Abstract
The envelope glycoprotein (GP) of lymphocytic choriomeningitis virus (LCMV) is posttranslationally cleaved into two subunits. We show here that this endoproteolytic processing is not required for transport to the cell surface but is essential for LCMV GP to mediate infectivity of pseudotyped retroviral vectors. By systematic mutational analysis of the LCMV GP cleavage site, we determined that the consensus motif R-(R/K/H)-L-(A/L/S/T/F)265 is essential for the endoproteolytic processing. In agreement with the identified consensus motif, we show that the cellular subtilase SKI-1/S1P cleaves LCMV GP.
Lymphocytic choriomeningitis virus (LCMV) is the prototype of the Arenaviridae family, which includes important human pathogens causing hemorrhagic fever, such as Lassa virus, Junin virus, Machupo virus, and Guanarito virus. LCMV has been widely used as an experimental model for the study of immunology, viral persistence, and viral pathogenesis (34, 50).
Virions of LCMV are composed of a nucleocapsid which is surrounded by a lipid envelope containing the envelope glycoprotein (GP). The initial steps in LCMV infection involve the interaction of GP with the cellular receptor of the target cells. Depending on the GP sequence, LCMV uses either alpha-dystroglycan or an alternative cellular protein as a receptor (15, 41, 42). After internalization of the virions within vesicles, LCMV GP mediates fusion of the viral and cellular membranes, resulting in delivery of the nucleocapsids into the cytoplasm (9, 18, 19).
LCMV GP is initially expressed as a precursor polypeptide, GP-C, which is posttranslationally cleaved into two subunits, GP-1 and GP-2 (12). Cleavage of LCMV GP-C by a yet-unidentified cellular protease occurs in the Golgi or a post-Golgi compartment (47), whereas the related Lassa virus GP-C was found to be cleaved early in the secretory pathway by the subtilase SKI-1/S1P (4, 31). The amino-terminal cleavage product GP-1 is a peripheral membrane protein and is noncovalently associated with the carboxy-terminal subunit GP-2, which is an integral membrane protein (14). GP-1 presumably interacts with the cellular receptor, whereas the GP-2 subunit most likely mediates fusion of the viral envelope with the cellular membrane (8, 13, 26, 27). The fusion peptide of GP-2 appears to be activated to a fusion-competent state by a pH-dependent conformational change of the LCMV GP subunits (9, 18, 19).
The GP of LCMV and other arenaviruses are cleaved between two uncharged amino acids (13, 30), whereas most other viral GPs are cleaved after a basic amino acid (29). In this study, we analyzed cleavage of LCMV GP in detail. We show that cleavage of LCMV GP is not required for transport to the cell surface but is required for mediating virus infectivity of retroviral pseudotypes. Furthermore, we have determined the consensus motif for cleavage of the LCMV GP, and we show that LCMV GP is cleaved in a late-Golgi or post-Golgi compartment by the cellular subtilase SKI-1/S1P.
MATERIALS AND METHODS
Construction of LCMV GP and Lassa virus GP expression plasmids.
The GPs of LCMV strain WE (WE-HPI sequence) and Lassa virus strain Josiah were expressed by using either the human cytomegalovirus immediate-early promoter, the rabbit beta-globin intron B and rabbit beta-globin polyadenylation sites of the pHCMV-vector (6), or the beta-actin promoter-driven pCAGGS vector (33). LCMV GP mutants were generated by site-directed mutagenesis with the QuikChange site-directed mutagenesis system (Stratagene, Amsterdam, The Netherlands). The sequences of the Lassa virus GP and LCMV GP open reading frames were confirmed by DNA sequencing.
Cell lines, cell culture, and transfection.
The cell lines 293T, TE671, HeLa, and Vero were obtained from the American Type Culture Collection. These cell lines were grown in Dulbecco's modified Eagle medium (DMEM) with 1 mM pyruvate, 4 mM glutamine, and 10% heat-inactivated (30 min, 56°C) fetal bovine serum. 293T cells were calcium phosphate transfected as previously described (7). HeLa cells were transfected by using FuGENE6 (Roche Diagnostics GmbH, Mannheim, Germany).
CHO-K1 and SRD-12B cells were a generous gift from J. L. Goldstein (35). CHO-K1 cells were grown in DMEM nutrient mixture F12 Ham (Gibco/Invitrogen, Karlsruhe, Germany) supplemented with 10% fetal bovine serum, 100 U of penicillin per ml, and 0.1 mg of streptomycin per ml. SRD-12B cells (35) were maintained as CHO cells with the addition of 5 μg of cholesterol (Sigma) per ml, 1 mM sodium mevalonate (Sigma), and 20 μM sodium oleate (Sigma). CHO, SRD-12B, and Vero cells were transfected with Lipofectamine 2000 (Gibco/Invitrogen).
Production and titration of retrovirus pseudotypes.
Pseudotyped retrovirus vectors were generated by cotransfection of 293T cells with plasmids encoding LCMV GP, MLVgagpol, and a retroviral vector encoding the enhanced green fluorescent protein (eGFP), as previously described (7). Supernatants of the packaging cells were harvested, and vector titers were measured by end point dilution on TE671 cells or HeLa cells, as described previously (7).
Immunoblot analysis.
Cells were lysed at 65 h posttransfection in radioimmunoprecipitation assay buffer (150 mM NaCl, 1% NP-40, 0.5% deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 50 mM Tris [pH 8.0]), mixed at a ratio of 1:2 in 2× protein loading buffer (1× loading buffer contains 60 mM Tris [pH 6.8], 10% glycerol, 2% SDS, 0.004% bromphenol blue, and 5% β-mercaptoethanol), and heated for 5 min at 95°C. The protein samples were separated by SDS-10% polyacrylamide gel electrophoresis (PAGE) and transferred to nitrocellulose membranes. The membranes were blocked with Tris-buffered saline containing 2% bovine serum albumin (Sigma) and incubated with the monoclonal rat antihemagglutinin (anti-HA) tag-specific antibody 3F10 (Roche). After washing of the filters with Tris-buffered saline and incubation with horseradish peroxidase-conjugated goat anti-rat antibody (Dianova, Hamburg, Germany), the HA-tagged proteins were detected by chemiluminescence with the ECL system (Amersham-Pharmacia, Freiburg, Germany).
Pulse-chase experiments and immunoprecipitation.
Plasmid-transfected Vero cells were starved for 1 h with DMEM lacking methionine and cysteine before cells were labeled for 30 min with [35S]methionine and [35S]cysteine (100 μCi of [35S]Promix; Amersham-Pharmacia). The radioactive label was removed from the cells and replaced by DMEM, and the cells were left for 3 h. Brefeldin A (Sigma) and monensin (Sigma) were added to the cells at final concentrations of 15 μg/ml and 10 μM, respectively, in the medium during starving, labeling, and chase periods. The cells were lysed in radioimmunoprecipitation assay buffer, sonicated, and freed from insoluble material by centrifugation at 10,000 × g for 30 min, and the supernatants were incubated with a monoclonal tetra-His antibody (Qiagen, Hilden, Germany) and protein A-Sepharose. Precipitated material was subjected to SDS-PAGE and fluorography.
Flow cytometry.
For staining of LCMV GP on the cell surface, transfected cells were resuspended in phosphate-buffered saline (PBS) at 65 h posttransfection and incubated for 30 min at 4°C with monoclonal anti-LCMV GP-1-directed antibody KL25 (11). The cells were washed three times with PBS containing 2% fetal calf serum and labeled for 30 min at 4°C with goat anti-mouse antibody conjugated to allophycocyanin (Dianova). Stained cells were subsequently analyzed by flow cytometry on a FACSCalibur (Becton Dickinson, San Jose, Calif.).
Immunofluorescence.
For detection of LCMV GP by immunofluorescence, HeLa cells were grown on coverslips and fixed 2 days after transfection. For cell surface staining, cells were fixed for 15 min in PBS containing 1% paraformaldehyde and 0.02% glutaraldehyde at room temperature. For intracellular staining, cells were incubated for 15 min in PBS containing 4% paraformaldehyde at room temperature, washed with PBS, and incubated with 0,2% Triton X-100 for 5 min at room temperature.
Fixed cells were stained for 30 min at room temperature with monoclonal mouse anti-LCMV GP-1-directed antibody KL25 (11) or with monoclonal rat anti-HA tag-directed antibody 3F10 (Roche). After incubation, cells were washed three times with PBS and incubated for 20 min at room temperature with fluorescein isothiocyanate-conjugated goat anti-mouse or goat anti-rat antibodies (Dianova). Cells were washed with PBS, mounted on microscope slides, and analyzed on an Axiovert Zeiss immunofluorescence microscope.
RESULTS
Cleavage is not essential for cell surface expression of LCMV GP.
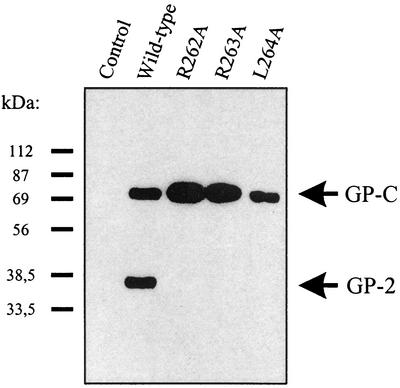
In order to determine whether endoproteolytic processing is a prerequisite for cell surface expression, we generated LCMV GP expression plasmids encoding GPs with mutations near the cleavage site. These constructs were transfected into 293T or HeLa cells, and cleavage of the C-terminal HA-tagged GPs was analyzed by Western blotting with an anti-HA tag-specific antibody. A loss of GP cleavage was observed when the arginine at amino acid 262 or 263 (positions P4 and P3) was replaced by an alanine (Fig. 1). Additionally, the mutation encoding an alanine instead of a leucine at amino acid 264 (position P2) impaired cleavage of the LCMV GP precursor GP-C (Fig. 1).
FIG. 1.
Endoproteolytic processing of wild-type and mutant LCMV GPs. 293T cells were transfected with pHCMV expression plasmids encoding C-terminally HA-tagged wild-type and mutant LCMV GPs, as indicated in Table 1, or untagged LCMV GP (control). At 48 h after transfection, proteins from lysed cells were analyzed by immunoblotting with the anti-HA tag-specific antibody 3F10. GP-C, precursor GP; GP-2, C-terminal cleavage product of GP-C containing the HA tag.
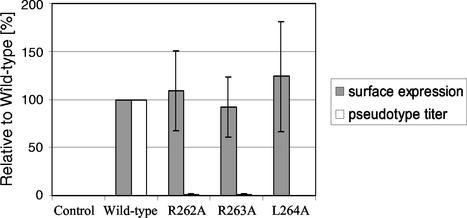
The uncleaved LCMV GP mutants were efficiently transported to the cell surface as shown by immunofluorescence and flow cytometry with the monoclonal anti-LCMV GP-1-directed antibody KL25 (Fig. 2) and other monoclonal GP-1-specific antibodies (data not shown). Indeed, uncleaved mutants resulted in slightly stronger cell surface staining of transfected cells than did wild-type, cleaved LCMV GP (Fig. 3). Furthermore, analysis of intracellular LCMV GP by immunofluorescence with an anti-HA tag-specific antibody, showed no difference in the cellular distribution of cleaved and uncleaved LCMV GP mutants (data not shown).
FIG. 2.
Cell surface expression of wild-type and mutant LCMV GPs. (A) HeLa cells were mock treated (control) or transfected with pHCMV expression plasmids encoding wild-type and mutant LCMV GPs, as indicated in Table 1. At 48 h after transfection, cells were analyzed by immunostaining. Cell surface LCMV GP was analyzed by immunostaining with the anti-LCMV GP-directed monoclonal antibody KL25. (B) 293T cells were mock treated (shaded) or transfected with pHCMV expression plasmids for LCMV GP (open). At 48 h after transfection, LCMV GP was detected by flow cytometry with antibody KL25.
FIG. 3.
Quantification of cell surface expression of wild-type and mutant LCMV GPs and pseudotyped retroviruses. Cell surface expression and pseudotyping efficiency of LCMV GP are shown in relation to those of wild-type LCMV GP, which were set 100%. Cell surface expression was quantified by flow cytometry with the KL25 antibody. Pseudotyping efficiency was determined by titration of virus from transfected retroviral packaging cell supernatants. Shown are the means and standard deviations from three independent transfections.
Proteolytic processing is a prerequisite for infectivity of retroviral pseudotypes with LCMV GP.
In order to determine whether uncleaved LCMV GPs can mediate virus infectivity, we generated retroviral vectors pseudotyped with either the cleaved wild-type LCMV GP or the uncleaved LCMV GP mutants. Therefore, we cotransfected 293T cells as previously described with plasmids encoding MLVgagpol, a retroviral vector encoding eGFP, and a vector coding for wild-type or mutant LCMV GP (7). Infectivity of the pseudotypes could be measured directly by gene transfer of the eGFP marker gene, encoded on the retroviral vector. More than 105 eGFP transfer units per ml could be measured in the supernatant of retroviral packaging cells expressing wild-type LCMV GP. In contrast, no eGFP gene transfer could be detected when the packaging cells expressed any of the uncleaved LCMV GP mutants (Fig. 3). This indicates that cleavage is a prerequisite for LCMV GP to mediate infectivity of the pseudotypes.
Consensus sequence at the cleavage site of LCMV GP-C.
To determine the sequence requirements at the cleavage site of LCMV GP-C in detail, we analyzed various GP mutants with single amino acid substitutions. In an alanine-scanning experiment, we introduced single alanine mutations between amino acids 258 and 268. The generated mutants and the corresponding GP characteristics are summarized in Table 1. All mutants were efficiently expressed on the cell surface. However, only the R262A, R263A, and L264A mutants were not cleaved. Reduced cleavage was observed for the F259A, L260A, and T261A mutants, whereas the K258A, G266A, T267A, and F268A mutants were efficiently cleaved.
TABLE 1.
LCMV GP sequence at the cleavage site and results of mutational analysis
| Expt | LCMV GP varianta | Sequence at the cleavage site | Cleavageb | Cell surface expressionc |
|---|---|---|---|---|
| Alanine scanning | Wild type | KFLTRRLA265 GTF268 | +++ | +++ |
| K258A | A.......... | +++ | +++ | |
| F259A | .A......... | −/+ | +++ | |
| L260A | ..A........ | + | +++ | |
| T261A | ...A....... | + | +++ | |
| R262A | ....A...... | − | +++ | |
| R263A | .....A..... | − | +++ | |
| L264A | ......A.... | − | +++ | |
| G266A | ........A.. | +++ | +++ | |
| T267A | .........A. | +++ | +++ | |
| F268A | ..........A | +++ | +++ | |
| Mutation at amino acids 262-264 | Wild type | KFLTRRLA265 GTF268 | +++ | +++ |
| R262K | ....K...... | − | +++ | |
| R263K | .....K..... | +++ | +++ | |
| R263H | .....H..... | ++ | +++ | |
| R263S | .....S..... | + | +++ | |
| R263N | .....N..... | + | +++ | |
| L264I | ......I.... | + | +++ | |
| L264V | ......V.... | − | +++ | |
| L264F | ......F.... | − | +++ | |
| Mutation at amino acid 265 | Wild type | KFLTRRLA265 GTF268 | +++ | +++ |
| A265L | .......L... | +++ | +++ | |
| A265I | .......I... | − | +++ | |
| A265V | .......V... | − | +++ | |
| A265T | .......T... | +++ | +++ | |
| A265F | .......F... | +++ | +++ | |
| A265E | .......E... | + | +++ | |
| 265Sd | .......S... | +++ | +++ |
293T cells were transfected with expression plasmids encoding wild-type or mutated LCMV GPs with the indicated amino acid substitutions.
Cells were lysed 2 days after transfection, and the cleavage of each mutant relative to that of wild-type LCMV GP was determined by immunoblot analysis. − undetectable cleavage; +++, cleavage equivalent to that of wild-type LCMV GP; ++, good clevage but significant less cleavage product than for the wild-type LCMV GP; +, very inefficient but detectable cleavage; −/+, trace amounts of the cleavage product detectable in only two of three experiments.
Cells were stained 2 days after transfection with anti-LCMV GP-directed antibody KL25 and analyzed by flow cytometry. +++, cell surface expression at least equivalent to that of wild-type LCMV GP.
LCMV GP variant WEP110L, which contains a serine at amino acid 265 (6).
To further determine the consensus sequence of the LCMV GP-C cleavage site, we analyzed additional amino acid substitutions at the most critical sites (Table 1). Replacement of the arginine at amino acid 262 with the basic amino acid lysine impeded GP cleavage, whereas this mutation at amino acid 263 allowed efficient cleavage. However, a histidine or, more severely, a serine or asparagine at amino acid 263 impaired the cleavage efficiency of LCMV GP-C. Less permissive was amino acid 264, because exchange of the leucine to the closely related amino acids isoleucine or valine strongly affected GP cleavage, as does exchange to the also hydrophobic amino acid phenylalanine.
We also introduced various amino acid substitutions of the amino acid alanine at position 265, which is located directly N terminal to the LCMV GP-C cleavage site (13) (Table 1). A leucine, serine, threonine, or phenylalanine at this amino acid position allowed efficient cleavage. However, isoleucine, valine, or glutamic acid at this position strongly impeded GP cleavage. Together, these results show that the complex consensus sequence R-(R/K/H)-L-(A/L/S/T/F)265 is responsible for cleavage of LCMV GP-C.
LCMV GP-C is cleaved by the subtilase SKI-1/S1P.
The requirements of the basic amino acid arginine at position P4, leucine at position P2, and nonbasic amino acids like alanine, leucine, or threonine at position P1 correlate well with known substrates and recognition sequences of the cellular subtilase SKI-1/S1P (38, 40). Therefore, we analyzed the role of SKI-1/S1P in the cleavage of LCMV GP-C. The SKI-1/S1P-deficient cell line SRD-12B (35) and its parental cell line CHO-K1 were transfected with expression plasmids encoding LCMV GP. No cleavage product was detected in the protease-negative cells, whereas the parental CHO cells efficiently cleaved LCMV GP. Furthermore, cotransfection of the protease-negative SRD-12B cells with LCMV GP and SKI-1/S1P expression plasmids restored GP cleavage (Fig. 4). Our data clearly show that SKI-1/S1P is responsible for endoproteolytic processing of LCMV GP-C.
FIG. 4.
SKI-1/S1P cleaves LCMV GP-C. SKI-1/S1P-deficient SRD-12B cells and the parental CHO-K1 cells were transfected with expression plasmids encoding C-terminally HA-tagged LCMV GP and SKI-1/S1P, as indicated. At 48 h after transfection, proteins from lysed cells were analyzed by immunoblotting with an anti-HA tag-specific antibody. GP-C, precursor GP; GP-C*, unglycosylated GP-C most likely due to overexpression (30, 31); GP-2, C-terminal cleavage product of GP-C containing the HA tag.
LCMV GP-C is cleaved late in the secretory pathway.
By using pulse-chase experiments with a temperature block, it has been observed that LCMV GP-C is cleaved in a Golgi or post-Golgi compartment (47). In contrast, by using a brefeldin A block, it was recently shown that Lassa virus GP-C is cleaved early in the secretory pathway (31). Brefeldin A is known to disassemble the cis- and medial-Golgi and to inhibit anterograde transport of secretory proteins. Since we have shown here that LCMV GP-C is cleaved by SKI-1/S1P, the same protease processing Lassa virus GP-C, we compared directly the effects of brefeldin A on the cleavage of LCMV and Lassa virus GPs.
Vero cells were transfected with empty vector or plasmids encoding a His6-tagged LCMV or Lassa virus GP. After pulse-chase and treatment with brefeldin A or monensin, the proteins were immunoprecipitated and analyzed by SDS-PAGE. As reported before, treatment of Lassa virus GP-C-expressing cells with brefeldin A had no effect on the cleavage of Lassa virus GP-C (Fig. 5, lane 3). In contrast, cleavage of LCMV GP-C was impaired by the addition of brefeldin A (Fig. 5, lane 6). The addition of monensin, an ionophore, blocking the transport from the Golgi to the late Golgi compartment, also inhibited the cleavage of LCMV GP-C (Fig. 5, lane 7). Our data clearly indicate that SKI-1/S1P cleaves GP-C of LCMV in a late Golgi or post-Golgi compartment, whereas Lassa virus GP-C is cleaved by the same protease in a compartment early in the secretory pathway.
FIG. 5.
Effects of brefeldin A and monensin on the cleavage of GP-C of LCMV and Lassa virus. Vero cells transfected with a pCAGGS plasmid encoding either Lassa virus GP (lanes 2 and 3), LCMV GP (lanes 5, 6, and 7), or empty vector (lanes 1 and 4) were labeled with [35S]methionine/[35S]cysteine at 24 h after transfection for 30 min, followed by a 3-h chase. GP-C and GP-2 were immunoprecipitated by using a tetra-His antibody and analyzed by SDS-PAGE and fluorography.
DISCUSSION
It was previously reported that both cleavage and cell surface expression of LCMV GP are impaired in parallel when LCMV GP is not N glycosylated or when it contains a proline at amino acid 110 (6, 47). However, these impairments may be due to protein folding problems in the endoplasmic reticulum targeting the misfolded LCMV GP to the protein degradation pathway (22). In this study, we have generated uncleaved LCMV GP mutants with mutations next to the cleavage site. All of these mutants were efficiently transported to the cell surface, showing that cleavage is not a requirement for cell surface expression of LCMV GP. Furthermore, uncleaved GPs did not show altered cellular distribution and could be detected with various conformation-dependent antibodies. Thus, endoproteolytic processing of LCMV GP is most likely not important for correct folding and cellular transport of the GP.
We also used cleaved and uncleaved GP mutants to generate retrovirus vector pseudotypes. High vector titers were obtained by using proteolytically processed LCMV GP, but uncleaved mutants did not mediate infectivity of retroviral vectors. Analysis of the supernatants of the packaging cells showed that uncleaved LCMV GP is present in preparations of retroviral particles (data not shown). Thus, uncleaved LCMV GP is most likely correctly incorporated into the assembling particles. We are currently analyzing the different steps involved in LCMV GP-mediated infectivity in wild-type virus and retroviral pseudotypes. For many viral envelope GPs from the retroviruses, othomyxoviruses, and paramyxoviruses, cleavage was found to be a prerequisite for the GP to mediate membrane fusion (28). Thus, uncleaved LCMV GP may also be impaired in mediating fusion of the viral and plasma membranes.
By systematic mutational analysis of the LCMV GP-C cleavage site, we determined that the consensus motif R-(R/K/H)-L-(A/L/S/T/F)265 is essential for GP-C cleavage, which has been described to occur between alanine 265 and glycine 266 (13). Thus, the LCMV GP-C cleavage site is located between uncharged amino acids and needs the basic amino acid arginine at position P4 and a leucine at position P2. This motif is very similar to the recognition sites of the mammalian subtilase SKI-1/S1P. This protease has been cloned as the site 1 protease (S1P) of the sterol regulatory element-binding proteins (SREBPs) (38), being involved in the cholesterol regulation pathway (17, 20, 37, 48). It also was independently cloned as a mammalian subtilisin-kexin isoenzyme (SKI-1), cleaving the brain-derived neurotrophic factor precursor (proBDNF) (40), and by sequencing of random cDNAs from a human myeloid cell library (32). In addition to SREBPs (16, 38) and proBDNF (40, 45), SKI-1/S1P also cleaves the membrane bound transcription factor ATF6 (49), the Lassa virus GP (4, 31), and the prodomain of SKI-1/S1P itself (16, 23, 45). Analysis of the cleavage sites of the identified substrates showed that SKI-1/S1P can mediate cleavage after uncharged amino acids such as leucine or threonine. Furthermore, the identified substrates always contain an arginine at position P4 and usually contain a leucine at position P2. These similarities between the determined consensus sequence of LCMV GP-C and the recognition motif of SKI-1/S1P, as well as the identification of SKI-1/S1P as the protease cleaving the homologues Lassa virus GP (30, 31), led us to analyze cleavage of LCMV GP-C by SKI-1/S1P.
Analysis of SKI-1/S1P-dependent cleavage of LCMV GP-C was facilitated by the cell line SRD-12B, which is deficient in the SKI-1/S1P protease (35). In these cells LCMV GP-C was not cleaved, whereas GP cleavage was found in the parental cell line or in the deficient cells after transfection of an SKI-1/S1P expression plasmid, thus showing that the viral GPs of LCMV and Lassa virus are cleaved by the same cellular protease.
Treatment of Lassa virus and LCMV GP-expressing cells with brefeldin A and monensin confirmed previous findings that while Lassa virus GP-C is cleaved by SKI-1/S1P early in the secretory pathway, LCMV GP-C is proteolytically processed in a late Golgi or post-Golgi compartment. Thus, LCMV GP-C is in contrast to Lassa virus GP-C, and the known cellular substrates SREBP, ATF6, and proBDNF are cleaved after leaving the cis/medial Golgi. SKI-1/S1P is activated by autocatalytic cleavage of the prodomain early in the secretory pathway before an active form of SKI-1/S1P is transported to the Golgi complex, and after further proteolysis, the ectodomain is secreted as an active protease in the extracellular space (21). The subcellular localization of cleavage may also be determined by particular substrates, as shown for furin, an SKI-1/S1P-related subtilase (5). Additionally, in vitro cleavage studies with SKI-1/S1P showed that the pH optimum of the proteolysis differs from substrate to substrate (45). Furthermore, we observed strongly reduced cleavage of LCMV GP-C after treatment with the lysosomotropic agent chloroquine, whereas this agent had no effect on the proteolytic processing of Lassa virus GP-C (data not shown). This indicates that SKI-1/S1P can cleave its substrates along the secretory pathway when the cellular conditions, such as pH, are favorable for the specific substrate.
LCMV and Lassa virus GP-C are cleaved by the same protease, SKI-1/S1P, although proteolytic processing of the GPs occurs in different subcellular compartments and the GPs contain different cleavage motifs. LCMV and Lassa virus GPs both share arginine at position P4, which is highly conserved among the arenaviruses (Fig. 6). This raises the possibility that all arenavirus GPs are cleaved by SKI-1/S1P or a closely related protease, although they show various amino acid substitutions within the cleavage motif.
FIG. 6.
Amino acid sequences at cleavage site of LCMV GP and GPs from other arenaviruses. The alignment shows the GP sequences between amino acid positions 258 and 268 (numbering of LCMV GP) of the Old World arenaviruses LCMV (1, 6, 36, 39, 44), Lassa virus (3), and Mopeia virus (46), as well as the New World arenaviruses Pichinde virus (2), Oliveros virus (10), Guanarito virus (43), Junin virus (25), Machupo virus (43), and Tacaribe virus (24). The arrow indicates the cleavage site, which has been confirmed for Lassa virus GP-C (30), LCMV GP-C (ARM53b), Pichinde virus GP-C, and Tacaribe virus GP-C (13). Amino acids which are conserved in all sequences are shaded in black, and those which are present in the majority of sequences are shaded in gray. GP-1, C-terminal sequence of GP-1; GP-2, N-terminal sequence of GP-2.
In summary, we have shown that cleavage of the LCMV GP is not a prerequisite for cell surface expression but is required for LCMV GP to mediate infectivity of retroviral pseudotypes. The determined sequence required for cleavage of LCMV GP-C varies from that of Lassa virus GP-C, and the two arenavirus GPs are cleaved in different compartments of the secretory pathway. However, the GPs of LCMV and Lassa virus both are cleaved by the subtilase SKI-1/S1P.
Acknowledgments
We thank H.-D. Klenk, J. ter Meulen, and W. Ostertag for helpful discussion and support, B. Abel for excellent technical assistance, and C. Stocking for critical reading of the manuscript. We are grateful to J. L. Goldstein and N. G. Seidah for kindly providing SRD-12B cells and pcDNA3.1-SKI-1/S1P plasmid, respectively.
This work was supported by the Deutsche Forschungsgemeinschaft (grants LA 1135/3-1 and GA 282/4-1), the Bundesministerium für Bildung und Forschung, and CellTec Biotechnologie GmbH (grant 00312173).
REFERENCES
- 1.Asper, M., P. Hofmann, C. Osmann, J. Funk, C. Metzger, M. Bruns, F. J. Kaup, H. Schmitz, and S. Gunther. 2001. First outbreak of callitrichid hepatitis in Germany: genetic characterization of the causative lymphocytic choriomeningitis virus strains. Virology 284:203-213. [DOI] [PubMed] [Google Scholar]
- 2.Auperin, D. D., V. Romanowski, M. Galinski, and D. H. Bishop. 1984. Sequencing studies of pichinde arenavirus S RNA indicate a novel coding strategy, an ambisense viral S RNA. J. Virol. 52:897-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auperin, D. D., D. R. Sasso, and J. B. McCormick. 1986. Nucleotide sequence of the glycoprotein gene and intergenic region of the Lassa virus S genome RNA. Virology 154:155-167. [DOI] [PubMed] [Google Scholar]
- 4.Basak, A., M. Chretien, and N. G. Seidah. 2002. A rapid fluorometric assay for the proteolytic activity of SKI-1/S1P based on the surface glycoprotein of the hemorrhagic fever Lassa virus. FEBS Lett. 514:333-339. [DOI] [PubMed] [Google Scholar]
- 5.Bass, J., C. Turck, M. Rouard, and D. F. Steiner. 2000. Furin-mediated processing in the early secretory pathway: sequential cleavage and degradation of misfolded insulin receptors. Proc. Natl. Acad. Sci. USA 97:11905-11909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beyer, W. R., H. Miletic, W. Ostertag, and D. von Laer. 2001. Recombinant expression of lymphocytic choriomeningitis virus strain WE glycoproteins: a single amino acid makes the difference. J. Virol. 75:1061-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beyer, W. R., M. Westphal, W. Ostertag, and D. von Laer. 2002. Oncoretrovirus and lentivirus vectors pseudotyped with lymphocytic choriomeningitis virus glycoprotein: generation, concentration, and broad host range. J. Virol. 76:1488-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borrow, P., and M. B. Oldstone. 1992. Characterization of lymphocytic choriomeningitis virus-binding protein(s): a candidate cellular receptor for the virus. J. Virol. 66:7270-7281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borrow, P., and M. B. Oldstone. 1994. Mechanism of lymphocytic choriomeningitis virus entry into cells. Virology 198:1-9. [DOI] [PubMed] [Google Scholar]
- 10.Brown, M. S., and J. L. Goldstein. 1999. A proteolytic pathway that controls the cholesterol content of membranes, cells, and blood. Proc. Natl. Acad. Sci. USA 96:11041-11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruns, M., J. Cihak, G. Muller, and F. Lehmann-Grube. 1983. Lymphocytic choriomeningitis virus. VI. Isolation of a glycoprotein mediating neutralization. Virology 130:247-251. [DOI] [PubMed] [Google Scholar]
- 12.Buchmeier, M. J., and M. B. Oldstone. 1979. Protein structure of lymphocytic choriomeningitis virus: evidence for a cell-associated precursor of the virion glycopeptides. Virology 99:111-120. [DOI] [PubMed] [Google Scholar]
- 13.Burns, J. W., and M. J. Buchmeier. 1993. Glycoproteins of the arenaviruses, p. 17-35. In M. S. Salvato (ed.), The Arenaviridae. Plenum Press, New York, N.Y.
- 14.Burns, J. W., and M. J. Buchmeier. 1991. Protein-protein interactions in lymphocytic choriomeningitis virus. Virology 183:620-629. [DOI] [PubMed] [Google Scholar]
- 15.Cao, W., M. D. Henry, P. Borrow, H. Yamada, J. H. Elder, E. V. Ravkov, S. T. Nichol, R. W. Compans, K. P. Campbell, and M. B. Oldstone. 1998. Identification of alpha-dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus. Science 282:2079-2081. [DOI] [PubMed] [Google Scholar]
- 16.Cheng, D., P. J. Espenshade, C. A. Slaughter, J. C. Jaen, M. S. Brown, and J. L. Goldstein. 1999. Secreted site-1 protease cleaves peptides corresponding to luminal loop of sterol regulatory element-binding proteins. J. Biol. Chem. 274:22805-22812. [DOI] [PubMed] [Google Scholar]
- 17.DeBose-Boyd, R. A., M. S. Brown, W. P. Li, A. Nohturfft, J. L. Goldstein, and P. J. Espenshade. 1999. Transport-dependent proteolysis of SREBP: relocation of site-1 protease from Golgi to ER obviates the need for SREBP transport to Golgi. Cell. 99:703-712. [DOI] [PubMed] [Google Scholar]
- 18.Di Simone, C., and M. J. Buchmeier. 1995. Kinetics and pH dependence of acid-induced structural changes in the lymphocytic choriomeningitis virus glycoprotein complex. Virology 209:3-9. [DOI] [PubMed] [Google Scholar]
- 19.Di Simone, C., M. A. Zandonatti, and M. J. Buchmeier. 1994. Acidic pH triggers LCMV membrane fusion activity and conformational change in the glycoprotein spike. Virology 198:455-465. [DOI] [PubMed] [Google Scholar]
- 20.Duncan, E. A., M. S. Brown, J. L. Goldstein, and J. Sakai. 1997. Cleavage site for sterol-regulated protease localized to a Leu-Ser bond in the lumenal loop of sterol regulatory element-binding protein-2. J. Biol. Chem. 272:12778-12785. [DOI] [PubMed] [Google Scholar]
- 21.Elagoz, A., S. Benjannet, A. Mammarbassi, L. Wickham, and N. G. Seidah. 2002. Biosynthesis and cellular trafficking of the convertase SKI-1/S1P. Ectodomain shedding requires SKI-1 activity. J. Biol. Chem. 277:11265-11275. [DOI] [PubMed] [Google Scholar]
- 22.Ellgaard, L., M. Molinari, and A. Helenius. 1999. Setting the standards: quality control in the secretory pathway. Science 286:1882-1888. [DOI] [PubMed] [Google Scholar]
- 23.Espenshade, P. J., D. Cheng, J. L. Goldstein, and M. S. Brown. 1999. Autocatalytic processing of site-1 protease removes propeptide and permits cleavage of sterol regulatory element-binding proteins. J. Biol. Chem. 274:22795-22804. [DOI] [PubMed] [Google Scholar]
- 24.Franze-Fernandez, M. T., C. Zetina, S. Iapalucci, M. A. Lucero, C. Bouissou, R. Lopez, O. Rey, M. Daheli, G. N. Cohen, and M. M. Zakin. 1987. Molecular structure and early events in the replication of Tacaribe arenavirus S RNA. Virus Res. 7:309-324. [DOI] [PubMed] [Google Scholar]
- 25.Ghiringhelli, P. D., R. V. Rivera-Pomar, M. E. Lozano, O. Grau, and V. Romanowski. 1991. Molecular organization of Junin virus S RNA: complete nucleotide sequence, relationship with other members of the Arenaviridae and unusual secondary structures. J. Gen. Virol. 72:2129-2141. [DOI] [PubMed] [Google Scholar]
- 26.Glushakova, S. E., I. S. Lukashevich, and L. A. Baratova. 1990. Prediction of arenavirus fusion peptides on the basis of computer analysis of envelope protein sequences. FEBS Lett. 269:145-147. [DOI] [PubMed] [Google Scholar]
- 27.Glushakova, S. E., V. G. Omelyanenko, I. S. Lukashevitch, A. A. Bogdanov, Jr., A. B. Moshnikova, A. T. Kozytch, and V. P. Torchilin. 1992. The fusion of artificial lipid membranes induced by the synthetic arenavirus ′fusion peptide.' Biochim. Biophys. Acta 1110:202-208. [DOI] [PubMed] [Google Scholar]
- 28.Klenk, H. D., and W. Garten. 1994. Activation cleavage of viral spike proteins, p. 241-280. In E. Wimmer (ed.), Cellular receptors for animal viruses. Monograph 28. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 29.Klenk, H. D., and W. Garten. 1994. Host cell proteases controlling virus pathogenicity. Trends Microbiol. 2:39-43. [DOI] [PubMed] [Google Scholar]
- 30.Lenz, O., J. ter Meulen, H. Feldmann, H. D. Klenk, and W. Garten. 2000. Identification of a novel consensus sequence at the cleavage site of the Lassa virus glycoprotein. J. Virol. 74:11418-11421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lenz, O., J. ter Meulen, H. D. Klenk, N. G. Seidah, and W. Garten. 2001. The Lassa virus glycoprotein precursor GP-C is proteolytically processed by subtilase SKI-1/S1P. Proc. Natl. Acad. Sci. USA 98:12701-12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagase, T., N. Miyajima, A. Tanaka, T. Sazuka, N. Seki, S. Sato, S. Tabata, K. Ishikawa, Y. Kawarabayasi, H. Kotani, et al. 1995. Prediction of the coding sequences of unidentified human genes. III. The coding sequences of 40 new genes (KIAA0081-KIAA0120) deduced by analysis of cDNA clones from human cell line KG-1. DNA Res. 2:37-43. [DOI] [PubMed] [Google Scholar]
- 33.Niwa, H., K. Yamamura, and J. Miyazaki. 1991. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108:193-199. [DOI] [PubMed] [Google Scholar]
- 34.Oldstone, M. B. 2002. Biology and pathogenesis of lymphocytic choriomeningitis virus infection. Curr. Top. Microbiol. Immunol. 263:83-117. [DOI] [PubMed] [Google Scholar]
- 35.Rawson, R. B., D. Cheng, M. S. Brown, and J. L. Goldstein. 1998. Isolation of cholesterol-requiring mutant Chinese hamster ovary cells with defects in cleavage of sterol regulatory element-binding proteins at site 1. J. Biol. Chem. 273:28261-28269. [DOI] [PubMed] [Google Scholar]
- 36.Romanowski, V., Y. Matsuura, and D. H. Bishop. 1985. Complete sequence of the S RNA of lymphocytic choriomeningitis virus (WE strain) compared to that of Pichinde arenavirus. Virus Res. 3:101-114. [DOI] [PubMed] [Google Scholar]
- 37.Sakai, J., A. Nohturfft, J. L. Goldstein, and M. S. Brown. 1998. Cleavage of sterol regulatory element-binding proteins (SREBPs) at site 1 requires interaction with SREBP cleavage-activating protein. Evidence from in vivo competition studies. J. Biol. Chem. 273:5785-5793. [DOI] [PubMed] [Google Scholar]
- 38.Sakai, J., R. B. Rawson, P. J. Espenshade, D. Cheng, A. C. Seegmiller, J. L. Goldstein, and M. S. Brown. 1998. Molecular identification of the sterol-regulated luminal protease that cleaves SREBPs and controls lipid composition of animal cells. Mol. Cell 2:505-514. [DOI] [PubMed] [Google Scholar]
- 39.Salvato, M., E. Shimomaye, P. Southern, and M. B. Oldstone. 1988. Virus-lymphocyte interactions. IV. Molecular characterization of LCMV Armstrong (CTL+) small genomic segment and that of its variant, clone 13 (CTL−). Virology 164:517-522. [DOI] [PubMed] [Google Scholar]
- 40.Seidah, N. G., S. J. Mowla, J. Hamelin, A. M. Mamarbachi, S. Benjannet, B. B. Toure, A. Basak, J. S. Munzer, J. Marcinkiewicz, M. Zhong, J. C. Barale, C. Lazure, R. A. Murphy, M. Chretien, and M. Marcinkiewicz. 1999. Mammalian subtilisin/kexin isozyme SKI-1: a widely expressed proprotein convertase with a unique cleavage specificity and cellular localization. Proc. Natl. Acad. Sci. USA 96:1321-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sevilla, N., S. Kunz, A. Holz, H. Lewicki, D. Homann, H. Yamada, K. P. Campbell, J. C. de La Torre, and M. B. Oldstone. 2000. Immunosuppression and resultant viral persistence by specific viral targeting of dendritic cells. J. Exp. Med. 192:1249-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smelt, S. C., P. Borrow, S. Kunz, W. Cao, A. Tishon, H. Lewicki, K. P. Campbell, and M. B. Oldstone. 2001. Differences in affinity of binding of lymphocytic choriomeningitis virus strains to the cellular receptor alpha-dystroglycan correlate with viral tropism and disease kinetics. J. Virol. 75:448-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spiropoulou, C. F., S. Kunz, P. E. Rollin, K. P. Campbell, and M. B. Oldstone. 2002. New World arenavirus clade C, but not clade A and B viruses, utilizes alpha-dystroglycan as its major receptor. J. Virol. 76:5140-5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stephensen, C. B., J. Y. Park, and S. R. Blount. 1995. cDNA sequence analysis confirms that the etiologic agent of callitrichid hepatitis is lymphocytic choriomeningitis virus. J. Virol. 69:1349-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toure, B. B., J. S. Munzer, A. Basak, S. Benjannet, J. Rochemont, C. Lazure, M. Chretien, and N. G. Seidah. 2000. Biosynthesis and enzymatic characterization of human SKI-1/S1P and the processing of its inhibitory prosegment. J. Biol. Chem. 275:2349-2358. [DOI] [PubMed] [Google Scholar]
- 46.Wilson, S. M., and J. C. Clegg. 1991. Sequence analysis of the S RNA of the African arenavirus Mopeia: an unusual secondary structure feature in the intergenic region. Virology 180:543-552. [DOI] [PubMed] [Google Scholar]
- 47.Wright, K. E., R. C. Spiro, J. W. Burns, and M. J. Buchmeier. 1990. Post-translational processing of the glycoproteins of lymphocytic choriomeningitis virus. Virology 177:175-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang, J., J. L. Goldstein, R. E. Hammer, Y. A. Moon, M. S. Brown, and J. D. Horton. 2001. Decreased lipid synthesis in livers of mice with disrupted Site-1 protease gene. Proc. Natl. Acad. Sci. USA 98:13607-13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ye, J., R. B. Rawson, R. Komuro, X. Chen, U. P. Dave, R. Prywes, M. S. Brown, and J. L. Goldstein. 2000. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol. Cell 6:1355-1364. [DOI] [PubMed] [Google Scholar]
- 50.Zinkernagel, R. M. 2002. Lymphocytic choriomeningitis virus and immunology. Curr. Top. Microbiol. Immunol. 263:1-5. [DOI] [PubMed] [Google Scholar]