Abstract
OBJECTIVE
To identify the factors that predict recovery in activities of daily living (ADLs) among disabled older persons living in the community.
DESIGN
Prospective cohort study with 2-year follow-up.
SETTING
General community.
PARTICIPANTS
213 men and women 72 years or older, who reported dependence in one or more ADLs.
MEASUREMENTS AND MAIN RESULTS
All participants underwent a comprehensive home assessment and were followed for recovery of ADL function, defined as requiring no personal assistance in any of the ADLs within 2 years. Fifty-nine participants (28%) recovered independent ADL function. Compared with those older than 85 years, participants aged 85 years or younger were more than 8 times as likely to recover their ADL function (relative risk [RR] 8.4; 95% confidence interval [CI] 2.7, 26). Several factors besides age were associated with ADL recovery in bivariate analysis, including disability in only one ADL, self-efficacy score greater than 75, Folstein Mini-Mental State Examination (MMSE) score of 28 or better, high mobility, score in the best third of timed physical performance, fewer than five medications, and good nutritional status. In multivariable analysis, four factors were independently associated with ADL recovery—age 85 years or younger (adjusted RR 4.1; 95% CI 1.3, 13), MMSE score of 28 or better (RR 1.7; 95% CI 1.2, 2.3), high mobility (RR 1.7; 95% CI 1.0, 2.9), and good nutritional status (RR 1.6; 95% CI 1.0, 2.5).
CONCLUSIONS
Once disabled, few persons older than 85 years recover independent ADL function. Intact cognitive function, high mobility, and good nutritional status each improve the likelihood of ADL recovery and may serve as markers of resiliency in this population.
Keywords: activities of daily living (ADLs), recovery, elderly, prospective cohort study, prognosis
A mong community-living older persons, the prevalence of disability in one or more activities of daily living (ADLs), such as bathing, dressing, and walking, increases substantially with age, from about 7% in those aged 65 to 74 years, to 14% in those aged 75 to 84 years, to over 24% in those aged 85 years or older. 1 Although useful in estimating the need for health care resources, prevalence estimates of disability do not convey that functional status and disability represent dynamic processes in older persons. Belying the stereotype of the older person as having an inexorable downhill course, from mild impairment to disability, longitudinal studies have found that a substantial minority (24% –30%) of elders, once disabled, recover independence in their ADL function. 1,2
Why some disabled elders recover and others do not is not known. Those who recover can be considered to be resilient. 3 If carefully studied and suitably characterized, these resilient elders might offer investigators valuable insights into the mechanisms of disability and dependence in older persons and facilitate the development of effective and efficient intervention programs to prevent, slow, or reverse ADL dependence. 4 Previous studies of functional recovery have focused almost exclusively on persons hospitalized after an acute event, such as a stroke or hip fracture. 5–9 Conversely, several community-based studies have sought to elucidate the determinants of ADL dependence, 10–12 but none has tried to identify the factors, besides age, that predict recovery of ADL function.
The purpose of this prospective, population-based cohort study was to identify the factors that predict recovery of ADL function among disabled older persons living in the community. As potential predictors, we evaluated not only traditional epidemiologic variables, such as age, gender, race, education, and the presence of chronic disease, but also factors, such as physical performance, cognitive status, nutrition, mood, and self-efficacy, that have recently been shown to predispose older persons to ADL dependence, 13–16 and that when impaired may impede recovery of ADL function, further threatening independent living.
METHODS
Subjects
Subjects were participants of Project Safety, a probability sample of community-living persons, aged 72 years and older, living in New Haven, Connecticut, in 1989. The sampling technique, described in detail elsewhere, 17 was similar to that used to establish the New Haven site of the Established Populations for Epidemiologic Studies of the Elderly (EPESE). 18 Originally 1,436 persons were contacted. Only 44 (3%) failed to meet the three eligibility criteria, which included the ability to speak English, Spanish, or Italian, to follow simple commands, and to walk across a room without the assistance of another person. Among those eligible, 1,103 (79%) agreed to participate and were enrolled in the cohort. Comprehensive assessments were performed in participants' homes by trained nurse researchers at baseline and 1 year later using standard instruments. A 3-year assessment was performed via a telephone interview. To be eligible for this study, participants had to report that they were dependent (“unable to do” or “require help from a person”) in one or more of the following ADLs at either the baseline or the 1-year interview: bathing, dressing, transferring, walking, eating, toileting, and grooming. 19,20
Of the participants, 138 were ADL dependent at the baseline interview (prevalent cases), and 100 were newly dependent 1 year later (incident cases). Fourteen of these latter participants (with incident disability) had moved to a nursing home and were excluded from the study because they were no longer living in the community. Of the 224 eligible participants, 11 (5%) were lost to follow-up, leaving 213 disabled, community-living elders to form the study population. Persons who were lost to follow-up did not differ significantly from those in the study population in terms of age, gender, race, education, and number of ADL disabilities.
Candidate Predictors
Predictors of recovery were identified from candidate variables representing the following domains: demographic, psychosocial, sensory, functional, physical performance, clinical, and nutrition. Psychosocial information included depressive symptoms, assessed with the Center for Epidemiologic Studies–Depression (CES-D) scale 21 ; self-efficacy, assessed with the Falls-Efficacy Scale, an instrument developed to assess one's degree of confidence in performing 10 nonstrenuous activities without falling 22 ; and instrumental and emotional social support, assessed with items adapted from the New Haven EPESE questionnaire. 18
Sensory information included hearing, assessed by the Whisper test, 23 and corrected near visual acuity, assessed with the Rosenbaum card and calculated as the percentage of visual impairment. 24 Functional information included mobility, determined from the number of blocks walked and the flights of stairs climbed on an average day; and cognitive status, assessed with the Folstein Mini–Mental State Examination (MMSE). 25 Physical performance skills were assessed by three timed tests: walking back and forth over a 10-foot course, turning in a full circle, and standing up and sitting down from a hard-back chair three times with arms folded. 26
Clinical data included self-perceived health, rated as excellent, good, fair, poor, or bad; several self-reported chronic conditions, such as diabetes, heart disease, stroke and arthritis; the presence of urinary incontinence; and the number of prescription and nonprescription medications. Nutritional status was assessed with the body mass index (BMI), calculated as the self-reported weight in kilograms divided by the square of height in meters, and a question about whether the participant had experienced a significant weight loss—more than 10 pounds—in the previous year.
Follow-up and Outcome
Zero-time, the time at which prognostic estimations are made, 27 was defined as the initial assessment for the 128 prevalent cases of ADL dependence and as the 1-year assessment for the 85 incident cases. The primary outcome was the complete recovery of independent ADL function, defined as requiring no personal assistance in any of the ADLs at the next follow-up interview. 1,2 For participants disabled at the initial assessment, the next follow-up interview occurred at 1 year (median 12.3 mo; range 5.6 –25.8 mo); for participants newly disabled at 1 year, the next follow-up interview occurred at 3 years (median 36.4 mo; range, 23.3–38.7 mo). We considered defining recovery more broadly to also include participants who, although still dependent, had a net improvement of two or more ADLs by their next follow-up interview, but we found that only four participants would have met this criterion. Twenty-eight participants died before their next follow-up interview and were presumed not to have recovered their ADL function based on the results of a previous epidemiologic study, 28 which found that the prevalence of disability within 1 year of death approached 90% in community-living elders.
Statistical Analysis
To facilitate clinical interpretation and allow for the determination of relative risks (RRs), all categorical and continuous variables were dichotomized, using clinically acceptable or meaningful cutoff points. For example, the CES-D score was dichotomized at <16 to indicate the absence of depressive symptoms, 29 and the Falls-Efficacy score was dichotomized at>75 to distinguish persons with medium to high self-efficacy from those with low self-efficacy. 14 When more than one cutoff point was possible, the candidate predictor was dichotomized to generate the largest possible risk gradient. When it made clinical sense, composite variables were created to reduce the number of candidate predictors. High mobility was defined as either walking one or more blocks or climbing one or more flights of stairs on an average day. A composite measure of physical performance was created from the three timed tests, 15 and participants were categorized into two groups as best one third versus lowest two thirds of timed performance. Nutritional status was defined as good for participants who had neither a low BMI (<20.7 kg/m 2 in men and <19.1 kg/m 2 in women), 30 nor significant weight loss. 31
For each candidate predictor, rates of ADL recovery and crude RR were calculated. To identify the factors that were independently associated with ADL recovery, multivariable binomial regression models were constructed using Generalized Linear Interactive Modeling (GLIM). 32 In GLIM, the natural logarithm of RR can be directly estimated by specifying the log link, and RR can then be computed by exponentiation. Variables were considered for inclusion in the multivariable models if they were associated with ADL recovery in bivariate analysis with a p value < .05.
RESULTS
The prevalence of the potential predictors among study participants is shown in Table 1 There were no significant differences in these factors between participants who were disabled at the initial assessment and those who were newly disabled at 1 year with one exception. Participants with prevalent disability were less likely to live alone (56% vs 73%; p= .01). Of the 213 participants, 142 (67%) were disabled in a single ADL, 46 (22%) were disabled in two ADLs, 14 (7%) were disabled in three ADLs, and 11 (5%) were disabled in four or more ADLs.
Table 1.
Factors Associated with Recovery in Activities of Daily Living (ADL) Among Disabled Older Persons Living in the Community (N=213)
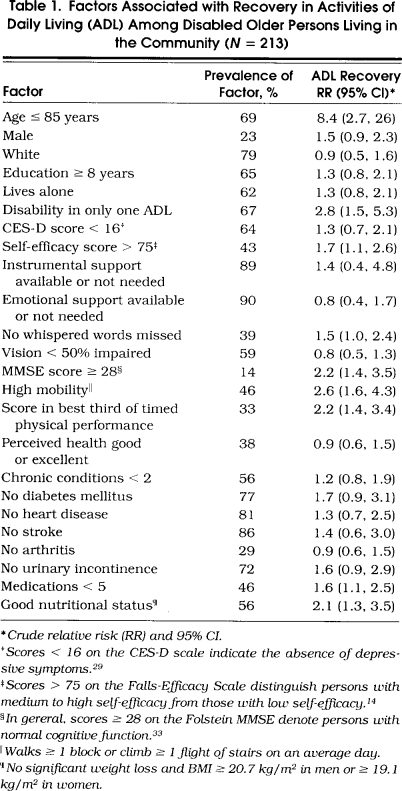
Of the 213 participants, 59 (28%) recovered their ADL function. Fifty recovered independence in one ADL, seven in two ADLs, and two in three ADLs. The rate of ADL recovery for participants with prevalent disability was nearly identical to that for participants with incident disability (27% vs 29%; p= .65). The length of follow-up among those who recovered and those who did not was identical for participants with prevalent disability (mean 13.2 mo) and differed little for participants with incident disability (mean 24.0 vs 24.5 mo; p= .14). Compared with those who remained disabled, participants who recovered their ADL function showed greater improvement in several measures of higher level function, including instrumental activities of daily living (p= .03), physical activity (p= .05), and social activity (p= .06).
The bivariate associations between the potential predictors and ADL recovery are shown in Table 1. Age 85 years or less was the strongest predictor of ADL recovery. Of the 66 participants older than 85 years, only 3 (5%) recovered their ADL function. Among the remaining participants, the rate of ADL recovery was relatively constant across age categories—42% for those aged 72 to 75 years, 38% for those aged 76 to 80 years, and 36% for those aged 81 to 85 years. Other factors significantly associated with ADL recovery included disability in only one ADL, self-efficacy score greater than 75, MMSE score of 28 or better, high mobility, score in the best third of timed physical performance, fewer than five medications, and good nutritional status. There were no significant two-way interactions among the bivariate predictors.
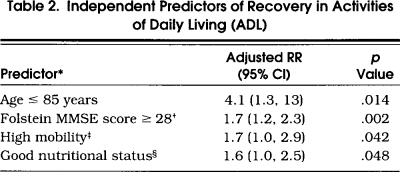
In multivariable analysis, age 85 years or less, MMSE score of 28 or better, high mobility, and good nutritional status were independently associated with ADL recovery (Table 2) Because cognitive impairment, low mobility, and poor nutrition are each associated with increased mortality in community-living elders, 34,35 we removed from the analysis the 28 participants who had died before their next follow-up interview and found that the associations between these predictors and ADL recovery remained strong and statistically significant. The results from these multivariable analyses did not change when an indicator variable was added to denote whether a participant was a prevalent or an incident case or when self-efficacy was left out of the models to reduce the number of missing observations.
Table 2.
Independent Predictors of Recovery in Activities of Daily Living (ADL)
DISCUSSION
In this prospective, population-based cohort study, 28% of disabled, community-living older persons recovered their ADL function within 2 years. This rate is comparable to rates reported in other longitudinal studies. 2,36 In the Massachusetts Health Care Panel Study, for example, Katz et al. found that 24% of disabled, noninstitutionalized persons 65 years or older regained independent ADL function within 15 months. 2 Using data from the National Long Term Care Surveys, Manton et al. reported a cumulative transitional probability for ADL recovery among disabled, community-living Medicare beneficiaries of about 30% over 2 years. 36 These results along with ours refute the popular perception that ADL disability leads invariably to further decline and increasing dependence, and offer hope to many newly disabled elders and to their caregivers and providers that a sizable minority of older persons, once disabled, will recover independent ADL function.
Not all disabled persons, however, are equally likely to recover ADL independence. In our study, few disabled persons older than 85 years recovered their ADL function. This strong association between advanced age and poor recovery persisted after we controlled for other important factors in multivariable analysis. Our findings are consistent with those of other studies which have reported that the likelihood of ADL recovery decreases substantially with age. 1,2 Older age, per se, may not impede recovery but, rather, may act as a proxy for other unmeasured factors, which in turn decrease the capacity of disabled persons to recover. Until these other factors are identified, it may be important to target persons older than 85 years for interventions to prevent the onset of ADL disability. Alternatively, these elderly persons, once disabled, might be offered more aggressive rehabilitative therapy. Further research on the mechanisms of ADL disability and recovery in this population is clearly warranted given current projections that the number of Americans older than 85 years could equal 15.3 million by 2050 —five times what it is currently. 37
In addition to age, we found that several other factors, representing a diverse array of functional and clinical features, were associated with ADL recovery. Three of these factors, MMSE score of 28 or better, high mobility, and good nutritional status, were independently associated with ADL recovery. The presence or absence of these factors may facilitate or impede ADL recovery in one of two ways. First, the likelihood of recovery may be related to the baseline level of physiologic capacity. 4 Reductions in physiologic capacity to extremely low levels, for example, likely hinder ADL recovery. Among the factors associated with successful recovery in this study, disability in only one ADL, MMSE score of 28 or better, high mobility, score in the best third of timed physical performance, and good nutritional status all represent higher levels of physiologic capacity. Second, some factors may directly impede ADL recovery regardless of one's physiologic capacity. Identified predictors that may operate by this mechanism are poor nutrition, overmedication, and low self-efficacy.
To our knowledge, this is the first community-based study that has tried to identify the factors, besides age, that predict recovery from ADL disability. In contrast, several previous studies have investigated the determinants of functional recovery among persons hospitalized after an acute event. Older age, cognitive impairment, depressive symptoms, and low social support have all been implicated as risk factors for poor recovery following a stroke, 5–7 and a hip fracture. 8,9 In our study, few participants reported insufficient emotional or instrumental social support, making any associations between social support and ADL recovery difficult to evaluate. Disabled elders who lack social support may be more likely than those with sufficient support to be admitted to a nursing home, making them unavailable, in turn, to participate in a community-based study.
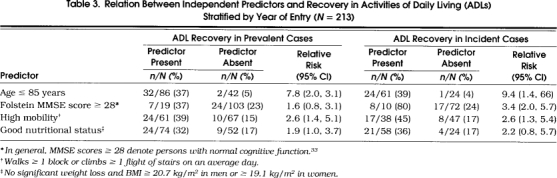
Our study has several potential limitations. First, to enhance our power to detect significant associations, we analyzed participants with prevalent disability and those with incident disability as a single group. Evidence suggests, however, that these persons do not represent distinct populations of disabled elders. The “baseline” characteristics of participants with prevalent disability, for example, were virtually identical to those with incident disability. Furthermore, participants with prevalent disability and those with incident disability had comparable rates of ADL recovery. Finally, as seen in Table 3 the magnitude of association between the independent predictors and ADL recovery was comparable in the two sets of participants. Although the follow-up periods differed between the two groups, length of follow-up was not associated with the likelihood of recovery in either group. With longer periods of follow-up, persons with ADL disability have more time not only to recover their ADL function, but also to develop recurrent disability. Because longitudinal studies of disability have traditionally collected follow-up data at annual interviews, relatively little is known about the transitions in ADL function that take place over shorter periods of time.
Table 3.
Relation Between Independent Predictors and Recovery in Activities of Daily Living (ADLs) Stratified by Year of Entry (N=213)
Second, ADL function was determined from ratings by elderly persons of their ability to perform ADL tasks without personal assistance. In community populations, the reliability of self-reported ADLs has been shown to be high and to be unaffected by age, cognitive status, or mode of interview (face-to-face vs. telephone). 38,39 The validity of self-reported ADLs, moreover, has been affirmed by several studies that have demonstrated high concordance between patient and proxy ratings and between patient ratings and direct observations of patients' performance. 40,41 Both the mode of ascertaining ADL function and our definitions of ADL dependence and recovery are consistent with those used in other epidemiologic studies. 1,2,15 In the current study, participants who recovered their ADL function showed greater improvement in several measures of higher-level function than participants who remained disabled, strongly suggesting that participants' self-reports of ADL recovery reflected true recovery rather than measurement error. Despite these improvements, however, ADL recovery for many persons may be due to personal adaptations (e.g., wearing clothes with Velcro fasteners instead of buttons) or to the use of special equipment (e.g., tub bench or bedside commode) rather than to improvements in physical capacity.
Third, we had little information on the events that may have precipitated ADL dependence. The likelihood of ADL recovery may depend, in part, on the severity or potency of the precipitating event. Older persons who become disabled after a major event such as a stroke or a hip fracture, for example, may be less likely to recover their ADL function than those who become disabled after a seemingly minor event such a noninjurious fall or a prolonged upper respiratory infection. It is not known what proportion of dependent elders become disabled after major events, which tend to be relatively uncommon, versus the proportion who become disabled after minor events, which tend to be fairly common. In our study, fewer than half the participants (39%) were hospitalized in the 12 months prior to their zero-time assessment, indicating that major events were unlikely to be the predominant cause of ADL disability. Because we were unable to determine the temporal relation between hospitalization and ADL disability, we did not evaluate hospitalization as a potential predictor of ADL recovery. To better elucidate the major pathways underlying functional dependence, future studies will need to focus more intently on the events that precipitate ADL dependence.
Fourth, information was not available on whether participants received interventions, such as physical or occupational therapy, after the onset of their disability. Therefore, we could not evaluate (or adjust for) the effect of these rehabilitative efforts on the likelihood of ADL recovery. Finally, spurious associations may result when data values are used to identify optimal cutoff points. Among the factors found to be significantly associated with ADL recovery in bivariate analysis, only the number of medications was dichotomized at the “best” cutoff point.
Our study has several important strengths. First, participants were drawn from a large, population-based sample of community-living older persons. Second, high-quality, detailed data were available from this cohort on state-of-the-art measures of cognitive status, physical performance, and psychological functioning, as well as on self-reported measures of physical function, social support, and nutrition. Third, follow-up for this cohort of frail elders was nearly 100% complete.
In summary, among disabled older persons living in the community, a sizable minority recover independent ADL function. Once disabled, however, persons older than 85 years are unlikely to recover ADL independence, suggesting that these elderly persons may need more aggressive restorative therapy or, alternatively, special preventive efforts to forestall the initial onset of ADL disability. Intact cognitive function, high mobility, and good nutritional status each improve the likelihood of ADL recovery and may serve as markers of resiliency in this population.
Acknowledgments
We thank Drs. Sharon K. Inouye and Stan V. Kasl for their critical review of an earlier draft of this manuscript.
References
- 1.Manton KG. A longitudinal study of functional change and mortality in the United States. J Gerontol Soc Sci. 1988;43:S153–61. doi: 10.1093/geronj/43.5.s153. [DOI] [PubMed] [Google Scholar]
- 2.Katz S, Branch LG, Branson MH, Papsidero JA, Beck JC, Greer DS. Active life expectancy. N Engl J Med. 1983;309:1218–24. doi: 10.1056/NEJM198311173092005. [DOI] [PubMed] [Google Scholar]
- 3.O'Leary VE, Ickovics JR. Resilience and thriving in response to challenge: an opportunity for a paradigm shift in women's health. Women's Health. 1995;1:121–42. [PubMed] [Google Scholar]
- 4.Buchner DM, Wagner EH. Preventing frail health. Clin Geriatr Med. 1992;8:1–17. [PubMed] [Google Scholar]
- 5.Parikh RM, Robinson RG, Lipsey JR, Starkstein SE, Fedoroff JP, Price TR. The impact of poststroke depression on recovery in activities of daily living over a 2-year follow-up. Arch Neurol. 1990;47:785–9. doi: 10.1001/archneur.1990.00530070083014. [DOI] [PubMed] [Google Scholar]
- 6.Dombovy ML, Sandok BA, Basford JR. Rehabilitation for stroke: a review. Stroke. 1986;17:363–9. doi: 10.1161/01.str.17.3.363. [DOI] [PubMed] [Google Scholar]
- 7.Glass TA, Matchar DB, Belyea M, Feussner JR. Impact of social support on outcome in first stroke. Stroke. 1993;24:64–70. doi: 10.1161/01.str.24.1.64. [DOI] [PubMed] [Google Scholar]
- 8.Magaziner J, Simonsick EM, Kashner TM, Hebel JR, Kenzora JE. Predictors of functional recovery one year following hospital discharge for hip fracture: a prospective study. J Gerontol Med Sci. 1990;45:M101–7. doi: 10.1093/geronj/45.3.m101. [DOI] [PubMed] [Google Scholar]
- 9.Marottoli RA, Berkman LF, Cooney LM Jr Decline in physical function following hip fracture. J Am Geriatr Soc. 1992;40:861–6. doi: 10.1111/j.1532-5415.1992.tb01980.x. [DOI] [PubMed] [Google Scholar]
- 10.Mor V, Murphy J, Masterson-Allen S, et al. Risk of functional decline among well elders. J Clin Epidemiol. 1989;42:895–904. doi: 10.1016/0895-4356(89)90103-0. [DOI] [PubMed] [Google Scholar]
- 11.LaCroix AZ, Guralnik JM, Berkman LF, Wallace RB, Satterfield S. Maintaining mobility in late life, II: smoking, alcohol consumption, physical activity, and body mass index. Am J Epidemiol. 1993;137:858–69. doi: 10.1093/oxfordjournals.aje.a116747. [DOI] [PubMed] [Google Scholar]
- 12.Guralnik JM, LaCroix AZ, Abbott RD, et al. Maintaining mobility in late life, I: demographic characteristics and chronic conditions. Am J Epidemiol. 1993;137:845–57. doi: 10.1093/oxfordjournals.aje.a116746. [DOI] [PubMed] [Google Scholar]
- 13.Tinetti ME, Inouye SK, Gill TM, Doucette JT. Shared risk factors for falls, incontinence, and functional dependence. Unifying the approach to geriatric syndromes. JAMA. 1995;273:1348–53. [PubMed] [Google Scholar]
- 14.Mendes de Leon CF, Seeman TE, Baker DI, Richardson ED, Tinetti ME. Self-efficacy, physical decline, and change in functioning in community-living elders: a prospective study. J Gerontol Soc Sci. 1996;51B:S183–90. doi: 10.1093/geronb/51b.4.s183. [DOI] [PubMed] [Google Scholar]
- 15.Gill TM, Williams CS, Richardson ED, Tinetti ME. Impairments in physical performance and cognitive status as predisposing factors for functional dependence among nondisabled older persons. J Gerontol Med Sci. 1996;51:M283–8. doi: 10.1093/gerona/51a.6.m283. [DOI] [PubMed] [Google Scholar]
- 16.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–61. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tinetti ME, Liu WL, Claus EB. Predictors and prognosis of inability to get up after falls among elderly persons. JAMA. 1993;269:65–70. [PubMed] [Google Scholar]
- 18.Cornoni-Huntley J, Ostfeld AM, Taylor JO, et al. Established Populations for Epidemiologic Studies of the Elderly: study design and methodology. Aging. 1993;5:27–37. doi: 10.1007/BF03324123. [DOI] [PubMed] [Google Scholar]
- 19.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. The Index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–9. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 20.Branch LG, Katz S, Kneipmann K, Papsidero JA. A prospective study of functional status among community elders. Am J Public Health. 1984;74:266–8. doi: 10.2105/ajph.74.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radloff LS. The CES-D Scale: a self report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 22.Tinetti ME, Mendes de Leon CF, Doucette JT, Baker DI. Fear of falling and fall-related efficacy in relationship to functioning among community-living elders. J Gerontol Med Sci. 1994;49:M140–7. doi: 10.1093/geronj/49.3.m140. [DOI] [PubMed] [Google Scholar]
- 23.Macphee GJ, Crowther JA, McAlpine CH. A simple screening test for hearing impairment in elderly patients. Age Ageing. 1988;17:347–51. doi: 10.1093/ageing/17.5.347. [DOI] [PubMed] [Google Scholar]
- 24.Spaeth EB, Fralick FB, Hughes WF. Estimates of loss of visual efficiency. Arch Ophthalmol. 1955;54:462–8. doi: 10.1001/archopht.1955.00930020468021. [DOI] [PubMed] [Google Scholar]
- 25.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 26.Seeman TE, Charpentier PA, Berkman LF, et al. Predicting changes in physical performance in a high-functioning elderly cohort: MacArthur studies of successful aging. J Gerontol Med Sci. 1994;49:M97–108. doi: 10.1093/geronj/49.3.m97. [DOI] [PubMed] [Google Scholar]
- 27.Feinstein AR, Pritchett JA, Schimpff CR. The epidemiology of cancer therapy, II: the clinical course: data, decisions, and temporal demarcations. Arch Intern Med. 1969;123:323–44. doi: 10.1001/archinte.123.3.323. [DOI] [PubMed] [Google Scholar]
- 28.Guralnik JM, LaCroix AZ, Branch LG, Kasl SV, Wallace RB. Morbidity and disability in older persons in the years prior to death. Am J Public Health. 1991;81:443–7. doi: 10.2105/ajph.81.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mulrow CD, Williams JW, Jr, Gerety MB, Ramirez G, Monteil OM, Kerber C. Case-finding instruments for depression in primary care settings. Ann Intern Med. 1995;122:913–21. doi: 10.7326/0003-4819-122-12-199506150-00004. [DOI] [PubMed] [Google Scholar]
- 30.Lawrence RH, Jette AM. Disentangling the disablement process. J Gerontol Soc Sci. 1996;51B:S173–82. doi: 10.1093/geronb/51b.4.s173. [DOI] [PubMed] [Google Scholar]
- 31.Moore AA, Siu AL. Screening for common problems in ambulatory elderly: clinical confirmation of a screening instrument. Am J Med. 1996;100:438–43. doi: 10.1016/S0002-9343(97)89520-4. [DOI] [PubMed] [Google Scholar]
- 32.Wacholder S. Binomial regression in GLIM: estimating risk ratios and risk differences. Am J Epidemiol. 1986;123:174–84. doi: 10.1093/oxfordjournals.aje.a114212. [DOI] [PubMed] [Google Scholar]
- 33.Greiner PA, Snowdon DA, Schmitt FA. The loss of independence in activities of daily living: the role of low normal cognitive function in elderly nuns. Am J Public Health. 1996;86:62–6. doi: 10.2105/ajph.86.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corti MC, Guralnik JM, Salive ME, Sorkin JD. Serum albumin level and physical disability as predictors of mortality in older persons. JAMA. 1994;272:1036–42. [PubMed] [Google Scholar]
- 35.Kelman HR, Thomas C, Kennedy GJ, Cheng J. Cognitive impairment and mortality in older community residents. Am J Public Health. 1994;84:1255–60. doi: 10.2105/ajph.84.8.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manton KG, Corder LS, Stallard E. Estimates of change in chronic disability and institutional incidence and prevalence rates in the U.S. elderly population from the 1982, 1984, and 1989 National Long Term Care Survey. J Gerontol Soc Sci. 1993;48:S153–66. doi: 10.1093/geronj/48.4.s153. [DOI] [PubMed] [Google Scholar]
- 37.Randall T. Demographers ponder the aging of the aged and await unprecedented looming elder boom. JAMA. 1993;269:2331–2. doi: 10.1001/jama.1993.03500180015006. [DOI] [PubMed] [Google Scholar]
- 38.Smith LA, Branch LG, Scherr PA, et al. Short-term variability of measures of physical function in older people. J Am Geriatr Soc. 1990;38:993–8. doi: 10.1111/j.1532-5415.1990.tb04422.x. [DOI] [PubMed] [Google Scholar]
- 39.Korner-Bitensky N, Wood-Dauphinee S. Barthel Index information elicited over the telephone. Is it reliable? Am J Phys Med Rehabil. 1995;74:9–18. doi: 10.1097/00002060-199501000-00003. [DOI] [PubMed] [Google Scholar]
- 40.Magaziner J, Bassett SS, Hebel JR, Gruber-Baldini A. Use of proxies to measure health and functional status in epidemiologic studies of community-dwelling women aged 65 years and older. Am J Epidemiol. 1996;143:283–92. doi: 10.1093/oxfordjournals.aje.a008740. [DOI] [PubMed] [Google Scholar]
- 41.Reuben DB, Siu AL, Kimpau S. The predictive validity of self-report and performance-based measures of function and health. J Gerontol Med Sci. 1992;47:M106–10. doi: 10.1093/geronj/47.4.m106. [DOI] [PubMed] [Google Scholar]


