Abstract
We have studied the induction of neutralizing antibodies by in vivo expression of the human immunodeficiency virus type 1 (HIV-1) envelope by using a Venezuelan equine encephalitis virus (VEE) replicon system with mice and rabbits. The HIV-1 envelope, clone R2, has broad sensitivity to cross-reactive neutralization and was obtained from a donor with broadly cross-reactive, primary virus-neutralizing antibodies (donor of reference serum, HIV-1-neutralizing serum 2 [HNS2]). It was expressed as gp160, as secreted gp140, and as gp160ΔCT with the cytoplasmic tail deleted. gp140 was expressed in vitro at a high level and was predominantly uncleaved oligomer. gp160ΔCT was released by cells in the form of membrane-bound vesicles. gp160ΔCT induced stronger neutralizing responses than the other forms. Use of a helper plasmid for replicon particle packaging, in which the VEE envelope gene comprised a wild-type rather than a host range-adapted sequence, also enhanced immunogenicity. Neutralizing activity fractionated with immunoglobulin G. This activity was cross-reactive among a panel of five nonhomologous primary clade B strains and a Chinese clade C strain and minimally reactive against a Chinese clade E (circulating recombinant form 1) strain. The comparative neutralization of these strains by immune mouse sera was similar to the relative neutralizing effects of HNS2, and responses induced in rabbits were similar to those induced in mice. Together, these results demonstrate that neutralizing antibody responses can be induced in mice within 2 to 3 months that are similar in potency and cross-reactivity to those found in the chronically infected, long-term nonprogressive donor of HNS2. These findings support the expectation that induction of highly cross-reactive HIV-1 primary virus-neutralizing activity by vaccination may be realized.
A major goal of efforts to develop a vaccine to prevent human immunodeficiency virus type 1 (HIV-1) infections is the induction of highly cross-reactive neutralizing antibodies. To date, candidate HIV-1 vaccines that have been tested in clinical trials have not induced broadly cross-reactive neutralizing antibodies or induced antibodies capable of neutralizing primary isolates of HIV-1. Since conserved neutralization epitopes on HIV-1 tend to be conformation sensitive, recent efforts have been directed toward immunization techniques that present envelope proteins in native form (14, 28, 32). Unfortunately, in vivo expression modalities alone have not induced HIV-1-neutralizing antibodies. Immunization of nonhuman primates with oligomeric, soluble envelope protein induces antibodies against conformation-sensitive epitopes that neutralize some strains of HIV-1 (14). Themes that have emerged include the need for alternative, more effective in vivo expression systems and for selection or manipulation of envelope genes or proteins to enhance immunogenicity of epitopes that are not commonly immunogenic on soluble HIV-1 envelope proteins.
Among the numerous approaches that have been used for in vivo expression of HIV-1 or simian immunodeficiency virus (SIV) gene products in experimental vaccines, promising results have been obtained by using a vector derived from Venezuelan equine encephalitis virus (VEE) (8). Immunization of monkeys with VEE replicons expressing SIV envelope and Gag proteins resulted in neutralizing antibody and other immune responses and substantial partial protection against a virulent, intravenous, heterologous SIV challenge (8). The same expression system has been used for in vivo expression of antigens of other viruses, including induction of neutralizing antibodies against viral glycoprotein immunogens (3-5, 7, 10, 25).
Primary virus-cross-reactive neutralizing antibodies have been infrequently described in sera from donors infected with HIV-1. Beirnaert et al. have described antibodies capable of neutralizing a number of primary viruses in the sera of approximately 10% of tested donors (1). Neutralizing antibodies that cross-react extensively with primary HIV-1 isolates of various clades have been described in a reference serum prepared from a donor infected in the United States with a clade B strain of HIV-1 (12, 33, 34). The serum was designated HIV-1-neutralizing serum 2 (HNS2). The donor had a long-term nonprogressive HIV-1 infection for more than 10 years. Relatively cross-reactive, primary virus-neutralizing antibodies have been described in sera from other donors with long-term nonprogressive HIV-1 infections (6). Envelope genes have been cloned from the donor of HNS2 and characterized. One representative envelope clone was designated R2 (26). HIV-1 particles pseudotyped with R2 envelope protein are neutralized cross-reactively by sera from donors infected with strains of HIV-1 from clades A, B, C, D, and F and circulating recombinant form 1 (CRF-1) but more commonly by sera from donors infected with clades A, B, and C than by sera from donors infected with clade D or CRF-1 (26). This cross-reactivity is seen even though the level of sensitivity of the envelope to neutralization is generally similar to that of other primary HIV-1 envelopes. Virus pseudotyped with R2 envelope can mediate CD4-independent infection and is sensitive to neutralization by monoclonal antibodies directed against conformational envelope protein epitopes (35). These properties are consistent with the possibility that the R2 envelope expresses one or more neutralization epitopes that were responsible for induction of primary virus-cross-reactive neutralizing antibodies in the donor of HNS2.
In this report, we describe results of efforts to develop a small-animal immunization model for induction of HIV-1-neutralizing antibodies. For this study, various forms of the R2 gene were expressed in vitro and in vivo by using the VEE replicon system. Neutralizing antibodies were induced in both mice and rabbits. The responses in mice were found to neutralize multiple primary viruses, including non-clade B strains. This correlation is evidence that the model is relevant to human immunization. The data demonstrate that neutralizing antibodies against HIV-1 can be induced by using an in vivo expression system and that such antibodies can be capable of cross-reactive, primary virus neutralization.
MATERIALS AND METHODS
VEE plasmids and clones.
VEE plasmids pRepX (also named pVR2), pCV, and pGP have been described previously (25). They were kindly provided by J. Smith, U.S. Army Medical Research Institute of Infectious Diseases, Frederick, Md., or by N. Davis, University of North Carolina. Mutations E1 (272A/T) and E2 (209E/K) were introduced into the pGP plasmid by site-directed mutagenesis (Stratagene Quick Change) by following the manufacturer's instructions. Mutations were verified by nucleotide sequence analysis. The introduced mutations reversed mutations that had been accumulated previously by the Trinidad donkey strain of VEE during adaptation of the virus to growth in baby hamster kidney (BHK) cells (25). They confer an attenuation phenotype for mice that affects lymphoid trafficking of replicon particles (16). The plasmid with the back mutations was designated pGPm.
The R2 env gene has been described previously. To prepare the full-length R2 gene for cloning into the VEE expression vector, it was necessary to introduce specific sequences at the 5′ and 3′ ends of the gene (25). Initially, the sequences were introduced at the ends of an HIV-1 envelope gene by PCR. At the 5′ end, a ClaI recognition sequence was introduced, followed by a 14-nucleotide VEE promoter sequence, followed immediately by the envelope gene initiation codon. At the 3′ end of the gene, a ClaI recognition sequence was introduced. Each primer contained an additional restriction enzyme recognition sequence for use in cloning of the gene into a cloning vector. The HIV-1 envelope gene was amplified by using the primers and cloned into a shuttle vector. The R2 gene segment extending from the KpnI recognition sequence at nucleotide 124 to the SalI recognition sequence at nucleotide 2299 was introduced to replace the corresponding sequences of the previously cloned gene. To construct R2 clone gp140 and gp160ΔCT coding sequences (lacking the sequences encoding the transmembrane sequences and cytoplasmic tail and the cytoplasmic tail only of the protein, respectively), premature termination codons, followed by ClaI sites, were introduced by mutagenesis (Stratagene Quick Change). The premature termination codons were introduced either immediately before or immediately after the transmembrane segment coding sequences of the R2 gene. The sequences of the resulting mutant genes were verified. Envelope coding sequences were excised from the respective shuttle vectors by using ClaI and transferred into the ClaI sites of VEE expression vector pRepX. Clones of each in the correct orientation for expression were identified.
Replicon particle preparation.
The two-helper system developed by Pushko et al. was used for construction of packaged VEE replicon particles (25). Replicon plasmids pRepX, pRepX-R2gp160, pRepX-R2gp160ΔCT, and pRepX-R2gp140 and helper plasmids pGP, pGPm, and pCV were linearized with NotI and transcribed in vitro by using T7 RNA polymerase in accordance with the manufacturer's (Ambion, Austin, Tex.) instructions. The products of transcription reactions using each HIV-1 env gene-containing plasmid were combined separately with the products of transcription reactions using pGP (or pGPm) and pCV, and each mixture was transfected by electroporation into 8 × 106 BHK21 cells by using a Bio-Rad Gene Pulser (Bio-Rad, Hercules, Calif.). The cells were seeded into 75-cm2 tissue culture flasks, fed with medium, and maintained for 24 to 48 h at 37°C in a 5% CO2-in-air atmosphere. The medium from each flask was harvested and then clarified by centrifugation at 10,000 rpm for 30 min at 4°C in a Sorvall RC5-B centrifuge. The clarified medium was transferred to a 35-ml centrifuge tube, underlayered with 5 ml of 20% sucrose in phosphate-buffered saline (PBS), and centrifuged at 24,000 rpm for 2 h in a Beckman L5-5E ultracentrifuge to pellet the particles. The medium and sucrose were removed, the pellet was covered with 0.5 ml of PBS at 4°C overnight and then scraped off into the PBS, and aliquots were stored at −70°C.
To determine the infectivity of replicon particle preparations, an immunofluorescence assay was performed. BHK21 cells were seeded at a density of 4 × 104/well into wells of 16-well LabTek tissue culture slides (Nalge Nunc International), incubated overnight at 37°C in 5% CO2, and inoculated with 50-μl aliquots of serial 10-fold dilutions of replicon particle preparations in PBS with Ca2+, Mg2+, and 0.1% fetal bovine serum. Medium was then added in 150-μl aliquots. After 24 h, the cells were fixed with cold acetone at −20°C for 20 min, air dried, and stored at −20°C. For assay, the cells were rehydrated in PBS with 0.1% bovine serum albumin (BSA), blocked with PBS containing 7.5% BSA for 15 min, developed by using the globulin fraction of human HIV-1 immune serum or negative serum that was diluted 1:200 in PBS containing 0.1% BSA for 1 h at room temperature. The wells were washed twice, and the assays were developed by using goat anti-human immunoglobulin G (IgG)-fluorescein isothiocyanate (Kirkegaard & Perry Laboratories Inc., Gaithersburg, Md.). The numbers of infected cells per well were determined by fluorescence microscopy, and the volumes inoculated and dilutions of replicon particles applied to the particular wells were used to calculate the number of infectious particles in the starting material. Each preparation was also tested for replication competence. Confluent BHK21 cell cultures in six-well plates were inoculated with 1:10 dilutions of replicon particle suspensions, allowed to adsorb for 1 h, washed three times with PBS, fed with medium, and incubated at 37°C for 24 h. The medium was harvested, filtered through 45-μm-pore-size filters, and stored at −70°C. After three serial blind passages, the medium was inoculated into wells of tissue culture slides for immunofluorescence assay.
HIV-1 envelope genes.
A number of the HIV-1 envelope genes used in this study have been described previously, including R2, SF162, MACS#3, MACS#4, MACS#6, and MACS#9 (26, 27, 36). These genes are all carried in the vector pSV7d (31). Chinese clade C and E HIV-1 env genes were obtained by PCR using genomic template DNA extracted from peripheral blood mononuclear cells (PBMC) from donors infected with the different clades (26, 27, 36). The DNA was cloned into pSV7d, and clones were screened for function in pseudotyped virus infectivity assays using HOS-CD4-CCR5 cells (11). The identities of functional clones were verified by partial sequencing, and they were used for pseudotyped virus preparation. The complete nucleotide sequences of these clones have been determined.
In vitro HIV-1 envelope protein expression.
HIV-1 envelope protein expression was tested by Western blotting and enzyme immunoassay (EIA). BHK21 cells were either transfected with pRepX, pRepX-R2gp160, pRepX-R2gp160ΔCT, or pRepX-R2gp140 RNA as described above or infected with replicon particles encoding the genes of interest. After 24 to 28 h, the medium was removed and cells were washed three times with PBS and lysed in cell lysis buffer (0.5% Triton X-100, 50 mM Tris-HCl [pH 7.4], 10 mM iodoacetamide, 10 μg of leupeptin per ml) (36). Cell extracts were prepared by three freeze-thaw cycles (−70°C and 37°C), followed by a brief centrifugation to remove cell debris. Proteins in cell extracts and medium were resolved by sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis (SDS-PAGE), transferred electrophoretically to nitrocellulose membranes, and detected by Western blot analysis with HIV-1-positive human serum and alkaline phosphatase-conjugated goat anti-human IgG (Bio-Rad).
The R2 gp140 envelope glycoprotein produced by using the VEE replicon system was characterized biochemically to evaluate its oligomeric status. Four 162-cm2 flasks of BHK 21 cells were transfected with VEE replicon RNA containing the R2 gpl40 env gene as described above. After 4 h, the culture medium was replaced with OptiMEM (GIBCO-BRL, Grand Island, N.Y.) without serum, the cells were incubated at 37°C for ∼72 h, and the gp140-containing supernatants were harvested at 24-h intervals and combined. The supernatant was clarified by centrifugation, and envelope protein was purified by lentil lectin affinity chromatography (Amersham Pharmacia Biotech AB, Uppsala, Sweden) as previously described (13). The envelope protein was concentrated with Centriprep-50 concentrators (Amicon, Beverly, Mass.). The envelope protein was further purified by size exclusion chromatography with Superdex-200 (Amersham Pharmacia Biotech AB), separating the preparation into oligomeric and monomeric fractions. The fractions were identified by Western blotting as previously described (14). Following purification, the oligomeric and monomeric preparations of purified, VEE replicon-produced R2 gp140 were analyzed by chemical cross-linking and SDS-PAGE. VEE replicon-produced R2 gp140 was compared to other HIV gp140 preparations produced by using recombinant vaccinia virus, including vaccinia virus-produced R2 gp140. Briefly, 100 mM ethylene glycol-bis(succinimidylsuccinate) (EGS) was prepared fresh in dimethyl sulfoxide. A reaction mixture containing 5 μg of envelope protein from the oligomeric or monomeric fractions and 5 mM EGS was incubated for 20 min at room temperature and quenched with 5 μl of 1 M glycine. Laemmli sample buffer was added, and the samples were heated for 5 min at 100°C. The cross-linked and non-cross-linked controls of R2 Env preparations were separated by SDS-4% PAGE and Western blotted with polyclonal antiserum R2143 raised against denatured gp140 (HIV-1 isolate IIIB). Protein bands were visualized with SuperSignal chemiluminescent substrates (Pierce, Rockford, Ill.) by using the manufacturer's protocol. We generally find that most of the envelope protein produced by using vaccinia virus for expression of primary HIV-1 envelope genes is oligomeric and consists of uncleaved gp140, while the monomeric envelope protein produced is primarily cleaved gp120.
The EIA for envelope antigen quantitation was performed as described previously (21). Briefly, Immulon II plates (Dynex Technologies, Inc.) were coated at 100 μl/well with human HIV-1 immune globulin diluted 1:1,000 in PBS-N (0.05% sodium azide in PBS) and incubated overnight at 4°C. After blocking with BLOTTO (5% milk in PBS) at 37°C for 2 h, the diluted cell extracts and medium in BLOTTO/T (5% milk-0.05% Tween 20 in PBS) were added to the plates, which were incubated at 37°C for 1 h. After washing, 100 μl of sheep anti-gp120 serum diluted 1:1,000 in BLOTTO/T was added; plates were incubated at 37°C for 1 h. After washing, 100 μl of avidin D-horseradish peroxidase diluted 1:2,000 and biotinylated anti-sheep Ig (Vector, Burlingame, Calif.) diluted 1:2,000 in BLOTTO/T were added per well, and the plates were incubated at 37°C for 1 h. The plates were washed, a 100-μl aliquot of 3,3′,5,5′-tetramethylbenzidine substrate solution (Kirkegaard & Perry) was added to each well, and the plates were kept in the dark at room temperature for 30 min. Color development was stopped by addition of 50 μl of 4 N H2SO4 to each well. The reactions were read at 492 nm with a Packard SpectraCount spectrophotometer.
Mouse and rabbit immunization.
Animals were used with approval of the Institutional Animal Care and Use Committee. BALB/c, C3H/He, and C57BL/6 mice were obtained from Jackson Laboratories and used at approximately 2 months of age for immunogenicity studies. A breeding pair of FVB × C57BL/6 mice transgenic for human CD4 and CCR5 was obtained from H. Goldstein (2). A breeding pair of 129Sv/Ev alpha/beta interferon receptor 1 knockout mice was obtained from R. Zinkernagel (30). These two mouse strains were bred under barrier conditions and used as young adults. The alpha/beta interferon receptor 1 knockout mice were bred in a facility utilizing HEPA-filtered air and sterilized cages, bedding, food, and water; precautions were used to prevent transmission of infections from handlers. All mice were immunized by footpad inoculation of 25-μl suspensions of replicon particles. Mice were bled from the tail vein, except at euthanasia, when they were bled from the axillae.
Adult New Zealand White rabbits (Jackson Laboratories) were immunized by inoculation of 25-μl volumes of replicon particle suspensions subcutaneously or intradermally into the lateral aspect of the lower hind leg. Rabbits were bled from ear veins.
Serum EIA.
An antigen capture EIA was used to determine serum Ig responses. Immulon II plates (Dynex Technologies, Inc.) were coated at 100 μl/well with human HIV-1 immune globulin diluted 1:1,000 in PBS-N overnight at 4°C. After blocking with BLOTTO at 37°C for 2 h, 100 μl of HIV-1 isolate IIIB gp140, at a concentration of 50 ng/ml in BLOTTO/T was added to each well. gp140 was purified from the medium of cell cultures infected with recombinant vaccinia virus as previously described (14). The plates were incubated at 37°C for 1 h. After washing, serially diluted mouse sera were added to the wells and the plates were incubated at 37°C for 1 h. Reaction mixtures were further developed by using biotinylated anti-mouse or -rabbit IgG as described above. Positive and negative control sera were included in each assay. Sera were assigned titers equal to the highest dilutions that produced reactions twice the level of the negative control serum.
Neutralization assays.
Neutralizing antibodies were assayed by using replication-competent viruses or by using luciferase reporter viruses pseudotyped with specific HIV-1 envelopes. The assays done with replication-competent viruses were performed with H9 cells or phytohemagglutinin-stimulated PBMC. T-cell line-adapted HIV-1 isolate IIIB was tested in H9 cells, and primary strains BZ167 and US1 were tested in phytohemagglutinin-stimulated PBMC as previously described (18, 20). The assays were run in triplicate by using single dilutions of sera with a final dilution in the virus-serum mixture of 1:40. The assay results were calculated by determination of p24 levels in culture supernatants and comparison of results obtained with immune mouse serum to those obtained with nonimmune mouse serum and serial dilutions of pooled human HIV-1-positive sera (18).
The ability of immune mouse or rabbit sera to inhibit pseudotyped virus infection of HOS-CD4-CCR5 cells was assayed as described previously (21-23, 26, 27, 36). Our experience with this assay system has shown that the results obtained are very similar to those obtained by using conventional virus neutralization assays of the same primary or laboratory-adapted HIV-1 strains (36). The pseudotyped virus assay is widely employed, rapid, quantitative, and cost effective. To prepare viruses for these assays, 293T cells were cotransfected with plasmids pNL4-3.luc.E-R− and pSV7d-HIV-1env (21). They included the R2 and SF162 envelope genes and other envelope genes described above. HOS-CD4-CCR5 cell cultures were prepared at approximately 60 to 80% confluency in 96-well opaque-walled tissue culture trays and then pretreated with medium containing Polybrene at 8 μg/ml for 30 min. Serial twofold serum dilutions were mixed with equal volumes of pseudotyped virus suspensions and incubated at 4°C for 1 h. The serum-virus mixtures were added to wells of the HOS-CD4-CCR5 cell tissue culture trays and allowed to adsorb for 1 h at 37°C. The wells were fed with medium containing Polybrene and incubated at 37°C in 5% CO2 for 2 days. The trays were then centrifuged at 1,700 rpm for 10 min in a Sorvall RT6000B ultracentrifuge, the cells were washed with PBS, and the trays were drained. Next, 15 μl of 1× Luciferase Assay System cell lysis buffer (Promega, Madison, Wis.) was added per well. The trays were shaken for 30 min at room temperature. Luciferase Assay System reporter lysis buffer (Promega) was added, and luciferase activity was measured by using a MicroLumat Plus Luminometer (Wallac, Gaithersburg, Md.).
Neutralizing antibody titers were calculated as 50% inhibitory endpoints (36). Assays were performed in triplicate. Sera from immunized mice were compared to sera from nonimmunized control mice maintained in parallel. The 50% inhibitory endpoints were determined by comparing the mean luminescence of test samples at each dilution to the mean luminescence of negative control sera. The highest dilution at which the test serum inhibited infectivity to less than 50% of the control luminescence was considered the endpoint. In most cases, interpolated 50% inhibitory endpoints were also calculated by regression analysis with Microsoft Excel. These calculated 50% inhibitory titers corresponded well to titers estimated by comparison of means at each dilution, and the results obtained by comparison of means are presented in this report. The mean number of luciferase units of the immune sera at the 50% neutralization endpoint was consistently significantly lower than the number of units obtained for the control serum at the same dilution by the Student t test (Excel). Moreover, there was a strong correlation between the serum dilution and the number of luciferase units in this assay (35). The 50% neutralization endpoints were approximately four- to eightfold higher dilutions than the 90% neutralization endpoints. Since the 50% endpoints were at the middle of the neutralization titration curves, the calculated values were less variable than the 90% endpoints. Also, because of the need to predilute sera substantially before testing (i.e., 1:40), there were some sera that did not achieve 90% neutralization but mediated statistically significant neutralization of ≥50%. Some inhibition of viral infectivity by control sera was often noted. Percent neutralizing activity in immune sera was determined by comparison of luciferase activities obtained in the presence of immune and control sera. HNS2 was included for comparison in all assays as a positive control.
Human sera.
All human serum samples were obtained in compliance with applicable human subject regulations. The reference human serum, HNS2, has been described previously (34). We had previously obtained sera from Thai donors with HIV-1 clade E infections and from African donors with clade C infections from J. Mascola and H. Shephard (26). We obtained sera from Chinese donors with clade C and E infections from the National HIV Epidemiologic Surveillance Program coordinated by the National AIDS Reference Laboratory, Beijing, China. All research samples were obtained with informed consent. Sera were heat treated at 56°C for 30 min prior to use. Serial dilutions were prepared in PBS.
Mouse serum IgG purification.
Protein A-Sepharose Fast Flow Beads (100 μl; Pharmacia) were washed twice with 1 ml of PBS. Mouse control or immune sera in 100-μl aliquots and 100 μl of PBS were added to washed protein A beads and incubated at room temperature for 1 h on a tube rotator. The serum-bead mixture was transferred to a 0.22-μm-pore-size cellulose acetate filter and filtered at 1,000 × g for 1 min. The beads were washed with 1 ml of PBS and centrifuged at 2,000 × g for 5 min to dry the beads. The bound mouse IgG was eluted with 45 μl of elution buffer (0.1 M glycine-HCl) and spun at 1,000 × g for 5 min into 10 μl of neutralization buffer (1 M Tris-HCl, pH 9.0) at the bottom of the tube. Elution was repeated with another 45 μl of elution buffer and spinning at 2,000 × g for 5 min. Purified mouse IgG was then dialyzed with sterile PBS.
Nucleotide sequence accession numbers.
The nucleotide sequences determined in this study have been submitted to the GenBank database and assigned accession no. AY217545 and AY217546.
RESULTS
In vitro expression of HIV-1 envelope protein.
Significantly more immunoreactive protein was detected in both cell lysates and supernatant medium from cultures of cells transfected with replicons expressing gp140 than in cultures of cells transfected with replicons expressing full-length gp160. The total amounts of envelope protein detected by ELISA in gp140- and gp160-expressing cell cultures were 339 and 62.9 μg/107 cells, respectively. Similar levels of envelope protein expression were found in cell cultures infected with the respective replicon particles. These ELISA results were consistent with the relative densities of bands seen on Western blots (not shown). The amount of envelope protein detected by Western blotting in lysates of cells expressing gp160ΔCT was similar to, or perhaps somewhat greater than, that produced by the gp160-expressing cells and substantially less than the amount detected in gp140-expressing cells (also not shown).
We also determined the oligomeric status of the R2 gp140 produced by using the VEE replicon system. Several flasks of BHK 21 cells were transfected with R2 gp140-encoding replicon transcripts. These transfected cells were allowed to produce protein for ∼72 h under serum-free conditions, the supernatants from these flasks were harvested and pooled, and the R2 envelope was purified by using a combination of lentil lectin affinity chromatography and size exclusion chromatography and established procedures (13, 14). Both oligomeric and monomeric fractions were collected and further analyzed by chemical cross-linking and SDS-PAGE (Fig. 1A). We found that VEE-produced R2 gp140 consisted primarily of uncleaved oligomeric gp140 (apparent dimers and trimers) along with a lesser amount of monomeric cleaved gp120. The characteristics of VEE-produced R2 gp140 oligomer were indistinguishable from those of other HIV-1 gp140s, as well as R2 gp140 produced by recombinant vaccinia virus (data not shown).
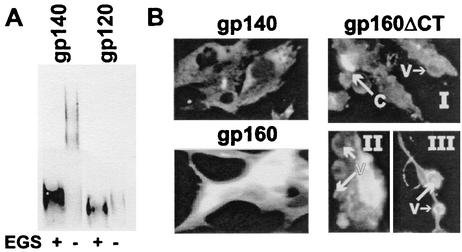
FIG. 1.
Expression and processing of the HIV-1 envelope by VEE replicons. (A) Protein produced by cells transfected with replicon transcripts expressing R2 gp140. Pooled fractions obtained by size exclusion chromatography containing predominantly either gp120 or gp140 were cross-linked with 100 mM EGS and analyzed by Western blotting as described in Materials and Methods. (B) BHK cells were inoculated with replicon particle preparations expressing R2 gp140, gp160, or gp160ΔCT and then assayed by immunofluorescence for HIV-1 envelope expression. Sites of gp160ΔCT antigen capping (C) and vesicle formation (V) are shown in subpanels I, II, and III.
In the course of conducting tests of replicon preparations to determine whether or not replication-competent virus was present, differences in patterns of immunofluorescence were observed in cells expressing each of the three forms of envelope protein, as shown in Fig. 1B. The immunofluorescence observed in the gp140-expressing cells was most prominent in the perinuclear regions of the cytoplasm corresponding to the approximate locations of the endoplasmic reticulum and the Golgi apparatus. The pattern of immunofluorescence observed in the gp160-expressing cells was more diffuse and generally consistent in appearance with distribution over the cell membrane and throughout the cytoplasm. The immunofluorescence observed in cells expressing gp160ΔCT was predominantly membrane associated. Areas of cell membrane were commonly seen where antigen capping had occurred, as shown in Fig. 1B, subpanel I. Immunofluorescence-positive membrane vesicles were frequently observed budding off the cell surface (subpanels I to III). After 24 h of infection with replicons expressing gp160ΔCT, the cytoplasm of the cells was virtually depleted, leaving cell remnants consisting of nuclei surrounded by membrane that extended away from the nuclei as filamentous projections (subpanel III). Large clusters of fluorescence-positive, membrane-bound vesicles could be seen dispersed throughout the former cell monolayer (not shown).
Immunogenicity of replicons expressing gp160 and gp140.
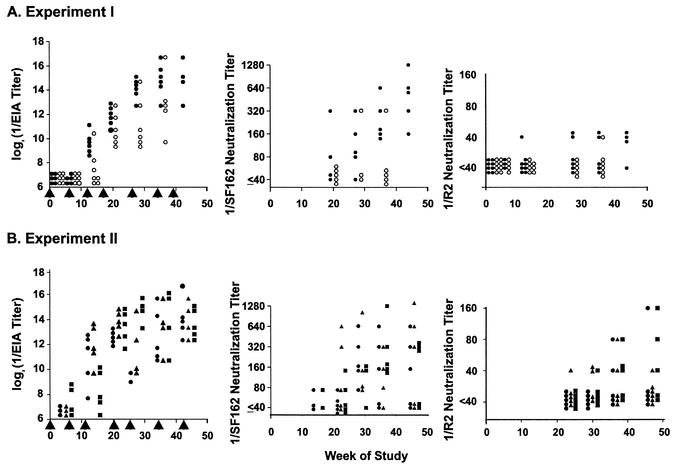
Initial immunogenicity experiments were conducted with BALB/c mice. In experiment I, the mice were given either 105 or 5 × 106 focus-forming units (FFU) of gp140-expressing replicon particles at intervals of approximately 1 to 2 months, as shown in Fig. 2. This dose range extended from a level that has induced potent immune responses against other antigens in mice (lower dose) to a higher level that was readily achievable based on the potency of the replicon particle preparations produced (25). Replicons expressing gp140 were used in the first experiment, since the amount of gp140 produced in vitro substantially exceeded the amount of gp160. No antibody responses were detected by EIA until after the third dose of replicons in experiment I (Fig. 2A). Increasing antibody titers were detected after each of the next two doses, but additional significant increases in antibody titers did not occur after either of two subsequent doses. Responses tended to occur earlier and titers were higher after the large dose than after the small dose of replicons.
FIG. 2.
Envelope protein binding antibodies and neutralizing activity in sera from BALB/c mice immunized by inoculation with VEE replicon particles expressing HIV-1 gp140 or gp160. Mice were injected in the footpad with replicon particle suspensions at the times indicated by the vertical arrowheads. Blood for assays was collected approximately 2 weeks after each immunization. Neutralization assays were performed with pseudotyped viruses. Neutralization titers were determined to be the highest immune serum dilutions at which >50% inhibition of luciferase activity was obtained, compared to nonimmune serum. In experiment I, mice were immunized repeatedly with either 5 × 106 FFU (closed circles) or 105 FFU (open circles) of replicon particles expressing gp140. In experiment II, mice were immunized with gp160-expressing replicons throughout (circles), gp140-expressing replicons throughout (squares), or gp160-expressing replicons for two doses, followed by gp140-expressing replicons for subsequent doses (triangles).
Neutralizing titers determined in the sera of these mice were defined as the highest serum dilutions that resulted in ≥50% inhibition of luciferase activity. Inhibition was determined by comparison to the average result obtained for virus treated with pooled serum from nonimmune mice maintained and bled in parallel with the immunized mice. Nonimmune mouse sera inhibited viral infectivity to various degrees, depending on the strain of virus being tested. The nonspecific inhibitory activity also tended to increase with increasing mouse age. For an immune serum to be considered neutralizing, the luciferase activity obtained at a particular dilution of immune serum could be no more than 50% of the activity obtained at the same dilution of nonimmune, age-matched serum tested in parallel. It is likely that this method of defining neutralizing titers yielded valid results since consistent results were obtained and because additional data (presented below) support the interpretation that the responses measured were mediated by virus-specific IgG.
Neutralizing responses were more readily detected in experiment I by using the SF162 strain than by using the R2 strain of HIV-1 envelope protein on pseudotyped viruses, as shown in Fig. 2A, middle and right parts. The kinetics of development of a neutralizing response against the SF162 strain was similar to the EIA response kinetics, and the larger replicon dose was more potent for induction of neutralizing antibodies. The highest titer detected in the strain R2 neutralization assays in experiment I was 1:40, the lowest serum dilution tested. The SF162 strain is generally considered neutralization resistant, similar to other primary isolates, but it is slightly more sensitive to neutralization than the R2 strain (19, 26).
In experiment II, we compared responses to gp140- and gp160-expressing replicon particles, each given in serial inoculations, to a regimen in which two inoculations with gp160-expressing replicon-expressing particles were followed by serial inoculations with gp140-expressing replicon particles. The first four inoculations given in experiment II each contained 105 infectious particles, while the remaining inoculations contained 106 infectious particles. The results of serum EIA are shown in the left part of Fig. 2B. Antibodies were detected in two of the mice that had received gp140-expressing replicons after the second dose. Antibodies were detected in other mice after the third dose. There were no significant differences in EIA titers achieved following receipt of the different types of replicon particle preparations. Maximum titers were achieved after the fifth immunization, with no significant increases after subsequent immunizations. The neutralizing responses resulting from the different regimens were also similar to each other and to the results achieved after the large-dose regimen in experiment I. Neutralizing titers against the R2 pseudotyped virus as high as 1:160 were achieved.
In a separate experiment (data not shown), BALB/c mice were immunized with 106 FFU of gp160-expressing replicons at 0, 3, 11, and 24 weeks or at 0, 3, and 24 weeks only. There were no significant differences in the EIA or neutralizing titers of serum obtained at week 26 of the study.
Effect of VEE envelope protein mutations on mouse immunogenicity.
The amino acid sequence of the envelope gene in the VEE envelope vector, pGP, corresponds to that of the V3014 clone of VEE (9). That clone was adapted from the Trinidad donkey strain of VEE by passage in BHK cells. During adaptation to growth in BHK cells, the virus developed host range mutations that favored growth in BHK cells and caused the virus to become attenuated for newborn mice (16). After subcutaneous inoculation of newborn mice, the mutations are associated with a reduction in the amount of virus replication in regional lymph nodes, subsequent decreases in the induction of cytokine secretion in lymphoid tissues, and dissemination of virus to the central nervous system (15, 16, 29). Since these effects of the host range mutations might be associated with reduced immunogenicity in mice, we studied replicon particle preparations coated with VEE envelopes in which the two attenuating mutations present in V3014 had reverted to the wild type (GPm). The reversion mutations were introduced by mutagenesis as described in Materials and Methods. The replicon particle preparations made and tested for infectivity in BHK cells were about 10-fold less infectious in BHK cells than were preparations made with the pGP sequence.
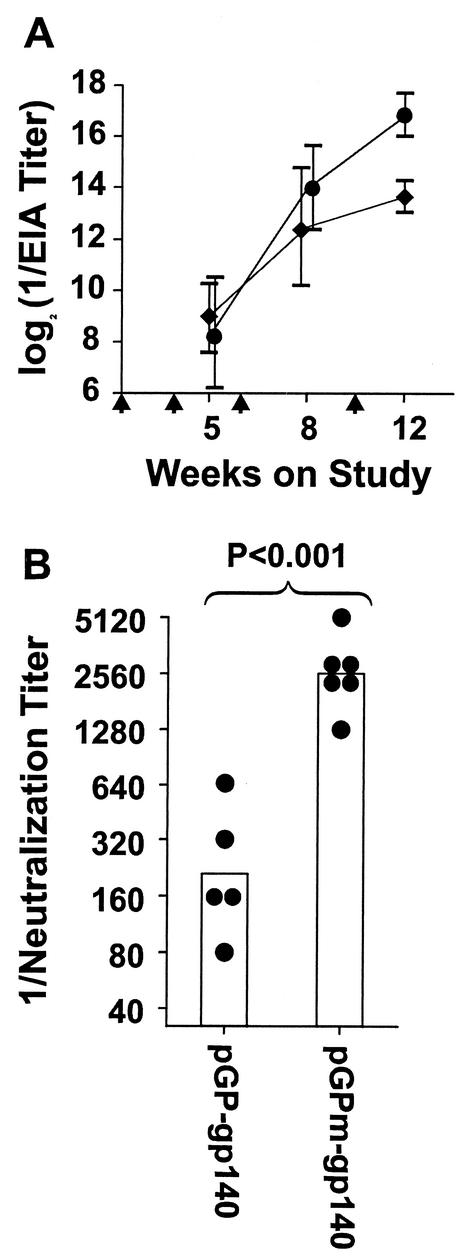
C3H/He mice were immunized with replicon preparations coated with the pGP envelope sequence or with the revertant VEE envelope sequence (pGPm) and expressing either gp160 or gp140 (comparative responses of different mouse strains are discussed below). The pGP-gp160 replicons were administered at 5 × 106 FFU/dose, while the pGPm-gp160 and pGPm-gp140 replicons were each administered at 5 × 105 FFU/dose. The smaller doses were used for the pGPm immunizations because of the lower infectivity titers of the pGPm replicons. Immunizations were given at 0, 3, 6, and 10 weeks. Responses are shown in Fig. 3. The antibody titers measured by EIA (shown in Fig. 3A) were significantly higher after 12 weeks in the group that received pGPm-gp140 than in the group that received pGP-gp140. All six sera from the pGPm-gp140 group inhibited luminescence resulting from SF162 strain infection by >50% at the 1:1,280 serum dilutions, and five of six inhibited by >50% at the 1:2,560 dilution. None of the sera from the pGP-gp140 group displayed consistent neutralizing activity at dilutions greater than 1:640. The neutralizing activity developed by the pGPm-gp140 mice at 8 weeks was similar to the maximal responses obtained in earlier experiments using pGP replicon particles after five or more immunizations over 30 to 40 weeks. The results demonstrate that the pGPm replicons induce significantly faster and more potent neutralizing responses to the HIV-1 envelope in mice than the pGP replicon particles, even when used at substantially lower infectivity titers based on assays of BHK cells.
FIG. 3.
Influence of VEE envelope protein host range mutations on immunogenicity of VEE replicons expressing HIV-1 envelope proteins. C3H/He mice were immunized by footpad inoculation at the times indicated by the vertical arrows. In panel A, EIA titers are presented as means ± standard deviations for groups of six mice each immunized with 5 × 106 FFU of pGP-gp140 per dose (⧫) or 5 × 105 FFU of pGPm-gp140 per dose (•). In panel B, the heights of the columns indicate the geometric mean neutralization titers of sera from mice immunized with either pGP-gp140- or pGPm-gp140-expressing replicons against strain SF162 pseudotyped virus. The sera were obtained after 8 weeks on study. The circles indicate the neutralization titers of the individual sera of mice in each of the groups. The P value shown is the result obtained by comparing the geometric means of the two groups by Student t test.
Effect of gp160 cytoplasmic tail deletion on antibody responses.
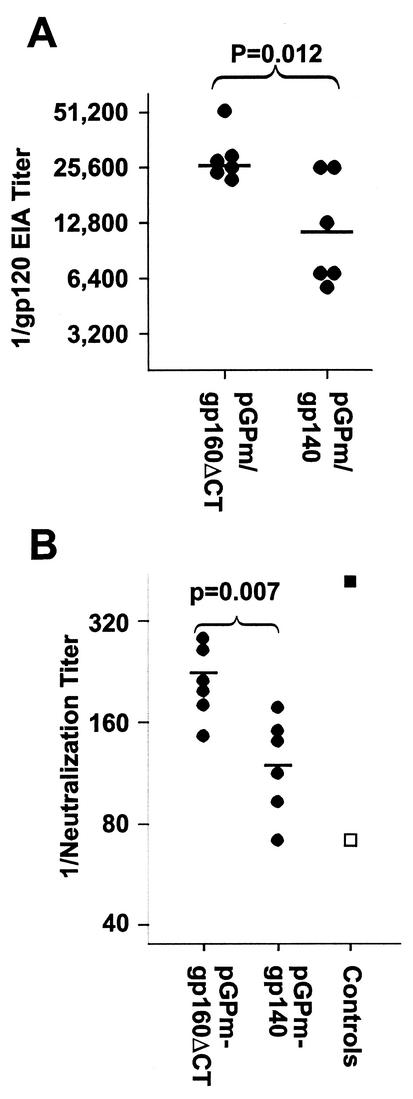
The possibility was tested that expression of membrane-anchored gp160ΔCT in vivo would induce greater antibody responses than expression of secreted gp140. Results of one experiment are shown in Fig. 4. The EIA titers obtained in the pGPm-gp160ΔCT group were significantly higher than those of the pGPm-gp140 group. Significantly greater neutralizing activity was also induced by pGPm-gp160ΔCT immunization than by pGPm-gp140 immunization. In a separate experiment (data not shown), we observed that immunization with pGP-gp160ΔCT induced EIA and neutralization responses that were similar in rate and magnitude to those induced by pGPm-gp140 and pGPm-gp160 and significantly greater than those induced by pGP-gp140. The results indicate that rapid, potent neutralization responses can be induced in mice by immunization with replicons coated with the wild-type VEE envelope and expressing the HIV-1 envelope with the cytoplasmic tail deleted.
FIG. 4.
Effect of gp160 cytoplasmic tail deletion on immunogenicity of VEE-HIV-1 env replicons. C3H/He mice were immunized with pGPm replicons expressing either gp140 or gp160ΔCT at 0, 2, 6, and 10 weeks, and sera were obtained at 12 weeks after the start of the study. Control sera were HNS2 (▪) and the negative reference serum (□).
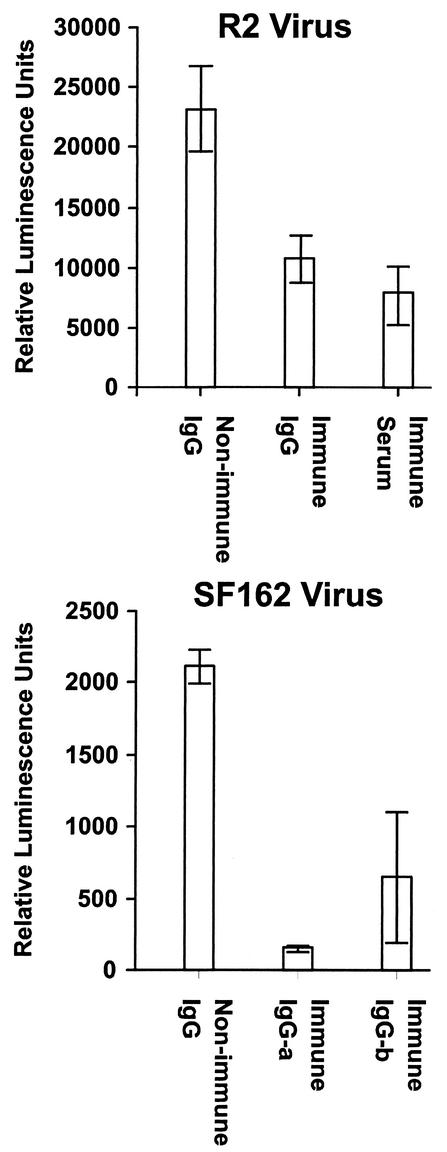
Neutralizing activity associated with the IgG fraction of serum.
We further evaluated whether the neutralizing responses obtained were the result of specific immune responses by testing whether they were mediated by IgG. Results of these experiments are shown in Fig. 5. An IgG fraction was prepared from serum pooled from gp140-immunized mice from experiment II (Fig. 2). This IgG preparation was compared to the serum pool from which it was derived and to IgG prepared from serum pooled from matched control mice for neutralizing activity against R2 pseudotyped virus. The concentration of the purified IgG was adjusted to be comparable to the concentration of IgG in serum. The results demonstrated similar significant inhibition of R2 virus infectivity by the immune IgG and immune serum pools. In a separate experiment, IgG fractions were prepared from serum pools from two separate groups of C3H mice immunized with gp140-expressing replicons and tested for neutralization of virus pseudotyped with SF162 envelope. These results are shown in the lower panel of Fig. 5 and demonstrate significant neutralizing activity in both IgG preparations.
FIG. 5.
Virus-neutralizing activity in sera of immunized mice is copurified with IgG. (Top) Sera from four immunized BALB/c mice from experiment II (Fig. 2) with comparatively high strain R2-neutralizing activity and from control mice in the same experiment (nonimmune IgG) were pooled, and IgG fractions were prepared as described in Materials and Methods. These materials were tested for neutralization of R2 envelope pseudotyped virus in comparison to the unfractionated immune serum pool. (Bottom) Similar immune globulin fractions were prepared from sera of C3H mice immunized with replicons expressing gp140 and tested for neutralization of SF162 envelope pseudotyped virus. The results shown in both panels are mean numbers of luminescence units obtained after virus incubation with the serum pool diluted 1:40 and IgG fractions of immune sera diluted to correspond to the concentrations in the serum pools from which they were derived. The nonimmune globulin preparations were tested at approximately the same IgG concentrations as the immune globulin preparations.
Comparative antibody responses of different mouse strains.
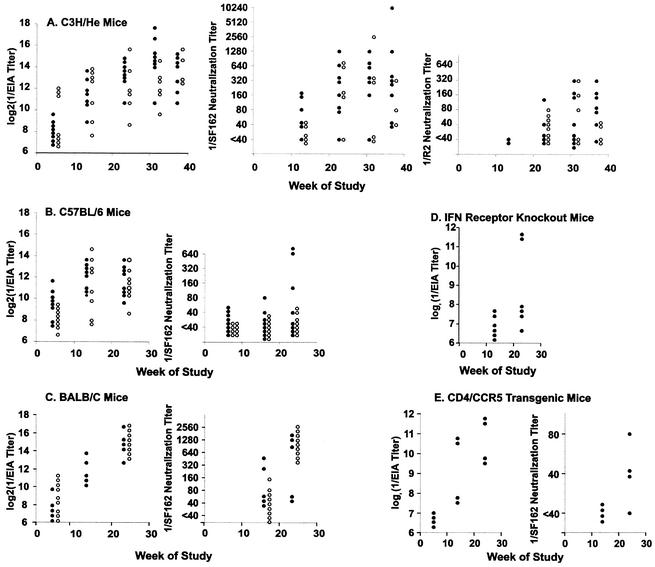
Antibody responses induced by gp140- and gp160-expressing replicons (106 FFU/dose) in various mouse strains are shown in Fig. 6. The responses measured by EIA in C3H/He, C57BL/6, and BALB/c mice were similar (Fig. 6A, B, and C), and the responses found in BALB/c mice were similar to those found in experiments I and II. Neutralizing antibody responses against the SF162 strain were similar in BALB/c and C3H/He mice and appeared stronger than those elicited in C57BL/6 mice. The EIA titers elicited in alpha/beta interferon receptor 1 knockout mice and CD4/CCR5 transgenic mice were lower than those elicited in the three inbred mouse strains (compare Fig. 6D and E to A, B, and C). The SF162-neutralizing antibody titers elicited in the transgenic mice were lower than those elicited in C3H/He and BALB/c mice (compare Fig. 6E to A and C).
FIG. 6.
Comparative EIA binding and neutralizing activities of sera of mice of different strains immunized with VEE replicons expressing gp160 (closed circles) or gp140 (open circles) at 106 FFU/dose. Immunizations were given at 0, 1, and 2 weeks on study and approximately every 2 months thereafter. Blood was obtained for assay approximately 2 weeks after the third and each subsequent immunization. Neutralization assays were done by using pseudotyped viruses. Neutralization titers were determined as the highest serum dilutions that caused reductions in mean luminescence results of >50%.
Cross-reactivity of neutralizing antibody responses among heterologous virus strains.
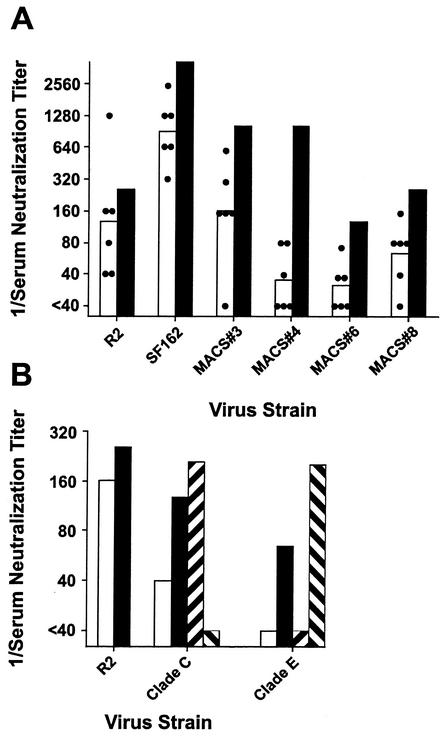
Sera were evaluated for neutralization of viruses pseudotyped with envelopes from clade B, C, and E (CRF-1) strains of HIV-1. Viruses pseudotyped with clade B primary envelopes from the R2 and SF162 strains and from four different donors from the Multicenter AIDS Cohort Study (MACS) were tested as shown in Fig. 7A. Results are shown for sera from six C3H/He mice immunized with pGPm-gp160 as described above. The infectivity of each of the four heterologous primary envelope pseudotyped viruses was inhibited by >50% at dilutions of 1:40 or greater, compared to sera from nonimmune control mice, by three, four, or five of the sera tested. The neutralizing activity in the mouse sera was somewhat less than the neutralizing activity in the HNS2 serum. These results demonstrated that the neutralizing response obtained by immunization with replicons expressing the R2 envelope protein cross-reacted among other primary HIV-1 clade B envelope proteins. Similar results (not shown) were obtained in tests of sera from additional groups of C3H mice immunized with pGP-gp140 or pGP-gp160.
FIG. 7.
Cross-reactivity of neutralizing activity in sera from gp160 immune C3H/He mice against primary HIV-1 envelope pseudotyped viruses. (A) The heights of the open columns indicate the geometric mean titers of six sera from immune mice against viruses pseudotyped with the R2 or SF162 envelope or one of four MACS donor envelopes. The circles indicate the 50% neutralization titers obtained with the individual sera. The closed columns indicate the titers obtained against the same viruses by using the HNS2 serum. In panel B, the results are shown for neutralization of viruses pseudotyped with the R2, Chinese clade C, and Chinese clade E (CRF-1) envelopes. The heights of the columns indicate the 50% neutralization titers of the immune mouse serum pool (□) and HNS2 (▪) and the geometric mean 50% neutralization titers of three sera from African donors infected with clade C viruses (▨) and three sera from Thai donors infected with clade E viruses (▧).
The neutralization of viruses pseudotyped with R2 and Chinese clade C and E envelopes was also compared. The cloning of these clade C and E envelopes is described in Materials and Methods. In preliminary experiments, the neutralization of viruses pseudotyped with these envelopes by human sera was evaluated. The clade C virus was neutralized by sera from Chinese donors infected with clade C viruses and by African donors infected with African clade C viruses (sera provided by H. Shephard and the HIVNET [26]). The clade E virus was neutralized by sera from Chinese and Thai donors infected with clade E viruses (the Thai sera were provided by J. Mascola [26]). The results of these preliminary experiments are not shown. Neutralization of these viruses by sera from mice immunized with pGPm-gp160 is shown in Fig. 7B. The 50% neutralizing dilution of the pool of mouse serum against R2 pseudotyped virus was 1:160, while the 50% neutralizing dilution of HNS2 against R2 pseudotyped virus was ≥1:256. The 50% neutralizing dilution of the pool of mouse serum against the clade C virus was 1:40, while the 50% neutralizing dilution of HNS2 against this virus was 1:128. The mouse sera did not neutralize the clade E virus at the dilutions tested, while the 50% neutralizing dilution of HNS2 was 1:16 (less than the lowest dilution of mouse serum that it was possible to test). These comparative neutralizing activities of HNS2 were similar to those reported previously (26). Additionally, virus pseudotyped with R2 envelope is neutralized more readily by sera from donors infected with clade C viruses than by sera from donors infected with clade E viruses (26). Thus, the interclade cross-reactivity of the mouse sera is consistent with the cross-reactivity of R2 and HNS2 (36).
The neutralization of heterologous, replication-competent clade B virus infections of cell cultures was studied, as shown in Fig. 8. Pooled sera from C3H/He mice immunized with pGPm-gp160 and control mice were compared for neutralization of isolate IIIB infection of H9 cells and of BZ167 and US1 strain infections of PBMC, as described in Materials and Methods. Both BZ167 and US1 are primary viruses. Compared to control sera, the immune sera significantly inhibited infection by isolate IIIB and strain BZ167.
FIG. 8.
Neutralization of replication-competent clade B virus strain infections of cell cultures. Isolate IIIB infections were assayed in H9 cells, and strain BZ167 and US1 infections were assayed in PBMC. Amounts of p24 in culture supernatants were assayed 1 week postinfection. Results are expressed as percentages of p24 in medium from cells challenged with virus preadsorbed to nonimmune sera. Results are from three separate assays, each done in triplicate. Bars indicate mean percent neutralization in triplicate experiments.
Induction of neutralizing antibodies in rabbits immunized with replicons expressing R2 envelope.
New Zealand White rabbits were immunized with R2 env-expressing replicons (Fig. 9). Sera were tested for neutralization of viruses pseudotyped with the R2 and SF162 envelopes. Sera from all three immunized rabbits inhibited infectivity of the SF162 strain by more than 50% at dilutions as high as 1:128 and 1:256, and sera from two of the three rabbits inhibited the infectivity of the R2 strain by more than 50% at dilutions as high as 1:64 and 1:128.
FIG. 9.
Neutralizing activity in sera from New Zealand White rabbits immunized with VEE replicons expressing the HIV-1 strain R2 envelope. Rabbits were immunized by subcutaneous or intradermal inoculation of replicons expressing R2 gp140 (first four immunizations) or R2 gp160ΔCT (remaining immunizations) at weekly intervals for three inoculations and then at approximately 6-week intervals for four inoculations. The results shown are the 50% neutralization titers of each serum against viruses pseudotyped with SF162 (closed columns) or R2 (open columns). The tests were performed in triplicate, and the assay was repeated.
DISCUSSION
In this report, we present evidence that an alphavirus expression system can serve as a versatile method for immunization of small animals with native HIV-1 envelope protein for induction of HIV-1-neutralizing antibodies. The same VEE-derived vector system has also been used extensively by others for induction of immunity to heterologous antigens, including induction of neutralizing antibodies in nonhuman primates immunized with SIV envelope protein (3-5, 7, 8, 25). Here we describe high-level expression of HIV-1 envelope protein in vitro, induction of potent, broadly cross-reactive neutralizing antibody responses in a murine model following in vivo expression of the envelope protein, and modification of the immunization approach so that it can be used for rapid induction of neutralizing antibody responses. The results indicate that induction of cross-reactive, primary virus-neutralizing antibodies by vaccination to prevent HIV-1 infection may be achievable.
The amount of envelope protein produced in BHK cells infected or transfected with replicons expressing HIV-1 gp140 or gp160 was similar to that reported for SIV (3). gp140 was produced at higher levels than gp160. Reasons for the different levels of expression were not investigated. We also expressed a form of env gene that retained the membrane anchor but lacked the cytoplasmic tail of the full-length protein, gp160ΔCT. BHK cells processed the three forms of the protein differently. The envelope protein produced by cells expressing full-length gp160 remained cell associated. Much of the envelope protein produced by cells expressing gp140 was secreted, and most of the secreted protein was uncleaved oligomer (dimer or higher-order forms). The envelope protein expressed by cells expressing gp160ΔCT was mostly secreted in the form of membrane-bound vesicles. The different fates of these different forms of the envelope protein may be relevant to their potential immunogenicity when expressed in vivo.
A number of variables were evaluated to search for methods to obtain enhanced immune responses. Dose-related differences in responses were observed after administration of large and small doses of VEE replicons, consistent with dose-response effects observed previously by others (17, 24, 25). The feasibility of administering multiple sequential doses of replicons to obtain repeated responses has also been established (25). The HIV-1 envelope-specific antibody responses, measured by ELISA and neutralization, were significantly enhanced, in both magnitude and rate of development, by the use of wild-type VEE envelope sequences for replicon packaging, consistent with results reported by J. F. Smith et al. (personal communication). No special advantage was achieved by varying the time course of booster dose administration or by use of a variety of mouse strains for immunization. The studies established that administration of three- or four-dose replicon immunization regimens over 8 to 10 weeks to BALB/c or C3H/He mice with replicons expressing gp160ΔCT and packaged with wild-type VEE envelope resulted in high-potency neutralizing antibody responses.
Certain difficulties must be addressed when studying induction of virus-neutralizing antibodies in animal models. In studies of induction of HIV-1-neutralizing antibodies, one particularly vexing difficulty is the presence of nonspecific virus inhibiting activity in animal sera. In the present study, we considered the inclusion of concurrent control animals an important factor, since the nonspecific inhibitory activity in mouse sera appeared to increase with mouse age. We believe our studies addressed the difficulty resulting from this nonspecific inhibitory effect and demonstrated the induction of HIV-1-neutralizing immunoglobulin for three reasons: (i) consistently greater activity could be found in sera from immune mice than in sera from nonimmune mice, (ii) inhibitory activity in the immune sera copurified with IgG, and (iii) the magnitude of the neutralizing response induced varied in relation to the form of HIV-1 envelope expressed. A theoretical concern regarding animal models is that they may mount neutralizing responses that differ in specificity from those that may occur in humans. While this concern may be valid, animal models are generally valuable tools for vaccine development. Evidence from our studies suggests that mice and rabbits may be useful models for evaluation of potential immune responses of humans to HIV-1 envelope. The evidence includes findings that mice and rabbits were similar to each other with respect to the nature of the responses developed and that the titers of antibodies that developed in mice against different strains of HIV-1 were similar to the titers of the human HNS2 serum against the same strains. These results indicate that the use of the high-potency in vivo VEE replicon expression system in mice and rabbits offers promise for evaluation of the antigenic requirements for induction of highly cross-reactive HIV-1-neutralizing antibodies.
The R2 env gene was used to construct the immunogen for this study because it had been obtained from a donor with highly cross-reactive HIV-1 primary virus-neutralizing antibodies and because the R2 envelope is neutralized cross-reactively by sera from donors infected with various clades of HIV-1 (26, 34). This cross-reactivity of R2 is unique compared to other strains to which it has been compared. The neutralizing responses induced extended to six other primary clade B strains and to a primary clade C strain but not to the clade D or E strains tested. We did not determine in the studies reported here if the cross-reactivity of the responses induced was dependent upon the unusual properties of the R2 envelope. However, our findings do indicate that the induction of primary virus-cross-reactive neutralizing antibodies by vaccination is an achievable goal.
Acknowledgments
This work was supported by National Institutes of Health grants AI37438, AI44339, AI48280, and TW00916 and Uniformed Services University of the Health Sciences grant RO87NC.
We are grateful to Nancy Davis for advice regarding the use of the VEE replicon system.
REFERENCES
- 1.Beirnaert, E., P. Nyambi, B. Willems, L. Heyndrickx, R. Colebunders, W. Janssens, and G. van Der. 2000. Identification and characterization of sera from HIV-infected individuals with broad cross-neutralizing activity against group M (env clade A-H) and group O primary HIV-1 isolates. J. Med. Virol. 62:14-24. [PubMed] [Google Scholar]
- 2.Browning, J., J. W. Horner, M. Pettoello-Mantovani, C. Raker, S. Yurasov, R. A. DePinho, and H. Goldstein. 1997. Mice transgenic for human CD4 and CCR5 are susceptible to HIV infection. Proc. Natl. Acad. Sci. USA 94:14637-14641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caley, I. J., M. R. Betts, N. L. Davis, R. Swanstrom, J. A. Frelinger, and R. E. Johnston. 1999. Venezuelan equine encephalitis virus vectors expressing HIV-1 proteins: vector design strategies for improved vaccine efficacy. Vaccine 17:3124-3135. [DOI] [PubMed] [Google Scholar]
- 4.Caley, I. J., M. R. Betts, D. M. Irlbeck, N. L. Davis, R. Swanstrom, J. A. Frelinger, and R. E. Johnston. 1997. Humoral, mucosal, and cellular immunity in response to a human immunodeficiency virus type 1 immunogen expressed by a Venezuelan equine encephalitis virus vaccine vector. J. Virol. 71:3031-3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charles, P. C., K. W. Brown, N. L. Davis, M. K. Hart, and R. E. Johnston. 1997. Mucosal immunity induced by parenteral immunization with a live attenuated Venezuelan equine encephalitis virus vaccine candidate. Virology 228:153-160. [DOI] [PubMed] [Google Scholar]
- 6.Connor, R. I., B. T. Korber, B. S. Graham, B. H. Hahn, D. D. Ho, B. D. Walker, A. U. Neumann, S. H. Vermund, J. Mestecky, S. Jackson, E. Fenamore, Y. Cao, F. Gao, S. Kalams, K. J. Kunstman, D. McDonald, N. McWilliams, A. Trkola, J. P. Moore, and S. M. Wolinsky. 1998. Immunological and virological analyses of persons infected by human immunodeficiency virus type 1 while participating in trials of recombinant gp120 subunit vaccines. J. Virol. 72:1552-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis, N. L., K. W. Brown, and R. E. Johnston. 1996. A viral vaccine vector that expresses foreign genes in lymph nodes and protects against mucosal challenge. J. Virol. 70:3781-3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis, N. L., I. J. Caley, K. W. Brown, M. R. Betts, D. M. Irlbeck, K. M. McGrath, M. J. Connell, D. C. Montefiori, J. A. Frelinger, R. Swanstrom, P. R. Johnson, and R. E. Johnston. 2000. Vaccination of macaques against pathogenic simian immunodeficiency virus with Venezuelan equine encephalitis virus replicon particles. J. Virol. 74:371-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis, N. L., N. Powell, G. F. Greenwald, L. V. Willis, B. J. Johnson, J. F. Smith, and R. E. Johnston. 1991. Attenuating mutations in the E2 glycoprotein gene of Venezuelan equine encephalitis virus: construction of single and multiple mutants in a full-length cDNA clone. Virology 183:20-31. [DOI] [PubMed] [Google Scholar]
- 10.Davis, N. L., L. V. Willis, J. F. Smith, and R. E. Johnston. 1989. In vitro synthesis of infectious Venezuelan equine encephalitis virus RNA from a cDNA clone: analysis of a viable deletion mutant. Virology 171:189-204. [DOI] [PubMed] [Google Scholar]
- 11.Deng, H., R. Liu, W. Ellmeier, S. Choe, D. Unutmaz, M. Burkhart, P. Di Marzio, S. Marmon, R. E. Sutton, C. M. Hill, C. B. Davis, S. C. Peiper, T. J. Schall, D. R. Littman, and N. R. Landau. 1996. Identification of a major co-receptor for primary isolates of HIV-1. Nature 381:661-666. [DOI] [PubMed] [Google Scholar]
- 12.D'Souza, M. P., P. Durda, C. V. Hanson, G. Milman, et al. 1991. Evaluation of monoclonal antibodies to HIV-1 by neutralization and serological assays: an international collaboration. AIDS 5:1061-1070. [DOI] [PubMed] [Google Scholar]
- 13.Earl, P. L., C. C. Broder, D. Long, S. A. Lee, J. Peterson, S. Chakrabarti, R. W. Doms, and B. Moss. 1994. Native oligomeric human immunodeficiency virus type 1 envelope glycoprotein elicits diverse monoclonal antibody reactivities. J. Virol. 68:3015-3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Earl, P. L., W. Sugiura, D. C. Montefiori, C. C. Broder, S. A. Lee, C. Wild, J. Lifson, and B. Moss. 2001. Immunogenicity and protective efficacy of oligomeric human immunodeficiency virus type 1 gp140. J. Virol. 75:645-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grieder, F. B., B. K. Davis, X. D. Zhou, S. J. Chen, F. D. Finkelman, and W. C. Gause. 1997. Kinetics of cytokine expression and regulation of host protection following infection with molecularly cloned Venezuelan equine encephalitis virus. Virology 233:302-312. [DOI] [PubMed] [Google Scholar]
- 16.Grieder, F. B., N. L. Davis, J. F. Aronson, P. C. Charles, D. C. Sellon, K. Suzuki, and R. E. Johnston. 1995. Specific restrictions in the progression of Venezuelan equine encephalitis virus-induced disease resulting from single amino acid changes in the glycoproteins. Virology 206:994-1006. [DOI] [PubMed] [Google Scholar]
- 17.Hevey, M., D. Negley, P. Pushko, J. Smith, and A. Schmaljohn. 1998. Marburg virus vaccines based upon alphavirus replicons protect guinea pigs and nonhuman primates. Virology 251:28-37. [DOI] [PubMed] [Google Scholar]
- 18.Kim, J. H., J. R. Mascola, S. Ratto-Kim, T. C. Vancott, L. Loomis-Price, J. H. Cox, N. L. Michael, L. Jagodzinski, C. Hawkes, D. Mayers, B. L. Gilliam, D. C. Birx, and M. L. Robb. 2001. Selective increases in HIV-specific neutralizing antibody and partial reconstitution of cellular immune responses during prolonged, successful drug therapy of HIV infection. AIDS Res. Hum. Retrovir. 17:1021-1034. [DOI] [PubMed] [Google Scholar]
- 19.Levy, J. A. 1993. Pathogenesis of human immunodeficiency virus infection. Microbiol. Rev. 57:183-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore, J. P., and D. C. Montefiori. 1997. Neutralization assays using the BZ167 strain of human immunodeficiency virus type 1. J. Infect. Dis. 176:1410-1412. [DOI] [PubMed] [Google Scholar]
- 21.Park, E. J., and G. V. Quinnan. 1999. Both neutralization resistance and high infectivity phenotypes are caused by mutations of interacting residues in the HIV-1 gp41 leucine zipper and the gp120 receptor- and coreceptor-binding domains. J. Virol. 73:5707-5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park, E. J., L. K. Vujcic, R. Anand, T. S. Theodore, and G. V. Quinnan, Jr. 1998. Mutations in both gp120 and gp41 are responsible for the broad neutralization resistance of variant human immunodeficiency virus type 1 MN to antibodies directed at V3 and non-V3 epitopes. J. Virol. 72:7099-7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park, E. J., S. Zolla-Pazner, and G. V. Quinnan, Jr. 2000. Distinct mechanisms mediating enhanced infectivity and envelope conformational change determine global neutralization resistance phenotype of HIV-1. J. Virol. 74:4183-4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pushko, P., M. Bray, G. V. Ludwig, M. Parker, A. Schmaljohn, A. Sanchez, P. B. Jahrling, and J. F. Smith. 2000. Recombinant RNA replicons derived from attenuated Venezuelan equine encephalitis virus protect guinea pigs and mice from Ebola hemorrhagic fever virus. Vaccine 19:142-153. [DOI] [PubMed] [Google Scholar]
- 25.Pushko, P., M. Parker, G. V. Ludwig, N. L. Davis, R. E. Johnston, and J. F. Smith. 1997. Replicon-helper systems from attenuated Venezuelan equine encephalitis virus: expression of heterologous genes in vitro and immunization against heterologous pathogens in vivo. Virology 239:389-401. [DOI] [PubMed] [Google Scholar]
- 26.Quinnan, G. V., P. F. Zhang, D. W. Fu, M. Dong, H. J. Alter, et al. 1999. Expression and characterization of HIV type 1 envelope protein associated with a broadly reactive neutralizing antibody response. AIDS Res. Hum. Retrovir. 15:561-570. [DOI] [PubMed] [Google Scholar]
- 27.Quinnan, G. V., P. F. Zhang, D. W. Fu, M. Dong, and J. B. Margolick. 1998. Evolution of neutralizing antibody response against HIV type 1 virions and pseudovirions in multicenter AIDS cohort study participants. AIDS Res. Hum. Retrovir. 14:939-949. [DOI] [PubMed] [Google Scholar]
- 28.Richardson, T. M. J., B. L. Stryjewski, C. C. Broder, J. A. Hoxie, J. R. Mascola, P. L. Earl, and R. W. Doms. 1996. Humoral response to oligomeric human immunodeficiency virus type 1 envelope protein. J. Virol. 70:753-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schoneboom, B. A., J. S. Lee, and F. B. Grieder. 2000. Early expression of IFN-α/β and iNOS in the brains of Venezuelan equine encephalitis virus-infected mice. J. Interferon Cytokine Res. 20:205-215. [DOI] [PubMed] [Google Scholar]
- 30.Steinhoff, U., U. Muller, A. Schertler, H. Hengartner, M. Aguet, and R. M. Zinkernagel. 1995. Antiviral protection by vesicular stomatitis virus-specific antibodies in alpha/beta interferon receptor-deficient mice. J. Virol. 69:2153-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stuve, L. L., S. Brown-Shimer, C. Pachl, R. Najarian, D. Dina, and R. L. Burke. 1987. Structure and expression of the herpes simplex virus type 2 glycoprotein gB gene. J. Virol. 61:326-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sugiura, W., C. C. Broder, B. Moss, and P. L. Earl. 1999. Characterization of conformation-dependent anti-gp120 murine monoclonal antibodies produced by immunization with monomeric and oligomeric human immunodeficiency virus type 1 envelope proteins. Virology 254:257-267. [DOI] [PubMed] [Google Scholar]
- 33.Vujcic, L., D. Katzenstein, M. Martin, and G. Quinnan. 1990. International collaborative study to compare assays for antibodies that neutralize human immunodeficiency virus. AIDS Res. Hum. Retrovir. 6:847-853. [DOI] [PubMed] [Google Scholar]
- 34.Vujcic, L. K., and G. Quinnan. 1995. Preparation and characterization of human HIV type 1 neutralizing reference sera. AIDS Res. Hum. Retrovir. 1:783-787. [DOI] [PubMed] [Google Scholar]
- 35.Zhang, P. F., P. Bouma, E. J. Park, J. B. Margolick, J. E. Robinson, S. Zolla-Pazner, M. N. Flora, and G. V. Quinnan, Jr. 2002. A variable region 3 (V3) mutation determines a global neutralization phenotype and CD4-independent infectivity of a human immunodeficiency virus type 1 envelope associated with a broadly cross-reactive, primary virus-neutralizing antibody response. J. Virol. 76:644-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang, P. F., X. Chen, D. W. Fu, J. B. Margolick, and G. V. Quinnan, Jr. 1999. Primary virus envelope cross-reactivity of the broadening neutralizing antibody response during early chronic human immunodeficiency virus type 1 infection. J. Virol. 73:5225-5230. [DOI] [PMC free article] [PubMed]