Abstract
Six human isolates of group B streptococci (GBS) were cultured on blood agar anaerobically at 37°C for 18 h and then at 4°C for 6 h and reincubated anaerobically at 37°C for 6 h. Three of the strains showed a marked enlargement of the hemolysis zone compared with that obtained after hot-only (37°C for 18 h) or hot-cold (37°C for 18 h and then 4°C for 6 h) treatment. Subsequent broth culture experiments revealed that enhanced hemolytic activity due to hot-cold-hot treatment was observed in all 6 GBS strains when cultured in the presence of starch.
Group B Streptococcus (GBS) is a major etiological agent causing serious infection in human newborns (1). The disease symptoms include pneumonia, sepsis, and meningitis with high mortality, especially for those born prematurely (18, 20). GBS is serotyped into seven types (I to VII) on the basis of the status of the capsular polysaccharide, with type III GBS being the most prevalent in isolates from neonatal infections (6). The majority of GBS are beta-hemolytic (4), but it has been suggested that the hemolysin is not an essential virulence factor in GBS infections (14). Nevertheless, continuing research interests are directed toward a possible link between beta-hemolysin produced by GBS and its virulence (5, 11, 13). Although the hemolysin was apparently cytolytic to a broad range of host cells (16), its role as a virulence factor remains obscure. Little is known also about the molecular properties of the hemolysin due to its instability (9). In the course of characterizing beta-hemolysis of GBS isolates, we have obtained results in which several GBS isolates cultured at 37°C demonstrated a marked enlargement in the zone of hemolysis on blood agar after a period of low temperature (0 to 4°C) followed by reincubation at 37°C. This phenomenon resembled that of hot-cold hemolysis as reported elsewhere for several toxin- or hemolysin-producing bacterial species such as Staphylococcus aureus (15) and Clostridium perfringens (10). Here we report a novel observation of hot-cold-hot hemolysis exhibited by GBS.
A total of six GBS strains were used in the present study. These included four isolates from the vaginas of healthy pregnant women, one isolate from the pharynx of a neonate with a GBS infection, and one isolate from the blood of a neonate with a GBS infection (Table 1). The isolates were serotyped by the Lancefield capillary precipitin method described elsewhere (8), and the results are shown in Table 1.
TABLE 1.
GBS strains used in the present study
| Strain | Serotype | Source of isolation | Yr of isolation |
|---|---|---|---|
| KB-1 | Ia | Vagina of womana | 1996 |
| KB-2 | Ia | Vagina of woman | 1996 |
| KB-4 | III | Blood of neonateb | 1997 |
| KB-6 | Ia | Vagina of woman | 1996 |
| KB-7 | III | Vagina of woman | 1997 |
| KB-8 | NT6 | Pharynx of neonate | 1995 |
The samples from vaginas were taken from healthy women.
The samples from neonates were taken from patients with GBS infections.
The GBS isolates were incubated in Todd-Hewitt broth (THB) (Difco Laboratories, Detroit, Mich.) at 37°C for 18 h, and then the exponential phase culture was diluted to 1/10 with phosphate-buffered saline (PBS), pH 7.2. The diluted cultures were then stabbed by needle into the following plate media: (i) THB with 1.5% Bacto agar (Difco) and defibrinated 5% sheep blood (THBA); (ii) THBA with 5% sheep red blood cells that had been washed and suspended in PBS to the original concentration (RBC); (iii) RBC supplemented with 5% sheep serum (RBC+serum); and (iv) RBC with 1% soluble starch (RBC+starch). Triplicate plates of the above three plate media were incubated and treated as follows: (i) anaerobic incubation at 37°C (hot-only treatment) using AnaeroPack (Mitsubishi Gas Chemical Co., Inc., Tokyo, Japan); (ii) anaerobic incubation at 37° for 18 h and subsequently at 4°C for 6 h (hot-cold treatment); and (iii) anaerobic incubation at 37° for 18 h and subsequently at 4°C for 6 h, with reincubation anaerobically at 37°C for 6 h (hot-cold-hot treatment). At the end of the treatments, the plates were examined for the presence of hemolysis zones surrounding GBS colonies.
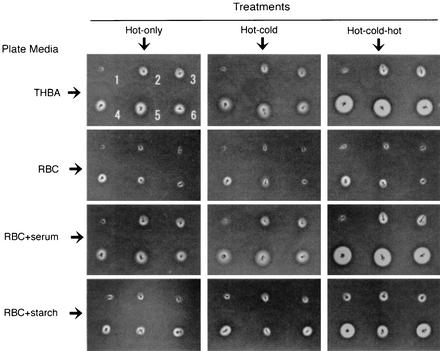
Three strains (KB-6, KB-7, and KB-8) showed a marked enlargement of the hemolysis zone on THBA with the hot-cold-hot treatment compared to that obtained with either hot-only or hot-cold treatment (Fig. 1). Meanwhile, such enlargement of the hemolysis zone was also observed for the same three strains after hot-cold-hot incubation on RBC+serum and RBC+starch but not RBC (Fig. 1). These observations suggest that serum and starch play an important role in activating GBS hemolysin in this process.
FIG. 1.
Hemolysis zone exhibited by six GBS strains on different plate media with different temperature treatments. The positions of the six GBS strains, KB-1, KB-2, KB-4, KB-6, KB-7, and KB-8, are indicated by the numbers 1 to 6, respectively, in the top left panel.
The hemolytic activity of GBS hemolysin was subsequently quantified in a liquid phase. Briefly, an appropriately diluted suspension (0.1 ml) of overnight culture containing 3.0 × 103 cells of each GBS strain was inoculated into 10 ml of (i) THB, (ii) THB supplemented with 5% sheep serum (THB+serum), and (iii) THB with 1% soluble starch (THB+starch). The media were then incubated anaerobically at 37°C for up to 18 h. A portion of each culture was collected at 6, 9, 12, 15, and 18 h of incubation and centrifuged (4°C, 10,000 × g) for 10 min. After centrifugation, twofold serial dilutions of the cell-free supernatants were made in PBS or PBS supplemented with 1 mM MgSO4 and 1 mM CaCl2. The serial dilutions of the supernatants were then mixed with an equal volume of 1% sheep blood cells suspended in PBS. The mixtures were treated as follows: (i) incubation at 37°C for 2 h (hot-only treatment); (ii) incubation at 37°C for 1 h and subsequently at 4°C for 6 h (hot-cold treatment); and (iii) incubation at 37°C for 1 h and subsequently at 4°C for 6 h, followed by reincubation at 37°C for 1 h (hot-cold-hot treatment). After treatment, the mixtures were centrifuged (3,000 × g, 15 min) and the absorbance at 540 nm of released hemoglobin in the supernatant was read to determine the highest dilution producing 50% hemolysis. The assay described above was performed in triplicate.
As the highest hemolytic activity in the broth media was recorded at 9 h for all strains tested, only these results are presented in Table 2. The culture supernatants of all strains grown in THB showed little hemolytic activity through all of the temperature treatments, whereas those of KB-6, KB-7, and KB-8 grown in THB+serum showed appreciable hemolytic activity through the treatments, with the hemolytic activity through the hot-cold-hot treatment being marginally higher. These observations were consistent with those obtained on solid medium (i.e., RBC+serum). Furthermore, the supernatants of all six strains grown in THB+starch showed marked hemolytic activity in which the hemolytic activities through hot-cold-hot treatment were approximately two- to fourfold higher than those through hot-only and hot-cold treatments. The evidence suggests that starch enhances production or activity of hemolysin by GBS strains. Alternatively, the starch is capable of stabilizing the hemolysin, thereby prolonging hemolytic activity, since the GBS hemolysin was reported to be short lived in its hemolytic activity and to bind readily to a carrier molecule like starch (9). The above possibilities should be evaluated in future study. It should be also noted that the observed phenomenon did not require the presence of Mg2+ or Ca2+.
TABLE 2.
Hemolytic activities of GBS culture supernatants in THB, THB + serum, and THB + starch
| Medium (strain) | Hot-cold-hot hemolysis on blood agar | Hemolytic activity of culture supernatants (HU)a
|
||
|---|---|---|---|---|
| Hot-only treatmentb | Hot-cold treatmentc | Hot-cold-hot treatmentd | ||
| THB | ||||
| KB-1 | −e | <2 | <2 | <2 |
| KB-2 | − | <2 | <2 | <2 |
| KB-4 | − | <2 | <2 | <2 |
| KB-6 | + | <2 | <2 | <2 |
| KB-7 | + | <2 | <2 | <2 |
| KB-8 | + | <2 | <2 | <2 |
| THB + serum | ||||
| KB-1 | − | <2 | <2 | <2 |
| KB-2 | − | <2 | <2 | <2 |
| KB-4 | − | <2 | <2 | <2 |
| KB-6 | + | 3.1 ± 0.9 | 3.5 ± 0.4 | 9.8 ± 1.8 |
| KB-7 | + | 3.0 ± 0.8 | 4.1 ± 1.1 | 4.9 ± 0.8 |
| KB-8 | + | 5.2 ± 0.9 | 5.3 ± 1.4 | 10.7 ± 1.7 |
| THB + starch | ||||
| KB-1 | − | 24.2 ± 4.7 | 26.1 ± 4.9 | 115.5 ± 13.9 |
| KB-2 | − | 20.1 ± 5.2 | 22.7 ± 3.1 | 96.3 ± 11.6 |
| KB-4 | − | 4.1 ± 1.1 | 6.2 ± 1.4 | 21.8 ± 3.9 |
| KB-6 | + | 226.0 ± 29.4 | 223.0 ± 38.2 | 641.4 ± 51.6 |
| KB-7 | + | 119.0 ± 16.4 | 109.3 ± 21.2 | 410.0 ± 50.7 |
| KB-8 | + | 237.8 ± 31.0 | 239.6 ± 29.4 | 767.6 ± 56.8 |
HU, hemolytic units.
Incubated at 37°C for 2 h.
Incubated at 37°C for 1 h followed by treatment at 4°C for 6 h.
After the 4°C treatment, reincubation was performed at 37°C for 1 h.
−, absence of hemolytic activity; +, presence of hemolytic activity.
Enhanced hemolysis with the hot-cold treatment has been reported in several bacterial species such as S. aureus (β-toxin) (15), C. perfringens (alpha-toxin) (10), Leptospira interrogans (2), and Bacillus cereus (7). The hemolytic activities of these bacterial strains was known to involve phospholipase C, which acts on sphingomyelin of blood cell membranes in the presence of Mg2+ or Ca2+ (17). In contrast, the GBS hemolysin required an additional hot treatment but did not require the presence of Mg2+ or Ca2+ for its enhancement. Although the GBS hemolysin has been reported to exhibit hemolysis in response to hot-only treatment (3, 19) which was independent of the presence of carrier molecules (9, 12), the hot-cold-hot hemolysis exhibited by the GBS was apparently pronounced in the presence of starch. The evidence indicates that the hot-cold-hot treatment results reflect yet another hemolytic property of the GBS which may be associated with its pathogenicity. Further investigation is in progress.
REFERENCES
- 1.Baker, C. J., and M. S. Edwards. 1995. Group B streptococcal infections, p. 980-1054. In J. Remington and J. O. Klein (ed.), Infectious diseases of the fetus and newborn infant, 4th ed. W. B. Saunders, Philadelphia, Pa.
- 2.Bernheimer, A. W., and R. F. Bey. 1986. Copurification of Leptospira interrogans serovar pomona hemolysin and sphingomyelinase C. Infect. Immun. 54:262-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christie, R., N. E. Atkins, and E. Munch-Petersen. 1944. A note on a lytic phenomenon shown by group B streptococci. Aust. J. Exp. Biol. Med. Sci. 22:197-200. [DOI] [PubMed] [Google Scholar]
- 4.Facklam, R. R., J. F. Padula, L. G. Thacker, E. C. Wortham, and B. J. Sconyers. 1974. Presumptive identification of groups A, B, and D streptococci. Appl. Microbiol. 27:107-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gibson, R. L., V. Nizet, and C. E. Rubens. 1999. Group B streptococcal beta-hemolysin promotes injury of lung microvascular endothelial cells. Pediatr. Res. 45:626-634. [DOI] [PubMed] [Google Scholar]
- 6.Harrison, L. H., J. A. Elliott, D. M. Dwyer, J. P. Libonati, P. Ferrieri, L. Billmann, and A. Schuchat. 1998. Serotype distributions of invasive group B streptococcal isolates in Maryland: implications for vaccine formulation. J. Infect. Dis. 177:998-1002. [DOI] [PubMed] [Google Scholar]
- 7.Ikezawa, H., M. Matsuhita, M. Tomita, and R. Taguchi. 1986. Effect of metal ions on sphingomyelinase activity of Bacillus cereus. Arch. Biochem. Biophys. 249:588-595. [DOI] [PubMed] [Google Scholar]
- 8.Lancefield, R. C. 1934. Serological differentiation of specific types of bovine hemolytic streptococci (group B). J. Exp. Med. 61:335-349. [DOI] [PMC free article] [PubMed]
- 9.Marchlewicz, B. A., and J. L. Duncan. 1980. Properties of hemolysin produced by group B streptococci. Infect. Immun. 30:805-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mollby, R., T. Wadstrom, C. J. Smyth, and M. Thelestam. 1974. The interaction of phospholipase C from Staphylococcus aureus and Clostridium perfringens with cell membranes. J. Hyg. Epidemiol. Microbiol. Immunol. 18:259-270. [PubMed] [Google Scholar]
- 11.Nizet, V., R. L. Gibson, and C. E. Rubens. 1997. The role of group B streptococci beta-hemolysin expression in newborn lung injury. Adv. Exp. Med. Biol. 418:627-630. [DOI] [PubMed] [Google Scholar]
- 12.Platt, M. W. 1995. In vitro hemolytic activity of group B streptococcus is dependent on erythrocyte-bacteria contact and independent of carrier molecules. Curr. Microbiol. 31:5-9. [DOI] [PubMed] [Google Scholar]
- 13.Ring, A., J. S. Braun, V. Nizet, W. Stremmel, and J. L. Shenep. 2000. Group B streptococcal β-hemolysin induces nitric oxide production in murine macrophages. J. Infect. Dis. 182:150-157. [DOI] [PubMed] [Google Scholar]
- 14.Rubens, C. E., M. R. Wessels, J. M. Kuypers, D. L. Kasper, and J. N. Weiser. 1990. Molecular analysis of two group B streptococcal virulence factors. Semin. Perinatol. 14:22-29. [PubMed] [Google Scholar]
- 15.Smyth, C. J., R. Mollby, and T. Wadstrom. 1975. Phenomenon of hot-cold hemolysis: chelater-induced lysis of sphingomyelinase-treated erythrocytes. Infect. Immun. 12:1104-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tapsall, J. W., and E. A. Phillips. 1991. The hemolytic and cytolytic activity of group B streptococcal hemolysin and its possible role in early onset group B streptococcal disease. Pathology 23:139-144. [DOI] [PubMed] [Google Scholar]
- 17.Titball, R. W. 1993. Bacterial phospholipase C. Microbiol. Rev. 57:347-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weisman, L. E., B. J. Stoll, D. F. Cruess, R. T. Hall, G. B. Merenstein, V. G. Hemming, and G. W. Fischer. 1992. Early-onset group B streptococcal sepsis: a current assessment. J. Pediatr. 121:428-433. [DOI] [PubMed] [Google Scholar]
- 19.Wilkinson, H. W., R. R. Facklam, and E. C. Wortham. 1973. Distribution by serological type of group B streptococci isolated from a variety of clinical material over a five-year period (with special reference to neonatal sepsis and meningitis). Infect. Immun. 8:228-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zangwill, K. M., A. Schuchat, and J. D. Wenger. 1992. Group B streptococcal disease in the United States, 1990: report from a multistate active surveillance system. Morb. Mortal. Wkly. Rep. 41(SS-6):25-32. [PubMed] [Google Scholar]