Abstract
We previously showed that the envelope glycoprotein (EnvA) of avian sarcoma/leukosis virus subtype A (ASLV-A) binds to liposomes at neutral pH following incubation with its receptor, Tva, at ≥22°C. We also provided evidence that ASLV-C fuses with cells at neutral pH. These findings suggested that receptor binding at neutral pH and ≥22°C is sufficient to activate Env for fusion. A recent study suggested that two steps are necessary to activate avian retroviral Envs: receptor binding at neutral pH, followed by exposure to low pH (W. Mothes et al., Cell 103:679-689, 2000). Therefore, we evaluated the requirements for intact ASLV-A particles to bind to target bilayers and fuse with cells. We found that ASLV-A particles bind stably to liposomes in a receptor- and temperature-dependent manner at neutral pH. Using ASLV-A particles biosynthetically labeled with pyrene, we found that ASLV-A mixes its lipid envelope with cells within 5 to 10 min at 37°C. Lipid mixing was neither inhibited nor enhanced by incubation at low pH. Lipid mixing of ASLV-A was inhibited by a peptide designed to prevent six-helix bundle formation in EnvA; the same peptide inhibits virus infection and EnvA-mediated cell-cell fusion (at both neutral and low pHs). Bafilomycin and dominant-negative dynamin inhibited lipid mixing of Sindbis virus (which requires low pH for fusion), but not of ASLV-A, with host cells. Finally, we found that, although EnvA-induced cell-cell fusion is enhanced at low pH, a mutant EnvA that is severely compromised in its ability to support infection still induced massive syncytia at low pH. Our results indicate that receptor binding at neutral pH is sufficient to activate EnvA, such that ASLV-A particles bind hydrophobically to and merge their membranes with target cells. Possible roles for low pH at subsequent stages of viral entry are discussed.
To infect a cell, an enveloped virus must bind to and fuse with a cellular membrane. There are two established routes of entry: fusion at low pH with the endosome (as for influenza virus, Sindbis virus, and vesicular stomatitis virus) and fusion at neutral pH (as for the retrovirus human immunodeficiency virus [HIV]) (27). Viral envelope glycoproteins mediate binding to cellular receptors as well as fusion.
The model retrovirus avian sarcoma/leukosis virus subtype A (ASLV-A) possesses a single envelope glycoprotein (EnvA). EnvA is synthesized as a precursor (Pr95) that is cleaved into two subunits (gp85 and gp37), which remain associated through a disulfide bond (18). The gp85 subunit (SU) possesses a receptor-binding domain, and the gp37 subunit (TM) possesses a transmembrane region and an internal hydrophobic fusion peptide. On the virion surface, EnvA exists as a trimer of SU/TM heterodimers, with the fusion peptides presumably buried within the trimer interface (14, 18, 22).
One or more “triggers” are required to activate viral fusion glycoproteins. In the cases studied, the trigger(s) causes the glycoprotein to undergo conformational changes that expose the fusion peptide for binding to the target membrane. Further conformational changes bring the apposed membranes together, causing the mixing of lipid leaflets and the opening of a fusion pore (27). For viruses such as influenza virus, low pH is the trigger (7, 17, 53, 59). For viruses that fuse at neutral pH, the trigger appears to be binding to one or more cell surface receptors (31).
The receptor for ASLV-A, Tva, possesses a 40-amino-acid low-density lipoprotein receptor (LDLR) motif that is sufficient to mediate viral entry (4, 47, 63). Previous data suggested that, like most retroviruses (37, 38, 54), ASLV is able to infect cells at neutral pH. For example, ASLV-C was shown to fuse with and infect cells in the presence of lysosomotropic agents (24). Also, soluble forms of the LDLR domain of Tva (2, 56) were shown to trigger a soluble trimer of the EnvA ectodomain to bind to liposomes at neutral pH. Liposome binding occurred at ≥22°C and was mediated by the fusion peptide (12, 28).
Recently, studies with ASLV have led to a third model for enveloped virus fusion with host cells (41). Key observations were that (i) lysosomotropic agents block ASLV-A and ASLV-B DNA synthesis, (ii) inhibition by lysosomotropic agents maps to ASLV Env, and (iii) cells expressing EnvA or EnvB only form large syncytia with receptor-expressing cells at low pH. Therefore, it was concluded that two consecutive triggers are required to activate ASLV Env for fusion: receptor binding at neutral pH and elevated temperature, followed by exposure to low pH. In view of this proposal, we have carefully evaluated the requirements for intact ASLV-A particles to bind to target bilayers and to merge their membranes with cellular membranes.
MATERIALS AND METHODS
EnvA-PI, cells, viruses, and receptor.
Phosphatidylinositol-phospholipase C (PI-PLC)-released EnvA (EnvA-PI) was prepared as described previously from NIH 3T3 cells that stably express glycosylphosphatidylinositol-anchored EnvA (23). DF-1 cells (American Type Culture Collection) were cultured in Dulbecco's modified Eagle medium-10% fetal bovine serum-1× antibiotic-antimycotic. ASLV-A was produced from DF-1 cells chronically infected with RCASBP(A)AP or RCASBP(A)GFP, as described previously (19). Thus the virus used here is equivalent to that used by Mothes and coworkers (41). At 18 h after being changed, the culture medium was collected, clarified (1,250 × g for 15 min), and concentrated by centrifugation through a 15% (wt/wt) sucrose cushion (2.5 h, 4°C, 82,700 × g) in an SW28 rotor. Viral pellets were resuspended in 200 μl of 20 mM MES (morpholineethanesulfonic acid)-20 mM HEPES (MES-HEPES), pH 7.4, overnight at 4°C. Pyrene-labeled ASLV-A was prepared by adding 1-pyrenehexadecanoic acid (Molecular Probes; 10 to 13 μg/ml) to the medium 24 h after plating the cells. After an additional 48 h, medium was replaced, and ASLV-A was harvested 18 h later as described above. To prepare pyrene-labeled Sindbis virus (51), DF-1 cells were plated and labeled as described above, except that, ∼19 h prior to harvest, cells were infected with Sindbis virus at a multiplicity of infection of 4 for 1 h. Fresh medium was added, and infected cells were incubated at 37°C. Eighteen hours later, medium was collected and clarified (1,250 × g for 15 min). Sindbis virus was concentrated by centrifugation (198,000 × g for 1 h at 4°C) in an SW41 rotor. Viral pellets were resuspended overnight in TNE (0.05 M Tris-HCl [pH 7.2], 0.1 M NaCl, 0.001 M EDTA) at 4°C and further purified on a Pfefferkorn gradient for 3 h at 198,000 × g and 4°C in an SW41 rotor (8). Pyrene-labeled Sindbis virus was harvested from the gradients and frozen in TNE. Only virus preparations with starting excimer/monomer ratios of 0.2 to 0.45 were used. The receptor (sTva47) was prepared as described previously (56).
Peptides.
Two peptides were synthesized at the University of Pennsylvania: (i) R102, with a sequence of SDHSESIQKKFQLMKEHVNKIGVDS (concentration for 50% inhibition of virus infectivity [IC50], ∼15 μg/ml), and (ii) R99, with a sequence of FNLSDHSESIQKKFQLMKEHVNKIG (IC50, 0.5 μg/ml). A preparation of R99 which was ∼6.5 times more potent was also made at the University of Virginia (see Fig. 5A). The difference in the efficacies of the two preparations may be related to their relative purities.
FIG. 5.
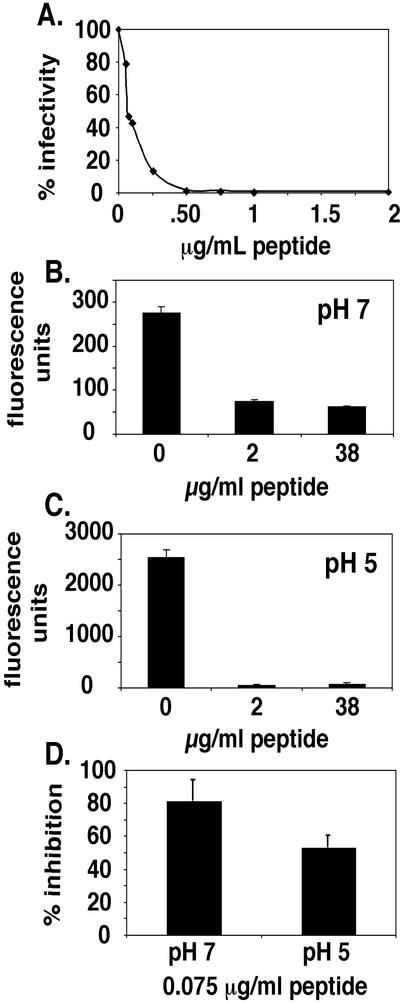
Characterization of the effects of a peptide designed to inhibit six-helix bundle formation in ASLV EnvA on infectivity and cell-cell fusion. (A) DF-1 cells were infected by ASLV-A in the presence of various concentrations of the University of Virginia preparation of R99. Cells were stained, and infected cells were counted; the graph indicates the percent infected cells in the absence of the peptide. (B) Cells expressing EnvA and cells expressing Tva were allowed to fuse in the presence of indicated concentrations of the University of Virginia preparation of R99 at pH 7. Results are expressed in terms of fluorescence units produced by the expression of β-galactosidase, with error bars representing the standard errors of the means (SEM). (C) Cell-cell fusion was performed as described for panel B, with the exception of a pH 5 shock (as described in Materials and Methods). (D) The IC50 of the University of Virginia preparation of peptide R99 (0.075 μg/ml) was tested for its effects on cell-cell fusion at pH 7 and 5. Results were quantitated as in panels B and C. Error bars, SEM.
Lipids and liposomes.
l-α-Phosphatidylcholine (PC; egg; Avanti) and cholesterol (chol; Sigma) were stored as described previously (28). Lipids (2:1 molar ratio of PC to chol) were mixed, dried under N2 gas in a glass tube, and lyophilized overnight. After addition of 1 ml of buffer (MES-HEPES; pH 7.4) with vortexing, liposomes were sonicated in a water bath sonicator and extruded 25 times through a 0.1-μm-pore-size filter in an Avanti Mini-Extruder. Liposomes were stored at 4°C and used within 1 week.
Virus-liposome binding assay.
Twenty-five microliters of ASLV-A and 0.5 μg of sTva47 were incubated on ice for 15 min. Twenty-five microliters of liposomes was added, and samples were incubated at 37°C for 30 min. Samples were layered on sucrose step gradients and centrifuged for 1 h at 197,000 × g in a TLA100 rotor. Three fractions were collected, resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose, and probed for matrix protein (MA) (16b).
EnvA-PI-liposome binding assay.
Twelve microliters of EnvA-PI and 0.29 μg of sTva47 were incubated on ice for 15 min. Eight microliters of liposomes was added, and samples were incubated at 37°C for 10 min. Samples were brought to a final concentration of 50% sucrose, and a step gradient was layered on top. Samples were centrifuged for 1 h at 197,000 × g in a TLA100 rotor. Six fractions were collected, resolved by SDS-PAGE, transferred to nitrocellulose, and probed for EnvA-PI with an antibody to the N terminus of the gp37 subunit (anti-Ngp37) (16b).
Pyrene virus-cell fusion assay.
The pyrene virus-cell fusion assay is adapted from that described by Smit et al. (51). DF-1 cells were plated at 106cells/3-cm-diameter dish. Twenty-one hours later, cells were washed twice with Hanks balanced salt solution-HEPES (H-H), pH 7.4 (32), and incubated in 1 ml of H-H for 15 min at 4°C. Pyrene-labeled ASLV-A (∼2.5 × 107 infectious units) was added to each dish in a final volume of 325 μl of H-H, pH 7.4. Following incubation at 4°C for 1 h (with rocking), the cells were washed twice with H-H. Approximately 8.5% (i.e., 2.125 infectious units/cell) of the ASLV-A particles remained bound. A set of cells was scraped in a final volume of 1 ml of H-H/dish, and fluorescence was immediately read in a Perkin-Elmer LS-5B fluorometer (excitation at 343 nm; emissions at 396 [monomer fluorescence] and 475 nm [excimer fluorescence]). A second set of cells was incubated at 37°C for 1 h. The buffer was replaced with 1 ml of H-H, the cells were removed, and fluorescence was analyzed. Lipid mixing was calculated as the percentage of excimer fluorescence decrease from 0 (at 37°C) to 60 min (at 37°C). Background cellular autofluorescence was subtracted. Bafilomycin (Sigma) was prepared as a 50 μM stock in dimethyl sulfoxide and stored in aliquots at −20°C.
Peptide IC50 determination.
DF-1 cells were plated in 12-well dishes at 100 μl/well (1/40 of total cell number) from a confluent 10-cm-diameter dish. Sixteen hours later, cells were washed two times with phosphate-buffered saline (with Ca2+ and Mg2+) (Cellgro). One milliliter of medium was added to each well. The R99 peptide (prepared at the University of Virginia) was added to each well. One hundred microliters of a 1:1,000 dilution of RCASBP(A)AP was added to each well, and the cells were incubated for 48 h at 37°C. Cells were developed for alkaline phosphatase expression according to previously published methods (30). Infected cells (expressing alkaline phosphatase) in each well were counted under a light microscope.
Pseudotype infectivity titers.
Infectivity assays were performed as described previously (14) except that receptor-bearing cells were incubated in media containing bafilomycin or NH4Cl for 1 h prior to addition of murine leukemia virus (MLV) particles pseudotyped with either ASLV EnvA or vesicular stomatitis virus G protein (VSV-G). At 5.5 h after addition of the virus, the cells were washed once with phosphate-buffered saline and once with medium and then incubated in inhibitor-free media for an additional 38 h prior to fixing and staining for β-galactosidase expression.
Expression of wild-type and dominant-negative dynamin.
Tetracycline-repressible HeLa cell line HtTA (13), stably expressing the ASLV-A receptor, Tva (HtTA/Tva), was generated by using vectors and methods previously described (22). Following single-cell cloning, cells expressing high levels of Tva were selected by fluorescence-activated cell sorter using an anti-Tva monoclonal antibody (C. Ochsenbauer-Jambor et al., unpublished data). HtTA/Tva cells were infected with recombinant adenovirus encoding either wild-type or dominant-negative (K44A) dynamin (under the control of a tetracycline-regulatable promoter) (1) at a multiplicity of infection of 25. Five hours later, cells were washed with cold H-H, pH 7.4, containing 1 μg of doxycycline/ml (H-H-D) to inhibit further dynamin expression. Cells were processed for lipid mixing with pyrene-labeled ASLV-A or Sindbis virus as described above in H-H-D.
Cell-cell fusion: β-galactosidase reporter assay.
A β-galactosidase reporter gene assay modified from that described by Nussbaum et al. (45) was used as previously described (15) to assess the ability of EnvA-expressing 293T cells to fuse with Tva-expressing NIH 3T3 cells. Briefly, 293T cells were transfected with 10 μg each of pOS8 (lacZ under the control of the T7 promoter) and pCB6-EnvA. Cells were induced with 10 mM sodium butyrate 30 h posttransfection (p.t.) and harvested 48 h p.t. Cells were plated onto PG950 cells which had been infected with MVA (avian vaccinia virus modified to express T7 polymerase) 16 h earlier and incubated at 31°C in the presence of 100 μg of rifampin/ml. The mixed cells were incubated for 4 h at 37°C in media containing 100 μg of rifampin and 150 μg of cytosine 1-β-d-arabinofuranoside (AraC) (RAM)/ml. For experiments utilizing the heptad repeat peptide inhibitor (see Fig. 5B to D), this incubation also included peptide R99 (prepared at the University of Virginia). Cells were washed and either exposed to pH 5.0 or maintained at neutral pH for 5 min (in the presence or absence of a peptide), reneutralized if necessary, washed, and incubated for a further 16 h at 37°C in RAM (containing the peptide). Cells were fixed and stained for β-galactosidase activity.
RESULTS
Receptor binding is sufficient to cause ASLV-A particles to bind hydrophobically to target membranes at neutral pH.
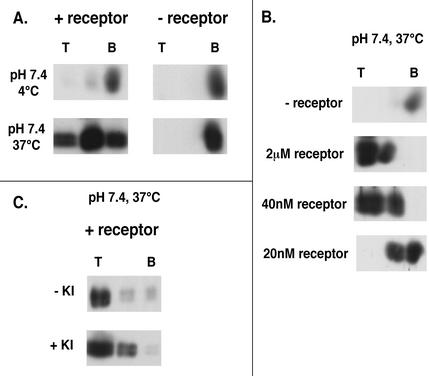
We previously showed that soluble forms of the EnvA receptor (sTva47 and sTva) can induce a soluble EnvA ectodomain (EnvA-PI) to bind hydrophobically to liposomes at neutral pH at ≥22°C (12, 28). Here, we asked whether sTva47 could induce intact ASLV-A particles to do the same. As seen in Fig. 1A, if samples containing virus and liposomes were maintained at 4°C and neutral pH, virus pelleted to the bottom of a sucrose gradient, whether or not it was preincubated with the receptor (top). ASLV-A that was not preincubated with the receptor but that was incubated at 37°C also pelleted to the bottom of the gradient (bottom right). In contrast, ASLV-A preincubated with sTva47 and then incubated at 37°C at neutral pH was largely retained in the upper fractions of the gradient (bottom left). As seen in Fig. 1B, the binding of ASLV-A to liposomes was triggered with very low concentrations of receptor (40 nM); the measured KD for receptor binding to EnvA-expressing cells is 17 ± 2 nM (14). Furthermore, receptor- and temperature-dependent binding of ASLV-A particles to liposomes at neutral pH was largely resistant to treatment with 1 M KI, indicating that it was a tight, hydrophobic interaction (Fig. 1C).
FIG. 1.
Characteristics of virus-liposome binding at neutral pH. (A) ASLV-A was incubated with (+ receptor) or without (− receptor) sTva47 at 4°C. Following addition of liposomes, samples were incubated at either 4 (top) or 37°C (bottom) for 30 min and then analyzed as described in Materials and Methods by using an antibody to the viral matrix protein (MA). This receptor-dependent interaction at 37°C was also observed when blots were probed with an antibody to the cytoplasmic tail of EnvA (data not shown). T and B, top and bottom gradient fractions, respectively. (B) ASLV-A was incubated with the indicated concentrations of receptor at 4°C for 15 min. After addition of liposomes, the samples were shifted to 37°C for 30 min and then analyzed as described for panel A. (C) Virus samples preincubated with sTva47 were processed as described for panel A except that, prior to being loaded onto sucrose step gradients, samples were (bottom) or were not (top) incubated with 1 M KI for 5 min on ice and then analyzed as described in Materials and Methods.
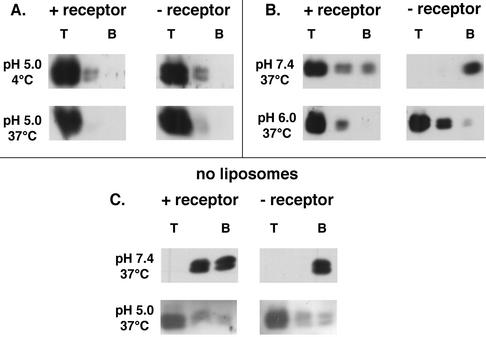
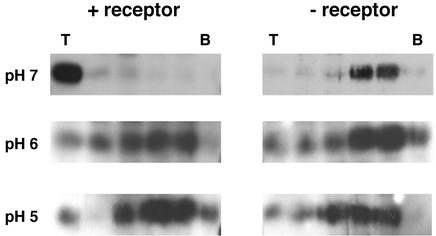
We next explored the behavior of ASLV-A in the presence of liposomes at pH 5.0. As seen in Fig. 2A, at pH 5.0, ASLV-A remained in the top fraction of the gradient whether or not it had been incubated with the receptor and whether the samples were maintained at 4°C (top) or incubated at 37°C (bottom). In samples treated at pH 6.0, ASLV-A was also largely retained in the top fraction (Fig. 2B). Even in the absence of liposomes, virus treated at pH 5.0, in the presence or absence of the receptor at 37°C, remained in the top fraction (Fig. 2C, bottom). As expected, virus that was maintained at pH 7.4 without the receptor or liposomes (at 37°C) pelleted to the bottom of the gradient; virus that was incubated with the receptor at pH 7.4 in the absence of liposomes (at 37°C) was not found in the top fraction, but some was found in the middle fraction (Fig. 2C, top). Therefore, receptor- and temperature-dependent binding of ASLV-A to liposomes occurred only when samples were maintained at or above neutral pH. Results at pH 8.0 were the same as those at pH 7.4 (data not shown). As seen in Fig. 3, similar results were obtained with the soluble form of the EnvA ectodomain, EnvA-PI, which bound to liposomes in a receptor-dependent manner at pH 7.4 (top) but in a receptor-independent manner at pH 6.0 (middle) and 5.0 (bottom).
FIG. 2.
Effect of low pH on the behavior of ASLV-A particles in the presence (A and B) and absence (C) of liposomes. (A) Samples were prepared and analyzed as described in the legend to Fig. 1A except that they were acidified to pH 5.0 with 0.1 M HCl immediately after the addition of liposomes and then reneutralized with 0.1 M NaOH after a 30-min incubation at 4 or 37°C. Samples were analyzed as described in Materials and Methods with an antibody to MA. Samples probed with an antibody to the cytoplasmic tail of EnvA gave similar results (data not shown). T and B are as defined for Fig. 1A. (B) Samples were prepared and analyzed as described in the legend to Fig. 1A and either maintained at pH 7.4 (top) or acidified to pH 6.0 (bottom). (C) Samples were prepared and analyzed as described in the legend to Fig. 1A except that 25 μl of MES-HEPES, pH 7.4, was substituted for liposomes and samples were either maintained at neutral pH (top) or acidified to pH 5.0 (bottom) for the 37°C incubation. Acidified samples were then reneutralized prior to loading onto sucrose step gradients, as in panel A.
FIG. 3.
The pH profile of EnvA-PI binding to liposomes. EnvA-PI, a soluble form of the EnvA ectodomain, was incubated with or without sTva47 at 4°C for 15 min. Following addition of liposomes, samples were either maintained at pH 7.4 (top) or acidified to pH 6.0 (middle) or 5.0 (bottom) and incubated at 37°C for 10 min. Samples were reneutralized, processed, and analyzed as described in Materials and Methods. T and B are as defined for Fig. 1A.
Low pH neither increases nor inhibits fusion (lipid mixing) of ASLV-A with host cells.
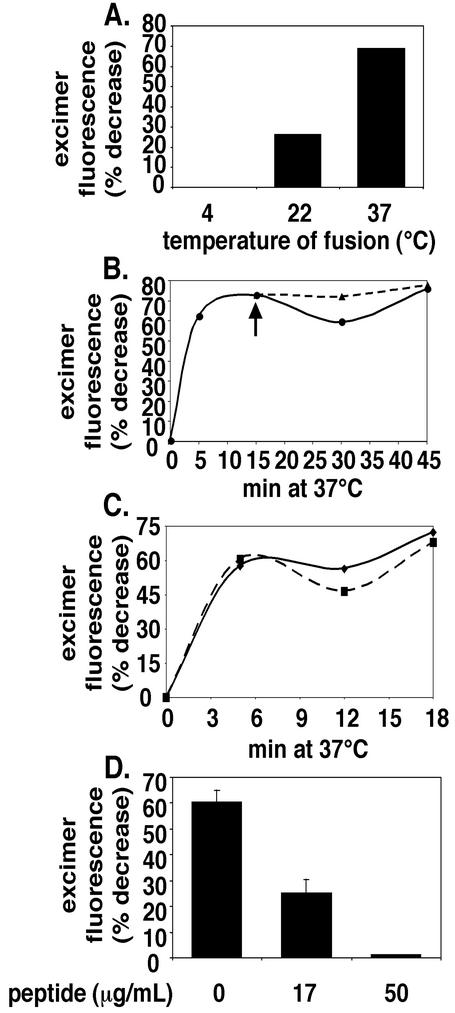
We next studied the fusion of pyrene-labeled ASLV-A with DF-1 cells by adapting methods developed to study the fusion of Sindbis and Semliki Forest viruses (32, 51). As seen in Fig. 4A, transfer of lipids from ASLV-A occurred optimally at >22°C; no lipid mixing occurred when samples were maintained at 4°C. Transfer of R18 from ASLV-C to chicken embryo fibroblast cells occurs with the same temperature profile (24). As seen in Fig. 4B, significant transfer of lipids from ASLV-A to DF-1 cells occurred within 5 min and transfer was complete within 10 to 15 min at 37°C at neutral pH. The extent of fusion was not enhanced if samples were shifted to pH 5.0 after 15 min at neutral pH (Fig. 4B). We incubated for 15 min at neutral pH before shifting to low pH, as Mothes and coworkers indicated that a 15-min neutral-pH incubation is required in order for low pH to overcome the bafilomycin block to ASLV DNA synthesis (41). As seen in Fig. 4C, a direct warm-up at pH 5.0 also did not significantly enhance or inhibit the extent of lipid transfer from prebound ASLV-A particles.
FIG. 4.
Characterization of fusion of pyrene-labeled ASLV-A with DF-1 cells. (A) Virus was bound to cells at 4°C. Unbound virus was removed by washing, and samples were incubated at the indicated temperatures for 1 h and then analyzed for lipid mixing. (B) Pyrene-labeled ASLV-A was bound to DF-1 cells at 4°C. Unbound virus was removed by washing, and samples were incubated at 37°C in neutral pH buffer for 15 min. Virus-cell complexes were then either maintained at pH 7.4 (solid line) or incubated at pH 5.0 (dashed line, arrow) for the remainder of the 37°C incubation. Samples were processed and analyzed for lipid mixing at the indicated times as described in Materials and Methods. (C) Samples were treated as in panel B, with the exception that, following the washing of unbound virus from cells, samples were warmed directly at pH 7.4 (solid line) or 5.0 (dashed line). (D) Virus-cell complexes were prepared at 4°C as for panel A, incubated for 1 h at 37°C in the absence or presence of the indicated concentrations of the University of Pennsylvania preparation of peptide R99 and then analyzed for lipid mixing. Error bars, standard errors of the means from two experiments.
A heptad repeat peptide inhibits fusion of ASLV-A with cells.
Peptides corresponding to the C-terminal heptad repeats within the six-helix bundles of several class I viral fusion proteins that function at neutral pH have been shown to inhibit virus infectivity and fusion. These include peptides from retroviruses (e.g., HIV, simian immunodeficiency virus, and feline immunodeficiency virus) (21, 35, 39, 40, 43, 61) and several paramyxoviruses (e.g., simian virus 5, respiratory syncytial virus, human parainfluenza virus 3, measles virus, and henipaviruses) (6, 34, 49). Therefore, we chose to examine the effects of a similar peptide (peptide R99), designed to inhibit six-helix bundle formation in ASLV EnvA on ASLV-A fusion and infection. The sequence of peptide R99 (given in Materials and Methods) was derived from the predicted C-terminal helix of the six-helix bundle of EnvA (20, 44). The peptide prepared at the University of Pennsylvania inhibited ASLV-A infectivity with an IC50 of 0.5 μg/ml (167 nM) (44). As seen in Fig. 5A, peptide R99 prepared at the University of Virginia inhibited virus infectivity with an IC50 of ∼0.075 μg/ml (25 nM). We presume that the higher potency (∼6.5-fold) of the latter peptide is due to its greater purity. We next tested whether the peptide has any effect on virus binding to cells by using pyrene-labeled ASLV-A. The peptide did not inhibit virus binding to target cells, as the number of pyrene fluorescence units bound to cells following removal of unbound virus in the presence of the peptide (9.63 ± 0.40 U) was equivalent to the number in its absence (9.4 ± 0.53 U). We next examined the effects of the peptide in the virus-cell fusion assay. As seen in Fig. 4D, peptide R99 inhibited the transfer of pyrene from prebound ASLV-A particles to DF-1 cells in a concentration-dependent manner. In addition to further defining the mechanism of ASLV-A fusion, this heptad repeat peptide thus served as a control to exclude the possibility of nonspecific transfer of pyrene from ASLV-A to DF-1 cells (Fig. 4D). We observed ∼60% inhibition of fusion of pyrene-labeled virus with cells with 17 μg of peptide R99 (from the University of Pennsylvania)/ml. Therefore, more of the peptide (∼30-fold) is needed to inhibit lipid mixing than to inhibit infectivity. Similar results were obtained with a heptad repeat peptide inhibitor of HIV entry (43). We also tested the effects of peptide R99 on EnvA-mediated cell-cell fusion with receptor-bearing cells. As seen in Fig. 5B and C, peptide R99 inhibited cell-cell fusion at pH 7 and 5 with similar efficiencies, even though more (∼10-fold) cell-cell fusion occurred at pH 5 than at pH 7. Note that the fluorescence units (representing β-galactosidase activity) were brought to similar background levels in the presence of the peptide at both pH 7 and 5. Similar results by using a modified gene reporter cell-cell fusion assay were obtained independently (44). When we compared the efficacies of the peptide at its IC50 for inhibiting cell-cell fusion, we found that it inhibited cell-cell fusion at neutral and low pHs by 81 and 53%, respectively (Fig. 5D).
Lipid mixing of ASLV-A particles with host cells does not require endosomal acidification.
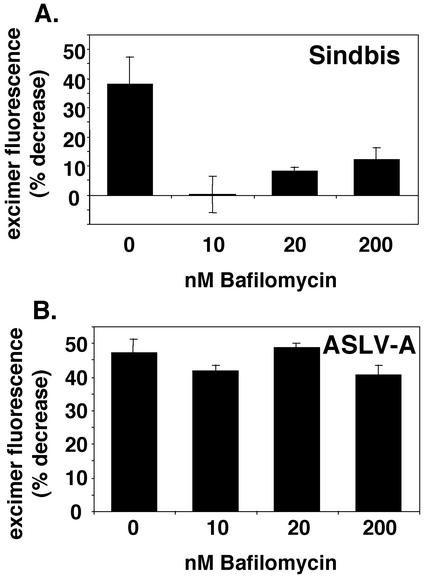
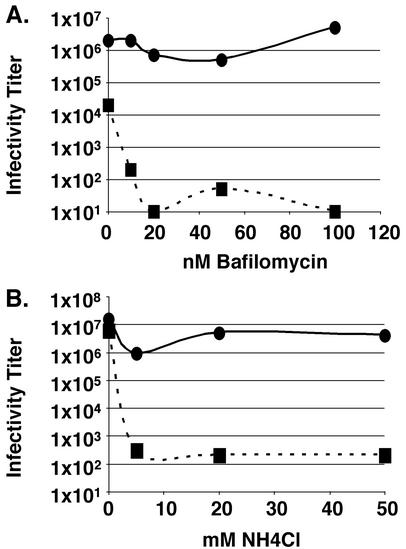
Low concentrations of bafilomycin (a vacuolar H+-transporting ATPase inhibitor) inhibit entry of viruses that require exposure to low pH to infect cells (25). As seen in Fig. 6A, as little as 10 nM bafilomycin potently inhibited transfer of pyrene-labeled lipids from Sindbis virus to DF-1 cells. In sharp contrast, even concentrations of bafilomycin as high as 200 nM had virtually no effect on the transfer of pyrene-labeled lipids from ASLV-A to DF-1 cells (Fig. 6B). We note that 10 nM bafilomycin is sufficient to neutralize the pH of DF-1 cell endosomes, as assessed by staining with acridine orange (L. J. Earp and J. M. White, unpublished data). Additionally, low concentrations of bafilomycin significantly inhibit the infectivity of MLV pseudotypes bearing the VSV-G protein (an envelope protein which requires low pH to initiate fusion), while having minimal effect on the infectivity of MLV pseudotypes bearing ASLV EnvA (Fig. 7A). As seen in Fig. 7B, patterns of infectivity similar to those observed for bafilomycin-treated samples were seen for MLV pseudotypes bearing ASLV EnvA or VSV-G in the presence of NH4Cl. We note that 5 mM NH4Cl is sufficient to neutralize the endosomes of DF-1 cells (L. J. Earp and J. M. White, unpublished data).
FIG. 6.
Effect of bafilomycin on fusion of pyrene-labeled Sindbis virus (A) and ASLV-A (B) with DF-1 cells. DF-1 cells were pretreated for 30 min at 37°C with the indicated concentration of bafilomycin. Cells were maintained in the indicated concentration of bafilomycin for the duration of the experiment. Pyrene-labeled Sindbis virus (A) or ASLV-A (B) was then bound to the cells for 1 h at 4°C. Following washing, the cells were incubated for 1 h at 37°C and then analyzed for lipid mixing as described in Materials and Methods. Error bars, standard errors of the means from two to eight replicate experiments.
FIG. 7.
Effect of bafilomycin and NH4Cl on the infectivity of MLV pseudotyped virus bearing ASLV EnvA or VSV-G. NIH 3T3 cells expressing the ASLV-A receptor, Tva, were pretreated with the indicated concentrations of bafilomycin (A) or NH4Cl (B) for 1 h prior to the addition of MLV pseudotypes bearing either ASLV EnvA (solid line) or VSV-G (dashed line). Bafilomycin or NH4Cl was removed 5.5 h postinfection, and samples were processed as described in Materials and Methods.
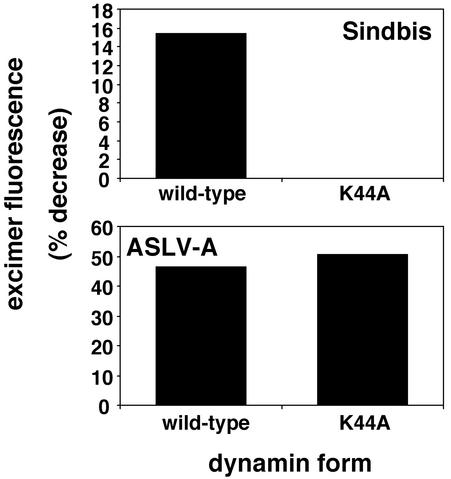
Dominant-negative dynamin inhibits lipid mixing of Sindbis virus, but not ASLV-A, with HtTA/Tva cells.
Dominant-negative dynamin inhibits entry of viruses, such as Sindbis virus and influenza virus, which require endocytosis in clathrin-coated vesicles (16, 60). Dominant-negative dynamin can also inhibit virus entry through caveolae (50). As seen in Fig. 8, whereas dominant-negative dynamin (K44A) inhibited transfer of pyrene-labeled lipids from Sindbis virus to HtTA/Tva cells (top), it did not inhibit transfer of pyrene-labeled lipids from ASLV-A to HtTA/Tva cells (bottom).
FIG. 8.
Effect of dominant-negative dynamin on fusion of pyrene-labeled Sindbis virus (top) and ASLV-A (bottom). Dominant-negative (K44A) or wild-type dynamin was expressed in cells by using an adenovirus expression system as described in Materials and Methods. Pyrene-labeled Sindbis virus (top) or ASLV-A (bottom) was bound to dynamin-expressing HtTA/Tva cells, and lipid mixing was assessed as described in Materials and Methods. The experiment was repeated three times with similar results.
The ability to form syncytia at low pH does not correlate with the ability to mediate infection.
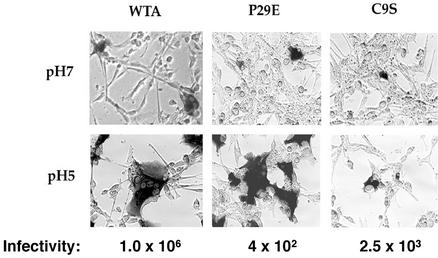
To further assess the role of pH in EnvA-mediated fusion, we examined the ability of cells expressing EnvA to form syncytia at neutral pH and after exposure to low pH. As seen in Fig. 9, at neutral pH, small syncytia of 2 to 4 cells formed. Low pH induced the formation of large syncytia. This correlates well with the greater extent (∼10-fold) of cell-cell fusion at low pH than at neutral pH seen in the β-galactosidase gene reporter assay (Fig. 5B and C). We next studied the cell-cell fusion properties of an EnvA bearing a substitution at the central proline of the fusion peptide (EnvA P29E) and an EnvA defective in forming a critical disulfide bond predicted to stabilize the fusion peptide (EnvA C9S). Both of these mutants were previously shown to be significantly impaired (∼3 to 4 log units) in their ability to support infection (14, 15); both showed reduced cell-cell fusion activity at neutral pH (approximately threefold for P29E and approximately sixfold for C9S) in the β-galactosidase gene reporter assay (S. E. Delos and J. M. White, unpublished results). EnvA P29E and EnvA C9S induced small syncytia (e.g., two to four nuclei) at neutral pH (Fig. 9, top), but the total number of fusion events was less than that observed for wild-type EnvA (WTA). In contrast, both WTA and EnvA P29E induced massive syncytia after treatment with low pH, whereas EnvA C9S did not (bottom).
FIG. 9.
Syncytium-forming activity and infectivity of wild-type and mutant EnvA proteins. Cells expressing either WTA (left), an EnvA fusion peptide mutant (P29E) (middle), or an EnvA mutant that cannot form a critical disulfide bond in the TM subunit (C9S) (right) were plated onto NIH 3T3 cells expressing Tva. Cells were either maintained at pH 7.4 (top) or subjected to pH 5.0 media for 5 min (bottom) and then processed for cell-cell fusion activity as described in Materials and Methods. The ability of each EnvA protein to support infection of MLV pseudotyped particles is indicated below each panel. Infectivity data are from references 14 and 15. The value for WTA is an average of the values reported in the two references.
DISCUSSION
Viral fusion proteins have been segregated into two classes based on structural criteria (26, 33). Class I viral fusion proteins, typified by influenza virus hemagglutinin (HA), employ a six-helix bundle fusion mechanism. Class II viral fusion proteins, typified by the E glycoprotein of tick-borne encephalitis virus, use a “dimer-to-trimer” fusion mechanism. Class I fusion proteins are further categorized into those that are activated by exposure to low pH (e.g., influenza virus HA) and those that are activated by interaction with receptors at neutral pH (e.g., the HIV Env glycoprotein). Recently, there was a proposal for a third type of class I fusion protein of Env, for which activation requires interaction with the receptor(s) at neutral pH, followed by exposure to low pH (41). The new model was based largely on studies using a PCR assay to monitor reverse transcript production following ASLV entry into cells, as well as microscopic analysis of syncytium formation between cells that express ASLV Env and cells that express the cognate ASLV receptor.
We previously showed that ASLV EnvA can be triggered to change conformation (22) and bind hydrophobically to target membranes (12, 28) solely by interaction with its receptor, Tva, at neutral pH (and at ≥22°C). We also showed that R18-labeled ASLV-C fuses with host cells at neutral pH (24). Possible caveats to our prior results were the use of a genetically engineered trimeric EnvA ectodomain (EnvA-PI) for the lipid binding studies and the use of exogenously labeled R18 ASLV-C in the fusion studies (41). In light of these caveats and the new model for ASLV fusion with cells, we examined whether the binding of intact ASLV particles or fusion of biosynthetically labeled ASLV particles with target membranes requires low pH. We found that (i) intact ASLV-A particles can be triggered to bind hydrophobically to target membranes by interaction with nanomolar concentrations of Tva at neutral pH and at ≥22°C (Fig. 1), (ii) intact ASLV-A particles biosynthetically labeled with pyrene require neither endosomal low pH (Fig. 6) nor dynamin-mediated endocytosis (Fig. 8) to fuse with cells, (iii) a peptide designed to inhibit six-helix bundle formation in the EnvA ectodomain fully inhibits membrane merger between ASLV-A particles and target cells (Fig. 4D) as well as cell-cell fusion occurring at either neutral pH or low pH (Fig. 5B to D), (iv) low-pH incubation neither enhances nor inhibits membrane merger of ASLV-A with target cells (Fig. 4B and C), and (v) cells expressing EnvA can fuse with cells expressing Tva at neutral pH (Fig. 5B and 9). Although more cell-cell fusion occurs at low pH (Fig. 5C and 9), the ability of EnvA-expressing cells to form large syncytia with Tva-expressing cells at low pH does not always correlate with the ability of EnvA to mediate infection (Fig. 9).
Model for activation of ASLV EnvA.
We interpret our results as evidence that the EnvA ectodomain can be activated to a fusogenic conformation at neutral pH. Particularly strong lines of evidence include the observations that classic inhibitors (i.e., bafilomycin and dominant-negative dynamin) of low-pH-activated viruses have no effect on fusion of pyrene-labeled ASLV-A with DF-1 cells (Fig. 6 and 8). Moreover, a peptide (R99) designed to inhibit six-helix bundle formation in EnvA completely blocks this process (Fig. 4D). Peptide R99 inhibits ASLV-A infection of DF-1 cells (Fig. 5A) but does not block the binding of ASLV-A to DF-1 cells (see Results) or Tva-induced binding of ASLV-A to liposomes (44; R. C. Netter and L. J. Earp, unpublished data). Hence, in the presence of peptide R99, the virus can make very close contact with the target bilayer, yet no lipid transfer occurs (i.e., the peptide inhibits lipid mixing after Env has been activated for fusion). In addition, peptide R99 is an effective inhibitor of content mixing as assessed by a cell-cell fusion assay (Fig. 5B). The peptide is similarly effective at inhibiting cell-cell fusion at neutral and low pH (Fig. 5B to D).
Therefore, we propose that, following interaction with Tva at neutral pH (at ≥22°C), EnvA is converted to a prehairpin intermediate which binds hydrophobically through its fusion peptides (3, 14) to target membranes and then folds back into a six-helix bundle, which mediates membrane merger. Conversion to the prehairpin intermediate is insensitive, while conversion to the six-helix bundle is sensitive, to the peptide. Our findings therefore suggest that the EnvA ectodomain does not require exposure to low pH to convert to a low-energy, fusogenic form (i.e., a six-helix bundle). Furthermore, the potency of peptide R99 in inhibiting virus infectivity (IC50, ∼25 nM) is similar to those of heptad repeat peptides that inhibit viruses (many retroviruses and paramyxoviruses) that fuse at neutral pH. In contrast, much higher concentrations (IC50, ∼1 mM) of the analogous heptad repeat peptide were needed to inhibit the infectivity of Ebola virus (57), a virus that is thought to infect cells through a low-pH compartment (10, 55, 62).
We note that ASLV-A is not precluded from fusing with cells (Fig. 4B and C) or inducing cell-cell fusion (Fig. 5C and 9) at low pH, indicating that at least some of the EnvA glycoproteins can undergo conformational changes that initiate fusion at low pH. This is supported by the fact that some Envs can bind to liposomes at low pH (Fig. 3). Some Envs can also form a TM oligomer at low pH (41) that resembles the TM oligomer formed at neutral pH in the presence of the receptor and target membranes at ≥22°C (44). However, Env also binds to liposomes (Fig. 3) and TM oligomers also form at low pH under nonfusogenic conditions (i.e., on ice or in the absence of the receptor) (41). These findings indicate that low pH may act as an alternate, nonessential means of activating EnvA. Previous studies have shown that denaturants (e.g., urea, heat, and low concentrations of SDS) can cause conformational changes in envelope glycoproteins that resemble those necessary for fusion (9, 22, 46, 48, 58).
Possible roles for low pH in the fusion and entry of avian retroviruses.
The continued presence of 200 nM bafilomycin, an inhibitor of the vacuolar H+-transporting ATPase, inhibits production of reverse transcripts from the ASLV genome (41). This finding led to the interpretation that endosomal pH is required for fusion and hence entry of the ASLV genome into cells (41). However, 200 nM bafilomycin is 20-fold higher than the concentration necessary to inhibit endosomal acidification in DF-1 cells (data not shown), and such high concentrations of bafilomycin can affect other cellular processes (5, 11, 52). Nonetheless, we consider four possible roles for low pH in ASLV entry (16a, 41).
The first possibility is that EnvA proceeds to hemifusion (27) at neutral pH but requires low pH to open a fusion pore. We consider this unlikely for several reasons. First, the pyrene labeling procedure should label both leaflets of the viral membrane equally, and our data reveal the same amount of lipid mixing (pyrene excimer decrease) regardless of whether ASLV-A particles are incubated with cells in the absence or presence of bafilomycin (Fig. 6B). Second, EnvA-mediated lipid mixing with Tva-expressing cells is relatively fast and is not enhanced or inhibited by treatment with low pH (Fig. 4B and C). Third, EnvA glycoproteins are capable of opening fusion pores at neutral pH of sufficient size to allow the passage of plasmids encoding β-galactosidase into target cells expressing the receptor (Fig. 5B and 9) (15, 29). Furthermore, our evidence suggesting that EnvA six-helix bundles form at neutral pH (in the presence of target membranes) coupled with recent evidence that six-helix bundle formation is only complete for HIV Env once robust fusion pores have formed (36) suggests that EnvA-mediated fusion proceeds to pore formation at neutral pH.
The second possibility is that small fusion pores form at neutral pH but that low pH is required to enlarge fusion pores or to enhance their enlargement. This might involve augmenting lateral interactions among activated EnvA trimers. The fact that low pH enhances the extent of syncytium formation between EnvA-expressing and Tva-expressing cells (Fig. 5C and 9) suggests a possible role for low pH in fusion pore enlargement; however, a caveat to this interpretation is that the cell-cell fusion activity of EnvA proteins does not always correlate with their ability to mediate infection (Fig. 9) (R. C. Netter and P. Bates, unpublished results; S. E. Delos and J. M. White, unpublished results).
The third possibility is that low pH is not required for fusion but rather for viral uncoating. For example, in a manner reminiscent of what is found for influenza virus, low pH may be required to alter internal viral proteins, thereby facilitating uncoating (42, 64). A first hint that this may be the case is that the sedimentation behavior of ASLV-A particles changes following exposure to low pH (in the absence of target membranes) (Fig. 2C). The fact that the bafilomycin sensitivity of reverse transcript production mapped to the Env glycoprotein in pseudotype experiments (41) is not inconsistent with this third possibility, as there may be an as yet undefined role for Env in viral uncoating.
The fourth possibility is that ASLV-A must enter at a specific site on the plasma membrane or through specific cellular compartments in order to optimize subsequent steps of replication (e.g., initiation of reverse transcription or transport to the nucleus). Thus, inhibition of ASLV DNA synthesis by bafilomycin or NH4Cl might be due to its effects on other cellular processes, including endocytic trafficking (5). This idea may also explain why the low-pH requirement mapped to Env (41); a different glycoprotein might direct virus entry at a different location in the cell.
In summary, our findings strongly indicate that interaction with low concentrations of the receptor (Fig. 1B) at neutral pH is sufficient to trigger EnvA on ASLV-A particles to bind tenaciously to target membranes and to mediate the lipid mixing stage of virus-cell fusion (Fig. 6), as well as allow the passage of a reporter gene through a fusion pore (Fig. 5C, 7, and 9). As low pH neither enhances nor inhibits lipid mixing of ASLV with target cells (Fig. 4B and C), our data indicate that, while ASLV-A is not precluded from fusing in a low-pH compartment, it does not need to be exposed to low pH in order to fuse. Future work will address whether low pH enhances the expansion of fusion pores that form during fusion between virus particles and target membranes or whether it is required for postfusion events.
Acknowledgments
We thank Jolanda Smit, Jan Wilschut, and Margaret Kielian for guidance in producing pyrene-labeled virus, Mark Federspiel for RCASBP(A)AP/DF-1 cells and RCASBP(A)GFP/DF-1 cells, Bob Johnston for Sindbis virus TR339, and David Agard for a construct encoding sTva47. We also thank Volker Vogt for anti-MA and helpful discussions, Yoram Altschuler for adenoviruses encoding wild-type and dominant-negative dynamin, Jim Casanova for HtTA cells, and Chang Hahn for guidance with the preparation of Sindbis virus.
This work was funded by NIH grants A122470 (J.M.W.) and CA76256 (P.B.). L.J.E. was supported in part by NIH training grants 5T32 CA09109 and 5T32 A107046.
REFERENCES
- 1.Altschuler, Y., S. M. Barbas, L. J. Terlecky, K. Tang, S. Hardy, K. E. Mostov, and S. L. Schmid. 1998. Redundant and distinct functions for dynamin-1 and dynamin-2 isoforms. J. Cell Biol. 143:1871-1881. [DOI] [PMC free article] [PubMed]
- 2.Balliet, J. W., J. Berson, C. M. D'Cruz, J. Huang, J. Crane, J. M. Gilbert, and P. Bates. 1999. Production and characterization of a soluble, active form of Tva, the subgroup A avian sarcoma and leukosis virus receptor. J. Virol. 73:3054-3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balliet, J. W., K. Gendron, and P. Bates. 2000. Mutational analysis of the subgroup A avian sarcoma and leukosis virus putative fusion peptide domain. J. Virol. 74:3731-3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bates, P., J. A. Young, and H. E. Varmus. 1993. A receptor for subgroup A Rous sarcoma virus is related to the low density lipoprotein receptor. Cell 74:1043-1051. [DOI] [PubMed] [Google Scholar]
- 5.Bayer, N., D. Schober, E. Prchla, R. F. Murphy, D. Blaas, and R. Fuchs. 1998. Effect of bafilomycin A1 and nocodazole on endocytic transport in HeLa cells: implications for viral uncoating and infection. J. Virol. 72:9645-9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bossart, K. N., L.-F. Wang, M. N. Flora, K. B. Chua, S. K. Lam, B. T. Eaton, and C. C. Broder. 2002. Membrane fusion tropism and heterotypic functional activities of the Nipah virus and Hendra virus envelope glycoproteins. J. Virol. 76:11186-11198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bullough, P. A., F. M. Hughson, J. J. Skehel, and D. C. Wiley. 1994. Structure of influenza haemagglutinin at the pH of membrane fusion. Nature 371:37-43. [DOI] [PubMed] [Google Scholar]
- 8.Burge, B. W., and E. R. Pfefferkorn. 1967. Temperature-sensitive mutants of Sindbis virus: biochemical correlates of complementation. J. Virol. 1:956-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carr, C. M., C. Chaudhry, and P. S. Kim. 1997. Influenza hemagglutinin is spring-loaded by a metastable native conformation. Proc. Natl. Acad. Sci. USA 94:14306-14313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chazal, N., G. Singer, C. Aiken, M. L. Hammarskjold, and D. Rekosh. 2001. Human immunodeficiency virus type 1 particles pseudotyped with envelope proteins that fuse at low pH no longer require Nef for optimal infectivity. J. Virol. 75:4014-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clague, M. J., S. Urbe, F. Aniento, and J. Gruenberg. 1994. Vacuolar ATPase activity is required for endosomal carrier vesicle formation. J. Biol. Chem. 269:21-24. [PubMed] [Google Scholar]
- 12.Damico, R. L., J. Crane, and P. Bates. 1998. Receptor-triggered membrane association of a model retroviral glycoprotein. Proc. Natl. Acad. Sci. USA 95:2580-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Damke, H., T. Baba, D. E. Warnock, and S. L. Schmid. 1994. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J. Cell Biol. 127:915-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delos, S. E., J. M. Gilbert, and J. M. White. 2000. The central proline of an internal viral fusion peptide serves two important roles. J. Virol. 74:1686-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delos, S. E., and J. M. White. 2000. Critical role for the cysteines flanking the internal fusion peptide of avian sarcoma/leukosis virus envelope glycoprotein. J. Virol. 74:9738-9741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeTulleo, L., and T. Kirchhausen. 1998. The clathrin endocytic pathway in viral infection. EMBO J. 17:4585-4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16a.Diaz-Griffero, F., S. A. Hoschander, and J. Brojatsch. 2002. Endocytosis is a critical step in entry of subgroup B avian leukosis viruses. J. Virol. 76:12866-12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16b.Earp, L. J., L. D. Hernandez, S. E. Delos, and J. M. White. Receptor-activated binding of viral fusion proteins to target membranes. In N. Duzgunes (ed.), Methods in enzymology: liposomes, in press. Academic Press, San Diego, Calif. [DOI] [PMC free article] [PubMed]
- 17.Eckert, D. M., and P. S. Kim. 2001. Mechanisms of viral membrane fusion and its inhibition. Annu. Rev. Biochem. 70:777-810. [DOI] [PubMed] [Google Scholar]
- 18.Einfeld, D., and E. Hunter. 1988. Oligomeric structure of a prototype retrovirus glycoprotein. Proc. Natl. Acad. Sci. USA 85:8688-8692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Federspiel, M. J., and S. H. Hughes. 1997. Retroviral gene delivery. Methods Cell Biol. 52:179-214. [PubMed] [Google Scholar]
- 20.Gallaher, W. R. 1996. Similar structural models of the transmembrane proteins of Ebola and avian sarcoma viruses. Cell 85:477-478. [DOI] [PubMed] [Google Scholar]
- 21.Gallo, S. A., A. Puri, and R. Blumenthal. 2001. HIV-1 gp41 six-helix bundle formation occurs rapidly after the engagement of gp120 by CXCR4 in the HIV-1 Env-mediated fusion process. Biochemistry 40:12331-12336. [DOI] [PubMed] [Google Scholar]
- 22.Gilbert, J. M., L. D. Hernandez, J. W. Balliet, P. Bates, and J. M. White. 1995. Receptor-induced conformational changes in the subgroup A avian leukosis and sarcoma virus envelope glycoprotein. J. Virol. 69:7410-7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilbert, J. M., L. D. Hernandez, T. Chernov-Rogan, and J. M. White. 1993. Generation of a water-soluble oligomeric ectodomain of the Rous sarcoma virus envelope glycoprotein. J. Virol. 67:6889-6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilbert, J. M., D. Mason, and J. M. White. 1990. Fusion of Rous sarcoma virus with host cells does not require exposure to low pH. J. Virol. 64:5106-5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glomb-Reinmund, S., and M. Kielian. 1998. The role of low pH and disulfide shuffling in the entry and fusion of Semliki Forest virus and Sindbis virus. Virology 248:372-381. [DOI] [PubMed] [Google Scholar]
- 26.Heinz, F. X., and S. L. Allison. 2001. The machinery for flavivirus fusion with host cell membranes. Curr. Opin. Microbiol. 4:450-455. [DOI] [PubMed] [Google Scholar]
- 27.Hernandez, L. D., L. R. Hoffman, T. G. Wolfsberg, and J. M. White. 1996. Virus-cell and cell-cell fusion. Annu. Rev. Cell Dev. Biol. 12:627-661. [DOI] [PubMed] [Google Scholar]
- 28.Hernandez, L. D., R. J. Peters, S. E. Delos, J. A. Young, D. A. Agard, and J. M. White. 1997. Activation of a retroviral membrane fusion protein: soluble receptor-induced liposome binding of the ALSV envelope glycoprotein. J. Cell Biol. 139:1455-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hernandez, L. D., and J. M. White. 1998. Mutational analysis of the candidate internal fusion peptide of the avian leukosis and sarcoma virus subgroup A envelope glycoprotein. J. Virol. 72:3259-3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holmen, S. L., D. W. Salter, W. S. Payne, J. B. Dodgson, S. H. Hughes, and M. J. Federspiel. 1999. Soluble forms of the subgroup A avian leukosis virus [ALV(A)] receptor Tva significantly inhibit ALV(A) infection in vitro and in vivo. J. Virol. 73:10051-10060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hunter, E. 1997. Viral entry and receptors, p. 71-121. In J. M. Coffin (ed.), Retroviruses. Cold Spring Harbor Laboratory Press, Plainview, N.Y. [PubMed]
- 32.Irurzun, A., J. L. Nieva, and L. Carrasco. 1997. Entry of Semliki forest virus into cells: effects of concanamycin A and nigericin on viral membrane fusion and infection. Virology 227:488-492. [DOI] [PubMed] [Google Scholar]
- 33.Kuhn, R. J., W. Zhang, M. G. Rossmann, S. V. Pletnev, J. Corver, E. Lenches, C. T. Jones, S. Mukhopadhyay, P. R. Chipman, E. G. Strauss, T. S. Baker, and J. H. Strauss. 2002. Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell 108:717-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lambert, D. M., S. Barney, A. L. Lambert, K. Guthrie, R. Medinas, D. E. Davis, T. Bucy, J. Erickson, G. Merutka, and S. R. J. Petteway. 1996. Peptides from conserved regions of paramyxovirus fusion (F) proteins are potent inhibitors of viral fusion. Proc. Natl. Acad. Sci. USA 93:2186-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malashkevich, V. N., D. C. Chan, C. T. Chutkowski, and P. S. Kim. 1998. Crystal structure of the simian immunodeficiency virus (SIV) gp41 core: conserved helical interactions underlie the broad inhibitory activity of gp41 peptides. Proc. Natl. Acad. Sci. USA 95:9134-9139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Markosyan, R. M., F. S. Cohen, and G. B. Melikyan. HIV-1 envelope proteins complete their folding into six-helix bundles immediately after fusion pore formation. Mol. Biol. Cell, in press. [DOI] [PMC free article] [PubMed]
- 37.McClure, M. O., M. Marsh, and R. A. Weiss. 1988. Human immunodeficiency virus infection of CD4-bearing cells occurs by a pH-independent mechanism. EMBO J. 7:513-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McClure, M. O., M. A. Sommerfelt, M. Marsh, and R. A. Weiss. 1990. The pH independence of mammalian retrovirus infection. J. Gen. Virol. 71:767-773. [DOI] [PubMed] [Google Scholar]
- 39.Medinas, R. J., D. M. Lambert, and W. A. Tompkins. 2002. C-terminal gp40 peptide analogs inhibit feline immunodeficiency virus: cell fusion and virus spread. J. Virol. 76:9079-9086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Melikyan, G. B., R. M. Markosyan, H. Hemmati, M. K. Delmedico, D. M. Lambert, and F. S. Cohen. 2000. Evidence that the transition of HIV-1 gp41 into a six-helix bundle, not the bundle configuration, induces membrane fusion. J. Cell Biol. 151:413-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mothes, W., A. L. Boerger, S. Narayan, J. M. Cunningham, and J. A. Young. 2000. Retroviral entry mediated by receptor priming and low pH triggering of an envelope glycoprotein. Cell 103:679-689. [DOI] [PubMed] [Google Scholar]
- 42.Mould, J. A., J. E. Drury, S. M. Frings, U. B. Kaupp, A. Pekosz, R. A. Lamb, and L. H. Pinto. 2000. Permeation and activation of the M2 ion channel of influenza A virus. J. Biol. Chem. 275:31038-31050. [DOI] [PubMed] [Google Scholar]
- 43.Munoz-Barroso, I., S. Durell, K. Sakaguchi, E. Appella, and R. Blumenthal. 1998. Dilation of the human immunodeficiency virus-1 envelope glycoprotein fusion pore revealed by the inhibitory action of a synthetic peptide from gp41. J. Cell Biol. 140:315-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Netter, R. C. 2002. Ph.D. thesis. University of Pennsylvania, Philadelphia.
- 45.Nussbaum, O., C. C. Broder, and E. A. Berger. 1994. Fusogenic mechanisms of enveloped-virus glycoproteins analyzed by a novel recombinant vaccinia virus-based assay quantitating cell fusion-dependent reporter gene activation. J. Virol. 68:5411-5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paterson, R. G., C. J. Russell, and R. A. Lamb. 2000. Fusion protein of the paramyxovirus SV5: destabilizing and stabilizing mutants of fusion activation. Virology 270:17-30. [DOI] [PubMed] [Google Scholar]
- 47.Rong, L., and P. Bates. 1995. Analysis of the subgroup A avian sarcoma and leukosis virus receptor: the 40-residue, cysteine-rich, low-density lipoprotein receptor repeat motif of Tva is sufficient to mediate viral entry. J. Virol. 69:4847-4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ruigrok, R. W. H., S. R. Martin, S. A. Wharton, J. J. Skehel, P. M. Bayley, and D. C. Wiley. 1986. Conformational changes in the hemagglutinin of influenza virus which accompany heat-induced fusion of virus with liposomes. Virology 155:484-497. [DOI] [PubMed] [Google Scholar]
- 49.Russell, C. J., T. S. Jardetzky, and R. A. Lamb. 2001. Membrane fusion machines of paramyxoviruses: capture of intermediates of fusion. EMBO J. 20:4024-4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shin, J. S., and S. N. Abraham. 2001. Caveolae as portals of entry for microbes. Microbes Infect. 3:755-761. [DOI] [PubMed] [Google Scholar]
- 51.Smit, J. M., R. Bittman, and J. Wilschut. 1999. Low-pH-dependent fusion of Sindbis virus with receptor-free cholesterol- and sphingolipid-containing liposomes. J. Virol. 73:8476-8484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sonawane, N. D., J. R. Thiagarajah, and A. S. Verkman. 2002. Chloride concentration in endosomes measured using a ratioable fluorescent Cl− indicator: evidence for Cl− accumulation during acidification. J. Biol. Chem. 277:5506-5513. [DOI] [PubMed] [Google Scholar]
- 53.Stegmann, T. 2000. Membrane fusion mechanisms: the influenza hemagglutinin paradigm and its implications for intracellular fusion. Traffic 1:598-604. [DOI] [PubMed] [Google Scholar]
- 54.Stein, B. S., S. D. Gowda, J. D. Lifson, R. C. Penhallow, K. G. Bensch, and E. G. Engleman. 1987. pH-independent HIV entry into CD4-positive T cells via virus envelope fusion to the plasma membrane. Cell 49:659-668. [DOI] [PubMed] [Google Scholar]
- 55.Takada, A., C. Robison, H. Goto, A. Sanchez, K. G. Murti, M. A. Whitt, and Y. Kawaoka. 1997. A system for functional analysis of Ebola virus glycoprotein. Proc. Natl. Acad. Sci. USA 94:14764-14769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tonelli, M., R. J. Peters, T. L. James, and D. A. Agard. 2001. The solution structure of the viral binding domain of Tva, the cellular receptor for subgroup A avian leukosis and sarcoma virus. FEBS Lett. 509:161-168. [DOI] [PubMed] [Google Scholar]
- 57.Watanabe, S., A. Takada, T. Watanabe, H. Ito, H. Kida, and Y. Kawaoka. 2000. Functional importance of the coiled-coil of the Ebola virus glycoprotein. J. Virol. 74:10194-10201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wharton, S. A., J. J. Skehel, and D. C. Wiley. 2000. Temperature dependence of fusion by Sendai virus. Virology 271:71-78. [DOI] [PubMed] [Google Scholar]
- 59.White, J. M. 1995. Membrane fusion: the influenza paradigm. Cold Spring Harbor Symp. Quant. Biol. 60:581-588. [DOI] [PubMed] [Google Scholar]
- 60.Whittaker, G. R., M. Kann, and A. Helenius. 2000. Viral entry into the nucleus. Annu. Rev. Cell Dev. Biol. 16:627-651. [DOI] [PubMed] [Google Scholar]
- 61.Wild, C. T., D. C. Shugars, T. K. Greenwell, C. B. McDanal, and T. J. Matthews. 1994. Peptides corresponding to a predictive alpha-helical domain of human immunodeficiency virus type 1 gp41 are potent inhibitors of virus infection. Proc. Natl. Acad. Sci. USA 91:9770-9774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wool-Lewis, R. J., and P. Bates. 1998. Characterization of Ebola virus entry by using pseudotyped viruses: identification of receptor-deficient cell lines. J. Virol. 72:3155-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Young, J. A., P. Bates, and H. E. Varmus. 1993. Isolation of a chicken gene that confers susceptibility to infection by subgroup A avian leukosis and sarcoma viruses. J. Virol. 67:1811-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zoueva, O. P., J. E. Bailly, R. Nicholls, and E. G. Brown. 2002. Aggregation of influenza virus ribonucleocapsids at low pH. Virus Res. 85:141-149. [DOI] [PubMed] [Google Scholar]