Abstract
Fusariosis is a hyalohyphomycosis due to Fusarium species that mainly occurs in immunocompromised hosts. The clinical spectrum of Fusarium infection comprises localized and disseminated forms. A case of localized cutaneous fusariosis caused by Fusarium solani in a renal transplant patient is described, and the skin manifestations of the disease are discussed.
CASE REPORT
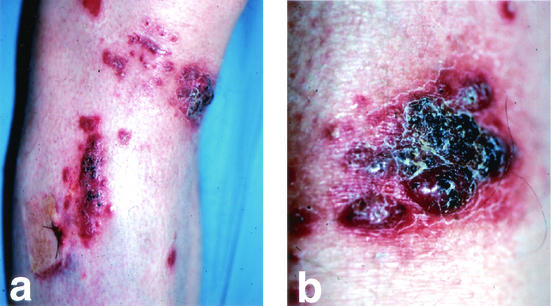
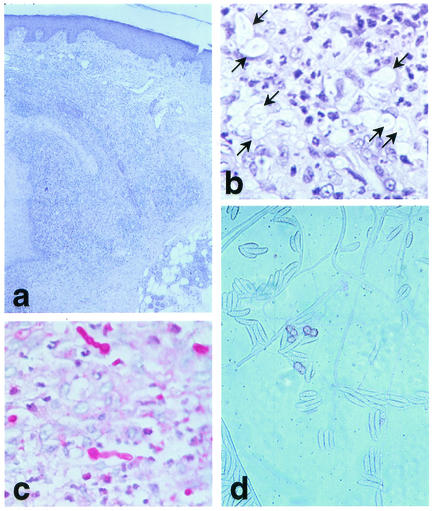
A 53-year-old man presented with a 2-month history of asymptomatic erythematous papules and nodules on his right lower leg. Similar lesions had also appeared more recently on the surgical scar of a previous skin biopsy. He had received a nonrelated cadaveric renal transplant in 1998 for end-stage renal failure due to Alport syndrome, and he was maintained on immunosuppression with prednisone at 10 mg daily and cyclosporine at 150 mg daily. On examination, there were scattered erythematous papules and nodules that sometimes coalesced in violaceous and focally necrotic plaques limited to the extensor aspect of the lower right leg (Fig. 1). No lymphadenopathy was noted, and the physical examination was otherwise normal. Histopathology of a skin sample revealed a granulomatous suppurative infiltrate extending to the entire dermis and numerous ectatic blood vessels (Fig. 2a). Many hyaline hyphae and unicellular fungal elements were sparsely seen in the infiltrate and within blood vascular spaces (Fig. 2b). Periodic acid-Schiff staining showed hyphae of more than 1 μm in diameter and reproductive structures represented by microconidia and chlamydospore-like structures, suggesting a presumptive diagnosis of non-Aspergillus hyalohyphomycosis (Fig. 2c). A biopsy sample was cultured for fungi on Sabouraud agar without cycloheximide and was incubated at 25°C in air for 4 days. It grew whitish gray cottony colonies suggestive of Fusarium spp. Successive subcultures performed on potato dextrose agar in the dark showed sickle-shaped multiseptated macroconidia; and one- to two-celled microconidia formed from unbranched phialides, conidiophores, and chlamydospores typical of Fusarium solani (Fig. 2d). Laboratory investigations revealed impaired renal function, with high serum creatinine levels (2.8 mg/dl; normal range, 0.3 to 1.1 mg/dl) and urea nitrogen levels (87 mg/dl; normal range, 12 to 36 mg/dl). The patient had anemia (hemoglobin concentration, 10.1 g/dl; normal range, 12 to 16 g/dl), whereas the white cell count and neutrophil numbers were within the normal ranges. Three consecutive hemocultures were negative. X ray of the chest and gastrointestinal tract, as well as an abdominal ultrasound, showed no abnormalities. Itraconazole was started at 100 mg twice daily, with marked improvement after 3 weeks. Three additional weeks of therapy resulted in subtotal clearing up of the cutaneous lesions. The patient died 2 months later of bronchopneumonia. Postmortem examination revealed a disseminated cytomegalovirus infection.
FIG. 1.
(a) Erythematous papulonodular lesions and focally necrotic plaques on the right lower leg. Note the presence of papular lesions on the surgical scar of a previous skin biopsy. (b) Closer view of a necrotic plaque.
FIG. 2.
(a) Suppurative granulomatous infiltrate and ectatic blood vessels in the dermis. Hematoxylin and eosin stain. Magnification, ×100. (b) At higher magnification, structures resembling fungal elements are visible around and within blood vessels. Hematoxylin and eosin stain. Magnification, ×200. (c) Periodic acid-Schiff stain-positive hyphae with reproductive fungal structures sparsely distributed in the dermis and within vascular spaces. Magnification, ×200. (d) Cultures from a biopsy sample growing whitish gray cottony colonies containing fusoid multiseptated macroconidia and microconidia formed from unbranched phialides, conidiophores, and chlamydospores typical of F. solani. Magnification, ×800.
Fusariosis is a rare infectious disease caused by species of the genus Fusarium that has been increasingly documented as an emerging agent of opportunistic infections in immunocompromised patients and, occasionally, immunocompetent hosts (1, 5, 10). Fusarium species are molds that are distributed worldwide and that may be recovered from a wide range of substrates. The portal of entry of Fusarium infection includes the respiratory and the gastrointestinal tracts, catheter tips and indwelling central venous catheters, and the skin (5, 10, 13). Infection occurs by direct contact with contaminated soil or plants, inhalation of airborne spores, or ingestion of contaminated food.
Patients with cutaneous diseases related to Fusarium spp. can present with superficial and deep infections as well as toxic reactions. In addition, Fusarium spp. may colonize wounds, burns, and ulcers. Localized superficial and deep fusarioses have been described in both healthy and immunocompromised hosts. Fusarium infection can be present in the skin with a variety of lesions, more commonly with erythematous papules and nodules with central necrosis, as in our patient, and subcutaneous nodular lesions. Less frequently, cutaneous fusariosis manifests as onychomycosis, intertrigo, finger cellulitis, pustules, ecthyma gangrenosum-like lesions and mycetoma, or lesions resembling granuloma anulare or facial granuloma (5, 7, 9, 11, 12). To our knowledge, only two cases of localized cutaneous fusariosis in renal transplant recipients have been reported previously. Those patients presented with a subcutaneous foot abscess and necrotic ulcers in the lower extremities, respectively (4, 15). In the immunocompromised host, disseminated disease may follow a superficial localized infection through lymphatic and/or hematological spreading because of the strong propensity of Fusarium for vascular invasion, thrombosis, and tissue necrosis (2, 3). Disseminated fusariosis is defined as involvement of two noncontiguous sites in association with more than one positive hemoculture (1, 2) and can affect almost any organ. It has usually been reported in neutropenic patients with hematological malignancy, especially acute leukemia, bone marrow transplant recipients, and, more rarely, patients with solid tumors (1, 3). In many instances, the skin is the initial clue to diagnosis, since cutaneous lesions are observed in about 85% of patients with disseminated Fusarium infection and often occur at an early stage of the disease with typical target lesions (1, 2, 9, 10). Disseminated infection carries a poor prognosis, which is related to various factors including the marked angiotropism of Fusarium and its capacity of adventitious sporulation in tissues (8), the underlying disease, the presence of neutropenia (<500 cells/μl), and late diagnosis and treatment. The mortality rate is estimated to be 70 to 90% in such cases (1, 2).
The diagnosis of Fusarium infection is principally based on mycology and histopathology. Recently, a PCR technique has also been developed for specific detection of Fusarium spp. from both culture and clinical samples (6). Cultures require incubation at 25°C on a Sabouraud glucose medium without cycloheximide. The most important microscopic features in species identification on culture are the conidia: the presence of fusoid macroconidia in which there are foot cells with some type of heel is accepted as the most definitive characteristic of the genus Fusarium (10). Histologically, diagnostic clues include the presence of adventitious sporulation consisting of phialides and phialoconidia and the presence of irregular hyphae with both 45- and 90-degree branching in a closed lesion (8, 14). Although culture remains the standard for identification of these fungi, presumptive histological identification of non-Aspergillus hyalohyphomycoses, as in our case, is of great value for several reasons. In fact, as suggested by Liu et al. (8), histopathologic evidence of hyalohyphomycoses is helpful when culture is not requested or is unsuccessful for technical reasons. Moreover, histology results can be obtained in less than 24 h, leading to the prompt institution of therapy. This is of importance because of both the rapid dissemination of in-fection in immunocompromised hosts and the frequent resistance of Fusarium species to antifungal drugs.
REFERENCES
- 1.Boutati, E. I., and E. J. Anaissie. 1997. Fusarium, a significant emerging pathogen in patients with hematologic malignancy: ten years' experience at a cancer center and implications for management. Blood 90:999-1008. [PubMed] [Google Scholar]
- 2.Bushelman, S. J., J. P. Callen, D. N. Roth, and L. M. Cohen. 1995. Disseminated Fusarium solani infection. J. Am. Acad. Dermatol. 32:346-351. [DOI] [PubMed] [Google Scholar]
- 3.Freidank, H. 1995. Hyalohyphomycoses due to Fusarium spp. Two case reports and review of the literature. Mycoses 38:69-74. [DOI] [PubMed] [Google Scholar]
- 4.Girardi, M., E. J. Glusac, and S. Imaeda. 1999. Subcutaneous Fusarium foot abscess in a renal transplant patient. Cutis 63:267-270. [PubMed] [Google Scholar]
- 5.Gupta, A. K., R. Baran, and R. C. Summerbell. 2000. Fusarium infection of the skin. Curr. Opin. Infect. Dis. 13:121-128. [DOI] [PubMed] [Google Scholar]
- 6.Hue, F. X., M. Huerre, M. A. Rouffault, and C. de Bievre. 1999. Specific detection of Fusarium species in blood and tissues by a PCR technique. J. Clin. Microbiol. 37:2434-2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landau, M., A. Srebrnik, R. Wolf, E. Bashi, and S. Brenner. 1992. Systemic ketoconazole treatment for Fusarium leg ulcers. Int. J. Dermatol. 31:511-512. [DOI] [PubMed] [Google Scholar]
- 8.Liu, K., D. N. Howell, J. R. Perfect, and W. A. Schell. 1998. Morphologic criteria for the preliminary identification of Fusarium, Paecilomyces, and Acremonium species by histopathology. Am. J. Clin. Pathol. 109:45-54. [DOI] [PubMed] [Google Scholar]
- 9.Mowbray, D. N., A. S. Paller, P. E. Nelson, and R. L. Kaplan. 1988. Disseminated Fusarium solani infection with cutaneous nodules in a bone marrow transplant patient. Int. J. Dermatol. 27:698-701. [DOI] [PubMed] [Google Scholar]
- 10.Nelson, P. E., M. C. Dignani, and E. J. Anaissie. 1994. Taxonomy, biology, and clinical aspects of Fusarium species. Clin. Microbiol. Rev. 7:479-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pereiro, M., M. T. Abalde, A. Zulaica, J. L. Caeiro, A. Flórez, C. Peteiro, and J. Toribio. 2001. Chronic infection due to Fusarium oxysporum mimicking lupus vulgaris: case report and review of cutaneous involvement in fusariosis. Acta Dermatol. Venereol. 81:51-53. [DOI] [PubMed] [Google Scholar]
- 12.Prins, C., P. Chavaz, K. Tamm, and C. Hauser. 1995. Ectyma gangrenosum-like lesions: a sign of disseminated Fusarium infection in the neutropenic patient. Clin. Exp. Dermatol. 20:428-430. [DOI] [PubMed] [Google Scholar]
- 13.Schell, W. A. 1995. New aspects of emerging fungal pathogens. A multifaceted challenge. Clin. Lab. Med. 15:365-387. [PubMed] [Google Scholar]
- 14.Watts, J. C., and F. W. Chandler. 1998. Morphologic identification of mycelial pathogens in tissue sections. A caveat. Am. J. Clin. Pathol. 109:1-2. [DOI] [PubMed] [Google Scholar]
- 15.Young, C. N., and A. M. Meyers. 1979. Opportunistic fungal infection by Fusarium oxysporum in a renal transplant patient. Sabouraudia 17:219-223. [PubMed] [Google Scholar]