Abstract
The need for improved diagnostic reagents to identify human long-term carriers of the zoonotic parasite Babesia microti is evidenced by numerous reported cases of transfusion-acquired infections. This report describes the identification and initial characterization of 27 clones representing seven genes or gene families that were isolated through serological expression cloning by using a technique that we specifically designed to screen for shed antigens. In this screen, sera from B. microti-infected SCID mice, putatively containing secreted or shed antigens from the parasites, were harvested and used to immunize syngeneic immunocompetent mice (BALB/c). After boosting, the sera from the BALB/c mice, containing antibodies against the immunodominant secreted antigens, were used to screen a B. microti genomic expression library. Analyses of the putative peptides encoded by the novel DNA sequences revealed characteristics indicating that these peptides might be secreted. Initial serological data obtained with recombinant proteins and a patient serum panel demonstrated that several of the proteins could be useful in developing diagnostic tests for detection of B. microti antibodies and antigens in serum.
Babesia species represent some of the most common infectious parasites among wild and domestic animals and are gaining increasing interest as emerging causes of zoonoses in humans. They require competent nonvertebrate and vertebrate hosts to maintain transmission cycles, infecting ixodid ticks and vertebrate erythrocytes. Several species have been shown to infect humans (10, 34), although Babesia microti is the species most frequently identified. The defined distribution of B. microti is constantly expanding; although the parasite was originally identified as being endemic to the northeastern United States and parts of the Midwest, new reports have expanded the distribution to as far south as New Jersey in the United States (6, 36) and many parts of Europe (4, 8, 15, 30, 31) and Japan (20, 27, 28, 35, 37).
In the United States, B. microti is naturally transmitted by the deer tick Ixodes scapularis (also called Ixodes dammini), which acquires its infection from the white-footed mouse, Peromyscus leucopus. Its perpetuation in nature is similar to that of other tick-transmitted agents that are now known to exist within congruent zoonotic cycles, including the Lyme disease spirochete, Borrelia burgdorferi, and the agent of human granulocytic ehrlichiosis (Anaplasma phagocytophila sp. nov.) (18, 32). Little is known about the mechanisms of persistence of B. microti in vertebrate hosts, but the existence of a long-term asymptomatic carrier state in babesial infections of domestic and wild animals has been recognized for years (3, 7, 26). The carrier state was recently recognized in humans as well, as evidenced by several reported cases of transfusion-acquired babesial infections. It is estimated that among eligible blood donors living in areas of endemicity, as many as 9 in 2,006 are infected at the time of donation (16). The study cited used multiple techniques, including PCR, indirect (immuno)fluorescent-antibody assay (IFA), and a peptide-based enzyme-linked immunosorbent assay (ELISA), to screen the donors and identify and confirm Babesia-positive carriers. These high prevalence rates, along with the increase in reported transfusion-acquired cases and worldwide distribution, suggest that the development of reliable, accurate testing methods for B. microti may become important. Here we report the discovery of several new B. microti-specific antigens that could be used to develop diagnostic tests to detect antibodies or circulating antigens in human serum.
MATERIALS AND METHODS
Preparation of anti-Babesia sera.
Infection with B. microti MN1 was established by intraperitoneal inoculation of 500 μl of cryopreserved hamster blood into 3-week-old 50-g female Golden Syrian hamsters (SASCO; Charles River Laboratories; Wilmington, Mass.). Infection was monitored by use of Giemsa-stained or acridine orange-stained blood smears over a 2-week period. Blood was harvested by cardiac puncture when the parasitemia levels reached 60 to 70%. Infected blood was diluted in saline to 108 infected red blood cells (RBCs)/ml. This blood was then used to inoculate several CB-17 SCID mice (Jackson Laboratories, Bar Harbor, Maine). Infection was monitored as described above. At 3 weeks postinoculation, the blood was harvested and found to exhibit a parasitemia level of ∼5%. Serum was obtained by centrifuging the harvested blood at ∼1,000 × g for 5 to 10 min and removing the serum from the top of the pelleted cells and debris. To further ensure that no parasites would be carried over to the next step, the serum was ultracentrifuged at 130,000 × g for 1 h. Syngeneic immunocompetent mice (BALB/c; Charles River Laboratories) were immunized with a total of 200 μl of a 1:1 (vol/vol) mixture of the SCID sera and monophosphoryl lipid A adjuvant monthly for a total of five injections. BALB/c mice were bled via the tail vein 12 days after the third and fourth immunizations and via cardiac puncture after the fifth immunization.
Library screening.
A pool of the mouse sera was used to screen a B. microti expression library. The library was constructed and screened as follows. B. microti genomic DNA (strain MN1; Mayo Clinic, Rochester, Minn.) was isolated from infected hamster blood with an ion-exchange column (Qiagen Inc., Valencia, Calif.). The DNA was sonicated to generate fragments of approximately 0.5 to 5.0 kbp. The fragments were blunt ended and then ligated to EcoRI adapters (Stratagene, La Jolla, Calif.). The inserts were size selected with a Sephacryl S-400-HR column (Sigma Chemical Co., St. Louis, Mo.) and then ligated to the lambda ZAP II vector (Stratagene). The ligation mixture was packaged with Gigapack III Gold packaging extract (Stratagene) (17).
Before the actual screening, the sera were adsorbed with filters that had been used to lift plaques of Escherichia coli containing the library vector with no inserted DNA. The library was plated on 11 large petri plates (150 by 15 mm) containing Luria-Bertani medium at a concentration of approximately 20,000 PFU (total number of plaques screened, 2.2 × 105). The plaques were lifted and thereby transferred to nitrocellulose filters and then were processed by established protocols (17) with the adsorbed SCID sera as the primary antibodies (17). Seventy plaques were picked upon the first screening of the library. These plaques were then processed and replated for secondary screening and in some cases tertiary screening, again with the SCID sera as the primary antibodies. Twenty-seven lambda clones were confirmed as being positive by the secondary and tertiary screenings. These phage clones were processed according to the protocols developed for the ZAP II vector by Stratagene for excision of the insert and subsequent cloning into the SOLR strain of E. coli (Stratagene). Individual clones were grown overnight in appropriate media. A small portion of the cultures was stored at −80°C as glycerol stocks, and the remainder was processed to extract DNA for analysis. The DNA from the insert in each clone was sequenced in both directions by using M13 forward, reverse, and internal DNA primers; ABI377XL automated sequencers (Applied Biosystems, Foster City, Calif.); and dRhodamine (Applied Biosystems)-labeled nucleotides.
Sequence analysis.
Nucleic acid and protein homology searches were performed against the GenBank, EST mouse, and GENESEQ databases by using the BLAST programs (1). Predicted polypeptides were analyzed by using the programs PSORT and PSORT II (Human Genome Center, IMS, Tokyo, Japan), Identify (Stanford University, Palo Alto, Calif.), TMpred (prediction of transmembrane regions and orientation) (13), Signal P (23), and Pfam (2).
Protein expression.
The expression of recombinant proteins was accomplished by amplifying the plasmid insert with Pfu polymerase (Stratagene) and unique phosphorylated primers; reverse primers were designed to also contain a second stop site and restriction enzyme site (either XhoI or EcoRI). The PCR product was digested with the appropriate enzyme and cloned into pPDM His (a modified pET 28 vector [Novagen, Madison, Wis.] with the His tag in frame) (Stratagene) that had been cut with the appropriate enzyme for each clone and Eco721. The correct construct was confirmed by sequence analysis, and the DNA was transformed into at least one of the following cell lines: BL21 pLysS (Novagen), BLR(DE3) pLysS (Novagen), or BLR(DE3) CodonPlus RIL (Novagen) and a plasmid from Stratagene.
Protein purification.
Methods for the purification of recombinant proteins have been described elsewhere (29). Proteins were checked for purity by sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by staining with Coomassie blue stain and by N-terminal protein sequencing (19) and were quantified with a micro-bicinchoninic acid assay (Pierce, Rockford, Ill.). Recombinant proteins were also assayed for endotoxin contamination with the Limulus assay (BioWhittaker, Walkersville, Md.).
ELISAs.
ELISAs were performed as previously described (17) by using 200 ng of recombinant protein to coat each well and patient serum at a dilution of 1:100. A serum panel from confirmed Babesia-positive patients was obtained from Imugen (Norwood, Mass.). Random donor sera were obtained from Corixa Corporation (Seattle, Wash.).
Immunodetection.
Free parasites were isolated from infected hamster RBCs as previously described (38). A lysate was made from isolated B. microti organisms by washing the pellet three times in phosphate-buffered saline, with a final resuspension in 2 ml of phosphate-buffered saline, for a starting blood volume of 4 ml (75% parasitemia). The resuspended parasites were then lysed by sonication. The protein lysate was run on duplicate 4 to 20% acrylamide- Tris-glycine gels. One gel was transferred to Hybond-C Extra nitrocellulose membranes (Amersham Pharmacia, Uppsala, Sweden) by using a Semidry Electroblotter (Genomic Solutions, Ann Arbor, Mich.), while the second gel was silver stained (see below). Sera from B. microti-infected patients were used to probe for immunoreactive bands, which were visualized by using alkaline phosphatase.
MS for protein sequencing.
A parasite lysate was run on 4 to 20% acrylamide- Tris-glycine gels and then silver stained. The protein band of interest, determined by cross-reactivity to patient sera on the immunoblot (see Fig. 2), was excised from the gels and digested with 0.5 μg of trypsin (Promega, Madison, Wis.). The digested peptide mixture was extracted three times with 5% acetic acid in 50% acetonitrile, and the combined extracts were dried and analyzed with a Capillary LC column coupled with Ion Trap mass spectrometry (MS) (LCQ-Finnigan, San Jose, Calif.) (11). The Capillary LC column (inner diameter, 100 μm; length, 12 cm) was filled with C18 resin. The peptide mixture was loaded into this column and washed. The concentrated peptides were then eluted with a gradient of 5 to 65% solution B over 20 min (solution A, 0.2% acetic acid in water; solution B, 80% acetronitrile in solution A). The eluted peptides were introduced into the Ion Trap mass spectrometer by electrospray via an electrospray ionization interface (Cytopeia, Seattle, Wash.) and analyzed by data-dependent MS and MS-MS scans. The collision-induced dissociation spectra (tandem mass spectra [MS-MS]) generated during the experiment were used to search the Corixa protein database with Sequest software (5) to identify possible sequence matches.
FIG. 2.
Identification of protein bands for mass spectrometry. (A) Silver-stained 4 to 12% acrylamide gel with a lysate of B. microti isolated from infected RBCs. (B) Immunoblot of an identical gel that was probed with sera from B. microti-infected patients. The band of interest is denoted with an arrow; both variants of BMN1-21 [BM11-5(a) and BM11-51(b)] were isolated from this band. MW, molecular weight, in thousands.
Nucleotide sequence accession numbers.
GenBank accession numbers for the sequences determined here are shown in Table 1.
TABLE 1.
Babesia clones submitted to GenBank
RESULTS
Expression library screening and sequence analyses.
A B. microti genomic expression library was screened with sera from BALB/c mice immunized with sera from B. microti-infected SCID mice. Twenty-seven clones were isolated, and complete sequences were obtained for all clones. Subsequent analyses of these sequences predicted open reading frames (ORFs) encoding at least 178 amino acids (aa) in 25 of the clones. Alignments of clone sequences showed that 18 of the clones represent partial or complete ORFs of seven genes or gene families, including two sequences that were identified previously (BMN1-10 and BMN1-15) (17). Table 2 shows a summary of the genes or gene families that were identified, along with some of the predicted characteristics of putative encoded polypeptides. All of the ORFs described in Table 2 contain upstream stop codons and potential start methionines, except for BM61 (part of the BM24 family), the BM4 family (which also contains BM4-12), and BM40; thus, the signal sequence analyses for these clones were inconclusive. The presence of upstream stop codons and potential start methionines supports the prediction that these sequences are actually transcribed and translated.
TABLE 2.
Summary of Babesia clones isolated through SCID mouse serum screening
| Name of family | No. of clones isolated | Size of product of largest ORF (amino acids) | N-terminal signal sequence | Repeat sequence (length of repeat, in amino acids) | Other prediction |
|---|---|---|---|---|---|
| BMN1-21 (BM11) | 3 | 309 | Cleavable signal | No | Type Ia membrane |
| BMN1-10 | 2 | 464 | Uncleavable signal | Yes (146) | Type IIa membrane |
| BMN1-15 | 7 | 1,312 | No | No | Membrane protein |
| BM4/12 | 2 | 620 | Unknown | Yes (23 and 6) | |
| BM24 | 2 | 292 | Unknown | Yes (6) | |
| BM31 | 1 | 268 | No | No | 2Fe-2S center |
| BM40 | 1 | 178 | Unknown | No |
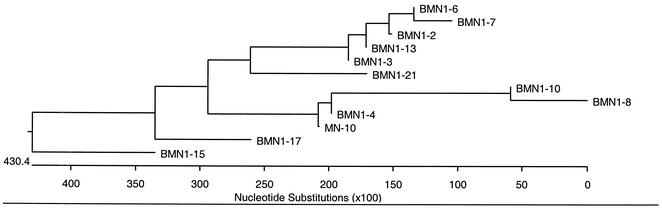
A new gene family related to the previously identified BMN1 families (17) was identified from three clones that comprise the new family: BMN1-21. Figure 1 shows the phylogenetic relationship of the new putative polypeptide sequence to previously identified BMN1 sequences; it is most closely related to the BMN1-3 sequence. Two of the clones predict identical ORFs encoding products of 309 aa [designated BM11-5(a)], and the third clone predicts a highly similar ORF encoding nine amino acid changes due to single-base-pair polymorphisms [designated BM11-51(b)]. Several protein analysis programs predict that the resulting polypeptides will have cleavable N-terminal signal sequences (aa 1 to 23) and will be type Ia membrane proteins with transmembrane domains from aa 290 to 306 and cytoplasmic tails from aa 307 to 308. Further, MS peptide sequence data demonstrate that both peptides are translated. Figure 2 shows the gel and immunoblot used to isolate immunoreactive bands for MS analysis. The mature BMN1-21 is predicted to have a mass of 31 kDa, a finding consistent with the migration of the bands isolated from the gel and immunoblot. Protein sequences identifed by MS were matched with both of the variants of BMN1-21 by a Sequest search against a protein database (in house) predicted from DNA sequences. Figure 3 shows the peptides identified from each variant by MS. The identified peptides from the regions with different residues confirm the existence of two translated genes.
FIG. 1.
Relationship between BMN1-21 and other BMN1 proteins. Relationships were determined with the MegAlign program from DNAStar, Inc. Alignment was made with the CLUSTAL W program in MegAlign. Branching order was determined by using the Jotun-Hein method in MegAlign. Branch length represents the average distance between sequence pairs, while units at the bottom indicate the numbers of substitution events.
FIG. 3.
Alignment of the two putative peptides encoded by the two BMN1-21 sequences. The peptide regions of each variant that were identified by MS sequencing are shown in boxes. The peptide residues that differed between the two variants are shaded.
One of the 27 clones isolated contains a probable ORF that is nearly identical to the previously described BMN1-10 sequence (17). Ten single nucleotide polymorphisms relative to the originally identified BMN1-10 sequence result in six amino acid changes in the putative peptide sequence. Sequence analysis of the putative polypeptide predicts an uncleavable N-terminal signal sequence and identifies the protein as a type II membrane protein (17). It is likely that there are multiple copies of BMN1-10 within the library because of the differences detected between the new and original BMN1-10 sequences. This could be due either to multiple copies within the genome of one subtype of B. microti or to the presence of multiple subtypes of B. microti containing different copies of BMN1-10 in the sample that was used to generate the library.
Six isolated clones show identity with the previously described BMN1-15 sequence. The longest clone is 3,524 nucleotides long, defining an ORF encoding 913 aa; however, the composite sequence of BMN1-15, including the overlap of isolated clones, is 4,723 nucleotides long and defines an ORF encoding 1,312 aa. This new BMN1-15 sequence adds a significant amount of sequence data to both the N and C termini of the originally reported BMN1-15 sequence (17). Some of the clones (BM26 and BM45) may define separate genomic copies of the BMN1-15 sequence or may indicate the presence of mixed isolates in the material that was used for library construction, as they show significant differences in the N terminus and in the coding or noncoding sequence, respectively. Sequence analysis with the TMpred program suggests that BMN1-15 may be a membrane protein with three transmembrane helices at aa 17 to 35, aa 745 to 767, and aa 1155 to 1173.
The remaining genes or families, BM4, BM24, BM31, and BM40, all appear to be novel sequences, and database searches have yielded no significant matches, except for BM31, which shows about 40% identity over 124 aa to a variety of tyrosine kinases. None is predicted to have an N-terminal signal sequence, but because the ORFs start with the beginning of the nucleotide sequence, it is possible that the N termini were not contained in the clones isolated. Two of the novel sequences contain repeat regions. The BM4 family (BM4 and BM4-12) contains two types of repeat regions; one region has short degenerate hexapeptide repeats flanked on both ends by sequences 23 nucleotides long and with 78% identity to each other. The BM24 family consists of two clones that have different lengths (BM24 and BM24-61) and different N and C termini but are identical over a span of 162 aa in the center, including a variable-length hexapeptide repeat sequence (A/T-T-S-T-T-A/S/T), that could be due to multiple inserts in the original library.
Serologic analyses.
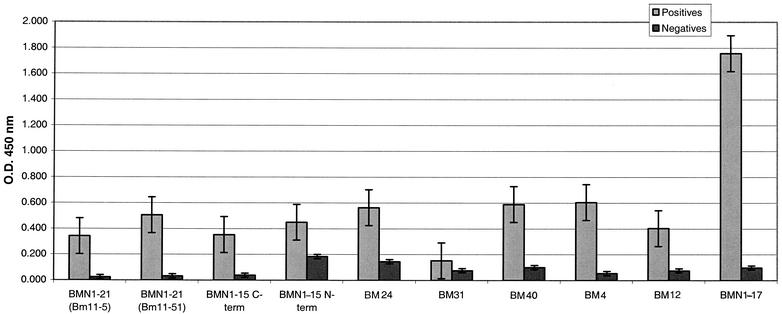
ELISAs were performed with all of the recombinant proteins that showed various diagnostic capabilities for a defined panel of patient sera. Babesia-positive samples were identified and confirmed with two or more of the following tests: blood smears, IFA, PCR, immunoblotting (immunoglobulin G [IgG] and IgM), and peptide-based ELISAs (14). Random donors were confirmed negative with two or more of these tests. Table 3 shows a summary of the ELISA results for the new recombinants in comparison to the more defined recombinant BMN1-17 (17). BMN1-21 is comparable to BMN1-17, detecting the same number of Babesia-positive samples and yielding only one additional false-positive result. When the reactivities of the recombinants are compared (Fig. 4), however, it is clear that BMN1-21 does not have the reactivity levels of BMN1-17; however, due to a low background reactivity, it can still maintain a reasonable diagnostic sensitivity. More analysis is required to determine whether any of these recombinants will be suitable for use in a diagnostic assay.
TABLE 3.
ELISA data for new Babesia antigens isolated through SCID mouse serum screening
| Antigen | No. of test serum samples from the following source/total no. of samplesa:
|
|
|---|---|---|
| Babesia positive | Random donor | |
| BMN1-17 | 52/53 | 1/42 |
| BMN1-21 | 52/53 | 2/42 |
| BM4 | 47/53 | 4/42 |
| BMN1-15 (C terminus) | 28/53 | 1/42 |
| BM4-12 | 27/53 | 1/42 |
| BM40 | 27/53 | 2/42 |
| BM24 | 24/53 | 0/42 |
| BMN1-15 (N terminus) | 18/53 | 1/40 |
| BM31 | 17/53 | 0/42 |
Babesia-positive samples were identified and confirmed with two or more of the following tests: blood smears, IFA, PCR, immunoblotting (IgG and IgM), and peptide-based ELISAs (14). Random donors were confirmed negative with two or more of these tests.
FIG. 4.
Reactivity of Babesia clones in ELISAs. The reactivity of recombinant antigens against sera from confirmed Babesia-positive and -negative patients or donors is shown. Cutoff values for each assay were determined from the mean for the random donor population plus three standard deviations of the mean. Bars indicate the mean optical density (O.D.) at 450 nm plus 1 standard error of the mean. term, terminus.
DISCUSSION
We have described several new antigen candidates for development and use in the diagnosis of B. microti infections. Present methods for the detection of B. microti include examination of blood smears, IFAs, PCR, hamster inoculation, and sometimes immunoblotting. These tests are generally insufficient to detect subclinical infections or are not feasible for large-scale (blood) screening due to cost or labor-intensiveness. Due to the increase in transfusion-acquired cases and recent data suggesting that as much as 1% of the population in areas of endemicity may be infected (16), the potential need for a high-throughput screening method has become apparent. A recently described peptide-based ELISA (14) uses peptides developed from the BMN1-17 and MN10 proteins (17). The antigens described here could be used to augment the BMN1-17-MN10 peptide-based ELISA or could be developed to detect antigens in sera. Several show preliminary results that are encouraging; for example, BMN1-21 shows sensitivity and specificity comparable to those of BMN1-17. Mapping and characterization of the epitopes on these antigens could yield diagnostic reagents that would improve upon those available now.
Previous screenings for immunodominant proteins from Babesia spp. typically have used sera from or monoclonal antibodies derived from infected specimens (e.g., see references 9, 17, and 24). These screenings have identified immunodominant proteins that are exposed to the host immune system as a natural process of infection. As discussed below, many of these proteins are variable membrane proteins with epitopes that are exposed. The procedure used in this study is different from others as it putatively selects, by the nature of the screening itself, for secreted or shed antigens (antigens in sera). This protocol has been used successfully to identify secreted tumor antigens (M. J. Lodes, unpublished data). Parasites infecting SCID mice likely shed or secrete proteins during their normal growth cycles. When sera from SCID mice are collected and processed to remove cellular debris and any whole cells or parasites, the shed or secreted proteins remain in the sera. These sera containing parasite proteins are inoculated into genetically matched BALB/c mice, which can elicit an appropriate immune response to the immunodominant proteins. Our use of these sera to screen an expression library thereby selects only for immunodominant proteins that were present in the SCID mouse sera when the BALB/c mice were immunized. In addition, analyses with protein translocation and motif software support the assumption that at least some of these novel antigens could be shed or secreted. Such antigens could be liberated into the serum, with splenic destruction of infected RBCs or RBC lysis during parasite replication, whereupon antigens secreted or shed into the interior of the RBC would be released. Determination of the appearance of any of these proteins may ultimately depend on identifying samples from infected animals and humans who are actively parasitemic. Further experimental evidence is necessary to establish whether or not these proteins are in fact detectable in serum and could therefore be used in the development of an assay, such as a capture ELISA, to detect antigens in sera.
The development of diagnostic tests for Babesia spp. has, in the past, focused mainly on species of veterinary or agricultural importance, such as Babesia bovis, Babesia equi, Babesia canis, and Babesia bigemina. Many of the antigens that have been developed into diagnostic tests or are being considered for vaccines have been identified as merozoite surface proteins or rhoptry-associated proteins, which are implicated in host evasion or erythrocyte invasion (22, 25). The hallmark features of many of these proteins are that they are encoded by multigene families that are often tandemly arranged (and expressed) in the chromosome (12, 21), and they contain short variable repeat regions that could be involved in clonal antigenic variation. Immunodominant epitopes are often mapped to variable repeat regions (14, 33). The BMN1 proteins and other novel proteins described here and previously (17) share these features and consequently could be involved in similar roles in the infection process. Future experiments should demonstrate the role of these antigens in infection and host evasion.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bateman, A., E. Birney, R. Durbin, S. R. Eddy, K. L. Howe, and E. L. Sonnhammer. 2000. The Pfam protein families database. Nucleic Acids Res. 28:263-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conrad, P., J. Thomford, I. Yamane, J. Whiting, L. Bosma, T. Uno, H. J. Holshuh, and S. Shelly. 1991. Hemolytic anemia caused by Babesia gibsoni infection in dogs. J. Am. Vet. Med. Assoc. 199:601-605. [PubMed] [Google Scholar]
- 4.Duh, D., M. Petrovec, and T. Avsic-Zupanc. 2001. Diversity of Babesia infecting European sheep ticks (Ixodes ricinus). J. Clin. Microbiol. 39:3395-3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eng, J. K., A. L. McCormack, and J. R. Yates III. 1994. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J. Am. Soc. Mass Spectrom. 5:976-989. [DOI] [PubMed] [Google Scholar]
- 6.Eskow, E. S., P. J. Krause, A. Spielman, K. Freeman, and J. Aslanzadeh. 1999. Southern extension of the range of human babesiosis in the eastern United States. J. Clin. Microbiol. 37:2051-2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Figueroa, J. V., L. P. Chieves, G. S. Johnson, and G. M. Buening. 1992. Detection of Babesia bigemina-infected carriers by polymerase chain reaction amplification. J. Clin. Microbiol. 30:2576-2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foppa, I. M., P. J. Krause, A. Spielman, H. Goethert, L. Gern, B. Brand, and S. R. Telford III. 2002. Entomologic and serologic evidence of zoonotic transmission of Babesia microti, eastern Switzerland. Emerg. Infect. Dis. 8:722-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fukumoto, S., X. Xuan, S. Shigeno, E. Kimbita, I. Igarashi, H. Nagasawa, K. Fujisaki, and T. Mikami. 2001. Development of a polymerase chain reaction method for diagnosing Babesia gibsoni infection in dogs. J. Vet. Med. Sci. 63:977-981. [DOI] [PubMed] [Google Scholar]
- 10.Gorenflot, A., K. Moubri, E. Precigout, B. Carcy, and T. P. Schetters. 1998. Human babesiosis. Ann. Trop. Med. Parasitol. 92:489-501. [DOI] [PubMed] [Google Scholar]
- 11.Gygi, S. P., G. L. Corthals, Y. Zhang, Y. Rochon, and R. Aebersold. 2000. Evaluation of two-dimensional gel electrophoresis-based proteome analysis technology. Proc. Natl. Acad. Sci. USA 97:9390-9395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hines, S. A., G. H. Palmer, D. P. Jasmer, T. C. McGuire, and T. F. McElwain. 1992. Neutralization-sensitive merozoite surface antigens of Babesia bovis encoded by members of a polymorphic gene family. Mol. Biochem. Parasitol. 55:85-94. [DOI] [PubMed] [Google Scholar]
- 13.Hofmann, H., and W. Stoffel. 1993. TMbase - A database of membrane spanning proteins segments. Biol. Chem. Hoppe-Seyler 347:166. [Google Scholar]
- 14.Houghton, R. L., M. J. Homer, L. D. Reynolds, P. R. Sleath, M. J. Lodes, V. Berardi, D. A. Leiby, and D. H. Persing. 2002. Identification of Babesia microti-specific immunodominant epitopes and development of a peptide EIA for detection of antibodies in serum. Transfusion 42:1488-1496. [DOI] [PubMed] [Google Scholar]
- 15.Hunfeld, K. P., A. Lambert, H. Kampen, S. Albert, C. Epe, V. Brade, and A. M. Tenter. 2002. Seroprevalence of Babesia infections in humans exposed to ticks in midwestern Germany. J. Clin. Microbiol. 40:2431-2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leiby, D. A., A. P. Chung, R. G. Cable, J. Trouern-Trend, J. McCullough, M. J. Homer, L. D. Reynolds, R. L. Houghton, M. J. Lodes, and D. H. Persing. 2002. Relationship between tick bites and the seroprevalence of Babesia microti and Anaplasma phagocytophila (previously Ehrlichia sp.) in blood donors. Transfusion 42:1585-1591. [DOI] [PubMed] [Google Scholar]
- 17.Lodes, M. J., R. L. Houghton, E. S. Bruinsma, R. Mohamath, L. D. Reynolds, D. R. Benson, P. J. Krause, S. G. Reed, and D. H. Persing. 2000. Serological expression cloning of novel immunoreactive antigens of Babesia microti. Infect. Immun. 68:2783-2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mather, T. N., and M. E. Mather. 1990. Intrinsic competence of three ixodid ticks (Acari) as vectors of the Lyme disease spirochete. J. Med. Entomol. 27:646-650. [DOI] [PubMed] [Google Scholar]
- 19.Matsudaira, P. 1987. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J. Biol. Chem. 262:10035-10038. [PubMed] [Google Scholar]
- 20.Matsui, T., R. Inoue, K. Kajimoto, A. Tamekane, A. Okamura, Y. Katayama, M. Shimoyama, K. Chihara, A. Saito-Ito, and M. Tsuji. 2000. First documentation of transfusion-associated babesiosis in Japan. Rinsho Ketsueki 41:628-634. (In Japanese.) [PubMed]
- 21.Mishra, V. S., T. F. McElwain, J. B. Dame, and E. B. Stephens. 1992. Isolation, sequence and differential expression of the p58 gene family of Babesia bigemina. Mol. Biochem. Parasitol. 53:149-158. [DOI] [PubMed] [Google Scholar]
- 22.Musoke, A. J., G. H. Palmer, T. F. McElwain, V. Nene, and D. McKeever. 1996. Prospects for subunit vaccines against tick-borne diseases. Br Vet. J. 152:621-639. [DOI] [PubMed] [Google Scholar]
- 23.Nielsen, H., S. Brunak, and G. von Heijne. 1999. Machine learning approaches for the prediction of signal peptides and other protein sorting signals. Protein Eng. 12:3-9. [DOI] [PubMed] [Google Scholar]
- 24.Nishisaka, M., N. Yokoyama, X. Xuan, N. Inoue, H. Nagasawa, K. Fujisaki, T. Mikami, and I. Igarashi. 2001. Characterisation of the gene encoding a protective antigen from Babesia microti identified it as eta subunit of chaperonin containing T-complex protein 1. Int. J. Parasitol. 31:1673-1679. [DOI] [PubMed] [Google Scholar]
- 25.Palmer, G. H., and T. F. McElwain. 1995. Molecular basis for vaccine development against anaplasmosis and babesiosis. Vet. Parasitol. 57:233-253. [DOI] [PubMed] [Google Scholar]
- 26.Ruebush, T. K., D. D. Juranek, A. Spielman, J. Piesman, and G. R. Healy. 1981. Epidemiology of human babesiosis on Nantucket Island. Am. J. Trop. Med. Hyg. 30:937-941. [DOI] [PubMed] [Google Scholar]
- 27.Saitoito, A., S. K. Rai, S. He, M. Kohsaki, M. Tsuji, and C. Ishihara. 1999. First demonstration of Babesia parasitizing in human in Japan. Kansenshogaku Zasshi 73:1163-1164. (In Japanese.) [DOI] [PubMed]
- 28.Shih, C. M., L. P. Liu, W. C. Chung, S. J. Ong, and C. C. Wang. 1997. Human babesiosis in Taiwan: asymptomatic infection with a Babesia microti-like organism in a Taiwanese woman. J. Clin. Microbiol. 35:450-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skeiky, Y. A., M. J. Lodes, J. A. Guderian, R. Mohamath, T. Bement, M. R. Alderson, and S. G. Reed. 1999. Cloning, expression, and immunological evaluation of two putative secreted serine protease antigens of Mycobacterium tuberculosis. Infect. Immun. 67:3998-4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skotarczak, B., and A. Cichocka. 2001. Isolation and amplification by polymerase chain reaction DNA of Babesia microti and Babesia divergens in ticks in Poland. Ann. Agric. Environ. Med. 8:187-189. [PubMed] [Google Scholar]
- 31.Skotarczak, B., and A. Cichocka. 2001. The occurrence DNA of Babesia microti in ticks Ixodes ricinus in the forest areas of Szczecin. Folia Biol. (Cracow) 49:247-250. [PubMed]
- 32.Spielman, A. 1976. Human babesiosis on Nantucket Island: transmission by nymphal Ixodes ticks. Am. J. Trop. Med. Hyg. 25:784-787. [DOI] [PubMed] [Google Scholar]
- 33.Suarez, C. E., G. H. Palmer, S. A. Hines, and T. F. McElwain. 1993. Immunogenic B-cell epitopes of Babesia bovis rhoptry-associated protein 1 are distinct from sequences conserved between species. Infect. Immun. 61:3511-3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Telford, S., III, and A. Speilman. 1998. Babesiosis of humans, p. 349-359. In L. Collier, A. Balows, and M. Sussman (ed.), Topley and Wilson's microbiology and microbial infections. Arnold, London, England.
- 35.Tsuji, M., Q. Wei, A. Zamoto, C. Morita, S. Arai, T. Shiota, M. Fujimagari, A. Itagaki, H. Fujita, and C. Ishihara. 2001. Human babesiosis in Japan: epizootiologic survey of rodent reservoir and isolation of new type of Babesia microti-like parasite. J. Clin. Microbiol. 39:4316-4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Varde, S., J. Beckley, and I. Schwartz. 1998. Prevalence of tick-borne pathogens in Ixodes scapularis in a rural New Jersey County. Emerg. Infect. Dis. 4:97-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei, Q., M. Tsuji, A. Zamoto, M. Kohsaki, T. Matsui, T. Shiota, S. R. Telford III, and C. Ishihara. 2001. Human babesiosis in Japan: isolation of Babesia microti-like parasites from an asymptomatic transfusion donor and from a rodent from an area where babesiosis is endemic. J. Clin. Microbiol. 39:2178-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wiser, M. F., and H. N. Lanners. 1992. Rapid transport of the acidic phosphoproteins of Plasmodium berghei and P. chabaudi from the intraerythrocytic parasite to the host membrane by using a miniaturized fractionation procedure. Parasitol. Res. 78:193-200. [DOI] [PubMed] [Google Scholar]