will.
Our study also provides a description of treatment preferences of persons living with HIV. For instance, 87% of respondents would want to receive antibiotics, and 64% would want to be put on a ventilator, in the acute or potentially reversible illness scenario in the HIV Living Will. These findings are consistent with those of Steinbrook et al., who found that 95% of patients with AIDS wanted antibiotic treatment and 55% wanted mechanical ventilation for Pneumocystis cariniipneumonia.16
Eighteen percent of the participants in our study said they had previously completed an advance directive. This is consistent with previous studies of persons living with HIV, which found that 28% had completed an advance directive,17 and 38% had discussed their life-sustaining treatment preferences with their physician.18 The rate of completion of advance directives by patients with HIV is considerably higher than the 2% rate among general internal medicine outpatients at the same hospital,19 or the 12% rate among the Ontario Public.20
The main potential limitation of this study is volunteer bias. Participants were not randomly sampled but volunteered in response to advertisements. This bias does not undermine the main conclusion because participants were randomized to receive one or the other (or both) advance directives. The bias does limit the generalizability of the findings to those who would complete an advance directive, but these are precisely the people for whom advance directive forms are designed. In addition, the generalizability of our findings is limited by our use of one specific patient population—persons living with HIV—and by the observation that advance directive documents are only one element of advance care planning, a “process of communication among patients, their health care providers, their families, and important others regarding the kind of care that will be considered appropriate when the patient cannot make decisions.”21
In conclusion, persons living with HIV prefer a disease-specific to a generic advance directive. They should be offered a disease-specific advance directive.
Acknowledgments
The authors thank Mina Gajjar, Marla Lueck, and Keitha McMurray for conducting the interviews, Shawna Mercer for data entry, and Eileen Lee for conducting the statistical analyses. Our primary debt of gratitude is to the persons with HIV who participated in this study.
References
- 1.Advance Directives Seminar Group [Principal Author: Singer PA]. Advance directives: are they an advance? Can Med Assoc J. 1992;146:127–34. [PMC free article] [PubMed] [Google Scholar]
- 2.Emanuel L. Advance directives: what have we learned so far? J Clin Ethics. 1993;4:8–15. [PubMed] [Google Scholar]
- 3.Emanuel LL, Emanuel EJ. The Medical Directive: a new comprehensive advance care document. JAMA. 1989;261:3288–93. doi: 10.1001/jama.261.22.3288. [DOI] [PubMed] [Google Scholar]
- 4.Molloy DW, Mepham V. Toronto, Ont: Penguin; 1992. Let Me Decide. [Google Scholar]
- 5.Singer PA. Toronto, Ont: Centre for Bioethics; 1994. University of Toronto Centre for Bioethics Living Will. [Google Scholar]
- 6.Doukas DJ, McCullough LB. The Values History: the evaluation of patient's values and advance directives. J Fam Prac. 1991;32:145–53. [PubMed] [Google Scholar]
- 7.Lambert P, Gibson JM, Nathanson P. The Values History: an innovation in surrogate medical decision-making. Law Med Health Care. 1990;18:202–12. doi: 10.1111/j.1748-720x.1990.tb00023.x. [DOI] [PubMed] [Google Scholar]
- 8.Singer PA. Disease-specific advance directives. Lancet. 1994;344:594–6. doi: 10.1016/s0140-6736(94)91971-2. [DOI] [PubMed] [Google Scholar]
- 9.Singer PA, Thiel EC, Naylor CD, et al. Life-sustaining treatment preferences of hemodialysis patients: implications for advance directives. J Am Soc Nephrology. 1995;6:1410–7. doi: 10.1681/ASN.V651410. [DOI] [PubMed] [Google Scholar]
- 10.Reinders M, Singer PA. Which advance directives do patients prefer? J Gen Intern Med. 1994;9:49–51. doi: 10.1007/BF02599143. [DOI] [PubMed] [Google Scholar]
- 11.Johnston SC, Pfeifer MP, McNutt R, for the End of Life Study Group The discussion about advance directives: patient and physician opinions regarding when and how it should be conducted. Arch Intern Med. 1995;155:1025–30. doi: 10.1001/archinte.155.10.1025. [DOI] [PubMed] [Google Scholar]
- 12.Schneiderman LJ, Pearlman RA, Kaplan RM, Anderson JP, Rosenberg EM. Relationship of general advance directive instructions to specific life-sustaining treatment preferences in patients with serious illness. Arch Intern Med. 1992;152:2114–22. [PubMed] [Google Scholar]
- 13.Mower WR, Baraff LJ. Advance directives: effect of type of directive on physicians' therapeutic decisions. Arch Intern Med. 1993;153:375–81. doi: 10.1001/archinte.153.3.375. [DOI] [PubMed] [Google Scholar]
- 14.SUPPORT Principal Investigators A controlled trial to improve care for seriously ill hospitalized patients: The Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments (SUPPORT) JAMA. 1995;274:1591–8. [PubMed] [Google Scholar]
- 15.Singer PA, Martin DK, Lavery JV, Thiel EC, Kelner M, Mendelssohn DC. Reconceptualizing advance care planning from the patient's perspective. Arch Intern Med. doi: 10.1001/archinte.158.8.879. In press. [DOI] [PubMed] [Google Scholar]
- 16.Steinbrook R, Lo B, Moulton J, Saika G, Hollander H, Volberding PA. Preferences of homosexual men with AIDS for life-sustaining treatment. N Engl J Med. 1986;314:457–60. doi: 10.1056/NEJM198602133140730. [DOI] [PubMed] [Google Scholar]
- 17.Teno J, Fleishman J, Brock DW, Mor V. The use of formal prior directives among patients with HIV-related disease. J Gen Intern Med. 1990;5:490–4. doi: 10.1007/BF02600877. [DOI] [PubMed] [Google Scholar]
- 18.Haas JS, Weissman JS, Cleary PD, et al. Discussion of preferences for life-sustaining care by persons with AIDS: predictors of failure in patient-physician communication. Arch Intern Med. 1993;153:1241–8. [PubMed] [Google Scholar]
- 19.Sam M, Singer PA. Canadian outpatients and advance directives: poor knowledge, little experience, but positive attitudes. Can Med Assoc J. 1993;148:1497–502. [PMC free article] [PubMed] [Google Scholar]
- 20.Singer PA, Choudhry S, Armstrong J. Public opinion regarding consent to treatment. J Am Geriatr Soc. 1993;41:112–6. doi: 10.1111/j.1532-5415.1993.tb02042.x. [DOI] [PubMed] [Google Scholar]
- 21.Teno JM, Nelson HL, Lynn J. Advance care planning: priorities for ethical and empirical research. Hastings Center Rep. 1994;24(suppl 12):S32–6. [PubMed] [Google Scholar]


