Abstract
In polarized Madin-Darby canine kidney epithelial cells, components of the plasma membrane fusion machinery, the t-SNAREs syntaxin 2, 3, and 4 and SNAP-23, are differentially localized at the apical and/or basolateral plasma membrane domains. Here we identify syntaxin 11 as a novel apical and basolateral plasma membrane t-SNARE. Surprisingly, all of these t-SNAREs redistribute to intracellular locations when Madin-Darby canine kidney cells lose their cellular polarity. Apical SNAREs relocalize to the previously characterized vacuolar apical compartment, whereas basolateral SNAREs redistribute to a novel organelle that appears to be the basolateral equivalent of the vacuolar apical compartment. Both intracellular plasma membrane compartments have an associated prominent actin cytoskeleton and receive membrane traffic from cognate apical or basolateral pathways, respectively. These findings demonstrate a fundamental shift in plasma membrane traffic toward intracellular compartments while protein sorting is preserved when epithelial cells lose their cell polarity.
INTRODUCTION
Traffic between membranous compartments is mediated by the soluble N-ethylmaleimide–sensitive factor attachment protein (SNARE) machinery in virtually all membrane traffic pathways investigated so far (Rothman and Warren, 1994; Hanson et al., 1997; Hay and Scheller, 1997; Nichols and Pelham, 1998). During vesicle docking, membrane proteins on the vesicle membrane (v-SNAREs) and the target membrane (t-SNAREs) bind to each other to form a complex that ultimately leads to fusion of the lipid bilayers. One aspect of the SNARE hypothesis is that successful membrane fusion requires the binding of matching combinations of v- and t-SNAREs, thereby ensuring the necessary specificity of vesicle fusion. Accordingly, each membrane organelle and each class of transport vesicles should be defined by a certain set of t- and v-SNARE isoforms. Many SNAREs have been identified to date, and protein sequence analysis has shown that v- and t-SNAREs of the currently known SNARE subfamilies are evolutionarily related to each other and belong to a common superfamily (Weimbs et al., 1997b, 1998). It is conceivable that the specificity of vesicle fusion is not directly determined by t-SNARE/v-SNARE interactions per se but rather by interactions involving larger complexes, including SNAREs and their regulatory proteins, such as those of the rab and sec1 protein families (Christoforidis et al., 1999; Gonzalez and Scheller, 1999; Pfeffer, 1999).
Epithelial cells display an additional layer of complexity in that they are typically polarized and possess two distinct plasma membrane domains (Louvard et al., 1992; Simons et al., 1992; Le Gall et al., 1995; Drubin and Nelson, 1996; Yeaman et al., 1999). The apical and basolateral plasma membrane domains have different protein and lipid compositions that reflect the different functions of these domains. This plasma membrane polarity is established and maintained by protein sorting and specific vesicle trafficking routes in the biosynthetic and endocytic pathways. In agreement with the SNARE hypothesis, the apical and basolateral plasma membrane domains of epithelial cells contain distinct t-SNAREs (Weimbs et al., 1997a). Two protein families have been identified as t-SNAREs, the syntaxin and SNAP-25 families. In the polarized renal epithelial Madin-Darby canine kidney (MDCK) cell line, syntaxins 3 and 4 are localized at the apical or basolateral plasma membrane, respectively (Low et al., 1996). Syntaxin 3 functions in transport from the trans-Golgi network (TGN) as well as the endosomal recycling pathway, both leading to the apical plasma membrane (Low et al., 1998a). Syntaxin 2 is localized to both domains of MDCK cells (Low et al., 1996), as is SNAP-23 (Low et al., 1998b), a ubiquitously expressed member of the SNAP-25 family (Ravichandran et al., 1996). SNAP-23 binds to syntaxins 3 and 4 in vivo (Galli et al., 1998; St-Denis et al., 1999) and is involved in biosynthetic and endocytic recycling and transcytotic pathways to both plasma membrane domains in MDCK cells (Leung et al., 1998; Low et al., 1998a). The subcellular localization of these SNAREs is generally very similar in other epithelial cell lines and tissues, although variations have been reported (Gaisano et al., 1996; Delgrossi et al., 1997; Weimbs et al., 1997a; Fujita et al., 1998; Galli et al., 1998; Riento et al., 1998).
Temporary or permanent loss of cell polarity is a common phenomenon during the development of epithelial tissues (Sorokin and Ekblom, 1992; Birchmeier et al., 1996) as well as in a number of pathological conditions (Louvard et al., 1992; Fish and Molitoris, 1994; Birchmeier et al., 1996). It is largely unknown how apical and basolateral membrane traffic pathways behave in epithelial cells that have lost or not yet acquired their cellular polarity under any of these circumstances. This is a fundamental question in cell biology. For example, changes in these pathways may play an important role in the acquisition of the invasive phenotype of tumor cells, e.g., by mistargeting of cell adhesion molecules or erroneous secretion of proteases that attack basement membrane and extracellular matrix proteins. It is well established that the malignancy of epithelium-derived tumors (carcinomas) correlates directly with the degree of dedifferentiation. A hallmark of dedifferentiation or anaplasia is the loss of cellular polarity. A better knowledge of the changes in membrane traffic pathways that occur when epithelial cells lose or gain cell polarity will help us understand normal epithelial function as well as pathological conditions.
In this work, we have investigated the subcellular localization of plasma membrane t-SNAREs as part of the machinery that controls membrane traffic in polarized versus nonpolarized MDCK cells. We identified syntaxin 11 as a novel plasma membrane t-SNARE in addition to syntaxins 2, 3, and 4 and SNAP-23. All plasma membrane t-SNAREs undergo dramatic changes in subcellular localization in MDCK cells depending on their state of cell polarity. Apical t-SNAREs relocalize to an intracellular vacuolar apical compartment (VAC), whereas basolateral t-SNAREs relocalize to a novel compartment. The presence of t-SNAREs in these intracellular compartments suggests that they function in the fusion of incoming transport vesicles and that these compartments are actively connected to cellular membrane traffic. Indeed, we find that the apical and basolateral intracellular compartments are functionally equivalent to the apical or basolateral plasma membranes of fully polarized cells, respectively, in that they receive membrane traffic from cognate apical or basolateral transport pathways.
These results suggest that fundamental rearrangements occur with respect to membrane traffic in epithelial cells that have lost their cellular polarity. Nevertheless, the localization of plasma membrane t-SNAREs does not become randomized; instead, the cells redirect plasma membrane transport pathways into intracellular compartments and preserve protein sorting.
MATERIALS AND METHODS
Materials
Cell culture media were from Cell Gro, Mediatech (Washington, DC). FBS was from Hyclone (Logan, UT). G418 was obtained from GIBCO-BRL (Gaithersburg, MD). Transwell polycarbonate cell culture filters were purchased from Corning Costar (Cambridge, MA). Canine apo-transferrin was purchased from Sigma Chemical (St. Louis, MO), loaded with iron, and dialyzed against PBS. The cDNA of human syntaxin 11 in the expression vector pcDNA3 has been described (Valdez et al., 1999). cDNAs for the expression of syntaxin–GST fusion proteins were gifts from Dr. Mark Bennett (University of California at Berkeley).
Antibodies
Polyclonal antibodies against rat syntaxins 2, 3, and 4 were raised in rabbits against GST fusion proteins of the cytoplasmic domains. The antibodies were affinity-purified with the use of the respective syntaxin cytoplasmic domains that were separated from GST by thrombin cleavage and coupled to Affigel (Bio-Rad, Richmond, CA). The rabbit polyclonal antibody against an N-terminal peptide of human SNAP-23 was affinity-purified as described previously (Low et al., 1998b). The affinity-purified rabbit polyclonal antibody against a peptide of the N-terminal 15 amino acids of human syntaxin 11 has been described (Valdez et al., 1999). The rat mAb against ZO-1 and the mouse mAbs against ubiquitin and α-fodrin (nonerythroid spectrin) were obtained from Chemicon International (Temecula, CA). The mouse mAbs against γ-tubulin and pan-cytokeratin were obtained from Sigma. AC17, a mouse mAb against the lysosomal/late endosomal membrane glycoprotein LAMP-2 (Nabi and Rodriguez-Boulan, 1993), was a gift from E. Rodriguez-Boulan (Cornell University Medical College, New York, NY). The mouse mAb against gp135, an endogenous apical plasma membrane protein in MDCK cells (Ojakian and Schwimmer, 1988), was a gift from G. Ojakian (State University of New York Health Science Center, Brooklyn, NY). The mouse mAb against E-cadherin, rr1 (Gumbiner and Simons, 1986), was donated by B. Gumbiner (Sloan-Kettering, New York, NY). The mouse mAb 6.23.3 against an endogenous MDCK basolateral plasma membrane protein of 58 kDa (Balcarova-Stander et al., 1984) was a gift from K. Matlin (Harvard Medical School, Boston, MA). The rabbit polyclonal antibody against canine gp80/clusterin (Urban et al., 1987) was a gift from C. Koch-Brandt (Universität Mainz, Mainz, Germany). Purified human polymeric immunoglobulin A (IgA) was kindly provided by J.-P. Vaerman (Catholic University of Louvain, Brussels, Belgium). Anti-Na+/K+-ATPase (α subunit, MA3-928) was from Affinity Bioreagents (Golden, CO). Fluorescein dichlorotriazine–labeled anti-human IgA antibody was from Organon Teknika (Durham, NC). The antibody against canine apo-transferrin has been described (Apodaca et al., 1994). The mouse mAb against Golgin-97 was from Molecular Probes (Eugene, OR). Secondary antibodies cross-absorbed against multiple species and conjugated to FITC, Texas Red, or Cy5 were from Jackson Immunoresearch (West Grove, PA).
SDS-PAGE and Immunoblotting
Total membrane fractions of MDCK, HepG2, HeLa, and HT29 cells were prepared by scraping the cells from confluent dishes in PBS containing protease inhibitors and homogenization by repeated passage through a 22-gauge needle. Nuclei and unbroken cells were removed by centrifugation at 500 × g for 2 min. The membranes were recovered by centrifugation at 16,000 × g for 10 min and dissolved in SDS-PAGE sample buffer. Equal amounts of protein were separated on a 12% SDS-polyacrylamide gel followed by transfer to nitrocellulose and incubation with the affinity-purified syntaxin 11 antibody. Bands were visualized by ECL.
Cell Culture
MDCK strain II cells were maintained in MEM supplemented with 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin in 5% CO2/95% air. For experiments with polarized MDCK cells, the cells were cultured on 12-mm, 0.4-μm pore size Transwell polycarbonate filters for the indicated periods. For experiments with nonpolarized MDCK cells, the cells were sparsely seeded onto glass coverslips in MEM without FBS and allowed to attach for 2 h. Afterward, the medium was changed to s-MEM (GIBCO-BRL) with three washes of 10 min each, and the cells were incubated overnight (i.e., 16–18 h). In some experiments, the cells were allowed to endocytose IgA or transferrin during this overnight incubation by adding 50 μg/ml polymeric IgA or 1 μg/ml iron-loaded canine transferrin, respectively.
Transfection
For expression of human syntaxin 11, MDCK cells were transfected with the syntaxin 11 cDNA in the expression vector pCDNA3 by the calcium phosphate method, followed by selection in medium containing 350 μg/ml G418 (as described by Breitfeld et al., 1989). For all experiments, a mixture of the G418-resistant cells, displaying a wide range of expression levels, was used. MDCK cells stably expressing rat syntaxins 2, 3, and 4 have been described (Low et al., 1996). MDCK cells expressing human SNAP-23 were described by Low et al. (1998b). MDCK cells expressing the wild-type rabbit polymeric immunoglobulin receptor (pIgR) (Mostov and Deitcher, 1986), signalless pIgR (Casanova et al., 1991), or glycosylphosphatidylinositol (GPI)-pIgR (Mostov et al., 1986) have been described previously.
Confocal Immunofluorescence Microscopy
Cells were fixed either in methanol at −20°C or with 4% paraformaldehyde, permeabilized with 0.025% (wt/vol) saponin (Sigma) in PBS, and then blocked with 10% FBS or 5% BSA followed by sequential incubations with primary antibodies and FITC- and/or Texas red–conjugated secondary antibodies. In some cases, nuclei were stained with 5 μg/ml propidium iodide (Vector Laboratories, Burlingame, CA) after treatment with 100 μg/ml RNAse A. The samples were analyzed with the use of a Leica (Bensheim, Germany) TCS-NT confocal microscope.
RESULTS
Syntaxin 11 Is Expressed at the Plasma Membrane in Polarized but Not in Nonpolarized MDCK Cells
The following t-SNAREs are localized to the plasma membrane in mammalian cells. The neuron-specific syntaxins 1A, 1B, and SNAP-25 function in the fusion of synaptic vesicles with the presynaptic plasma membrane. The more widely expressed syntaxins 2, 3, and 4 and SNAP-23 have been studied in various cell types, in which they are generally localized at the plasma membrane (Bennett et al., 1993; Low et al., 1996; Wang et al., 1997; Galli et al., 1998; Low et al., 1998b). All other syntaxin homologues studied so far are localized to various intracellular organelles where they are believed to be functionally involved in membrane trafficking pathways directed to these organelles.
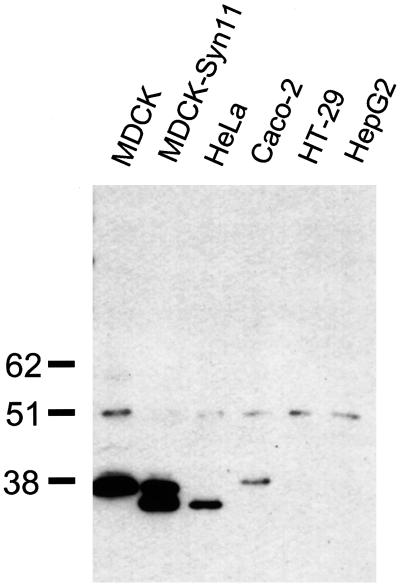
The recently discovered syntaxin 11 has an unusual primary structure in that it lacks a C-terminal transmembrane domain (Advani et al., 1998; Tang et al., 1998; Valdez et al., 1999). Nevertheless, syntaxin 11 is membrane-bound. In transiently transfected, nonpolarized NRK or HeLa cells, syntaxin 11 was found in intracellular vesicles that partially colocalized with endosomal and TGN markers (Advani et al., 1998; Valdez et al., 1999). Syntaxin 11 is widely expressed in several tissues, including tissues rich in epithelia such as lung, placenta, liver, and kidney, whereas it is absent in brain (Advani et al., 1998; Tang et al., 1998; Valdez et al., 1999). This tissue distribution prompted us to investigate whether syntaxin 11 is expressed in several pure epithelial cell lines. By Western blotting, syntaxin 11 can be detected in total membrane fractions of MDCK cells and Caco-2 colon carcinoma cells as well as in HeLa cells, but it is undetectable in the intestinal epithelial cell line HT-29 and in hepatocyte-derived HepG2 cells (Figure 1). The gel mobility of endogenous syntaxin 11 in HeLa cells is slightly higher than that in MDCK and Caco-2 cells. The reason for this difference is unknown, but it may result from differences in posttranslational modification.
Figure 1.
Syntaxin 11 is endogenously expressed in epithelial cell lines. Total membrane lysates were prepared from the following cultured cell lines: canine kidney epithelium–derived MDCK cells, MDCK cells stably transfected with human syntaxin 11, human colon carcinoma–derived Caco-2 and HT-29 cells, human liver epithelial HepG2 cells, and human HeLa cells. Equal amounts of protein were separated on a 12% SDS-polyacrylamide gel, transferred to nitrocellulose, and probed with an affinity-purified polyclonal antibody against an N-terminal peptide of the human syntaxin 11 sequence. Endogenous syntaxin 11 could be detected in MDCK, Caco-2, and HeLa cells. Identical signals were obtained with Western blots probed with antisera from two additional rabbits. All bands disappeared after competition with the syntaxin 11 peptide (our unpublished results). The electrophoretic mobility of syntaxin 11 in HeLa cells and exogenously expressed syntaxin 11 in MDCK cells is higher than that of endogenous syntaxin 11 in MDCK and Caco-2 cells.
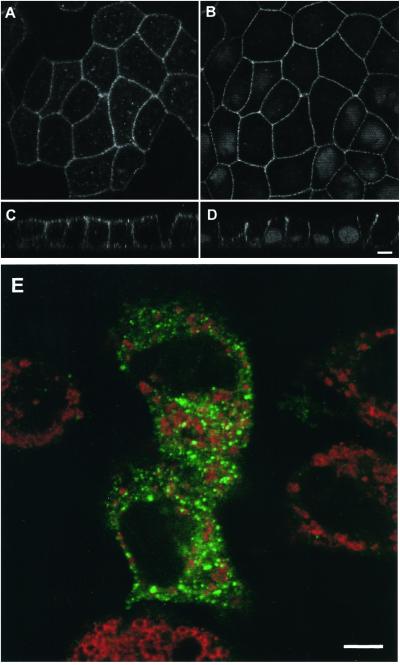
Next, we studied the subcellular localization of syntaxin 11 in MDCK cells because this epithelial cell line has been most extensively studied with respect to the localization and function of t-SNAREs. Because our syntaxin 11 antibody did not react well for immunocytochemistry with the endogenous canine protein, MDCK cells were stably transfected with the cDNA of the human protein. Confocal immunofluorescence microscopy revealed that syntaxin 11 is localized predominantly at the plasma membrane in polarized MDCK cells rather than in intracellular compartments, as reported previously for nonpolarized cells. The majority of syntaxin 11 localizes to both the apical and basolateral plasma membranes, with some additional intracellular punctate staining mostly in the apical cytoplasm (Figure 2). This localization was independent of the level of syntaxin 11 expression found by comparing individual cells with a wide range of expression levels in a mixed population of stably transfected cells. Also, as shown in Figure 1, the level of exogenous human syntaxin 11 expression was similar to the endogenous level in MDCK cells.
Figure 2.
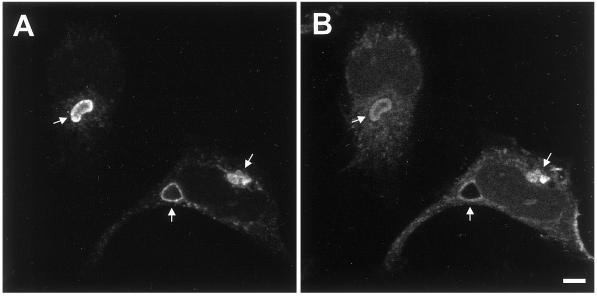
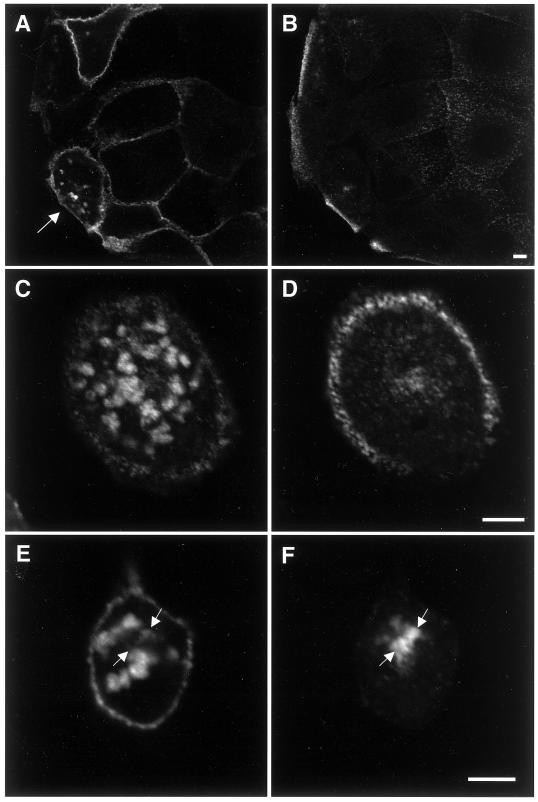
Syntaxin 11 is localized at the plasma membrane in polarized but not in nonpolarized MDCK cells. A mixed population of MDCK cells stably expressing human syntaxin 11 was grown either for 3 d on polycarbonate filters (A–D) or for 2 h on glass coverslips (E). The cells were stained with antibodies against syntaxin 11 (A, C, and E, green), the lateral plasma membrane protein E-cadherin (B and D), or the late endosomal/lysosomal protein LAMP-2 (E, red). B and D also show nuclear DNA staining with propidium iodide. A and B show horizontal confocal optical sections just above the nuclei. C and D show vertical optical sections with the apical plasma membrane at the top. Syntaxin 11 colocalizes with E-cadherin at the lateral plasma membrane. E shows a representative horizontal optical section through the middle of the cells. Syntaxin 11 does not significantly colocalize with LAMP-2. The absence of a syntaxin 11 signal in neighboring nonexpressing cells (A and E) demonstrates the specificity of the antibody. Bars, 5 μm.
Surprisingly, in nonpolarized MDCK cells, e.g., soon after plating and not allowing the cells enough time to form cell–cell interactions, syntaxin 11 was found to be intracellular, with very little if any plasma membrane staining (Figure 2E). Under these conditions, syntaxin 11 localizes to bright punctate vesicles, as reported previously in nonpolarized fibroblastic cells (Advani et al., 1998; Valdez et al., 1999). Costaining of syntaxin 11 with an antibody against the lysosomal/late endosomal protein LAMP-2 shows no significant overlap, indicating that syntaxin 11 is not simply being degraded in nonpolarized MDCK cells (Figure 2E). The observed dramatic change in the localization of syntaxin 11 depending on the state of cellular polarity of MDCK cells suggests that it normally functions at the plasma membrane in polarized epithelial cells, while it may have a different function in nonpolarized cells.
The Subcellular Localization of All Plasma Membrane t-SNAREs Changes during the Development of Epithelial Polarity
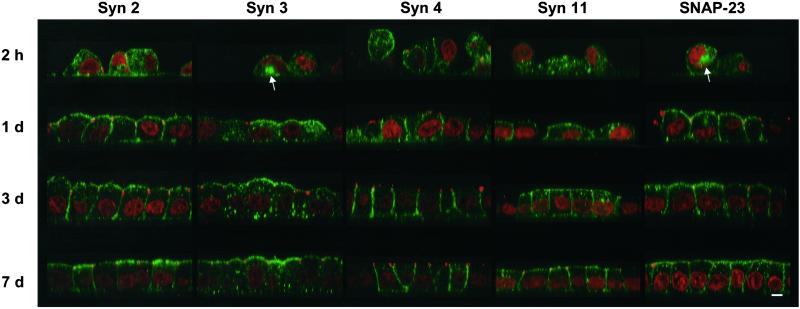
As with syntaxin 11, we observed that the previously characterized plasma membrane t-SNAREs in MDCK cells also undergo similar dramatic changes in subcellular localization depending on the degree of cellular polarity. Figure 3 shows a time course of MDCK cells at various stages during the establishment of a fully polarized monolayer. The cells were plated at high density onto polycarbonate filters, and the localizations of syntaxins 2, 3, 4, and 11 and SNAP-23, as well as the tight junction protein ZO-1, were monitored at different times after plating. After 2 h, the cells are irregularly shaped and start to form cell–cell contacts. At this stage, all plasma membrane t-SNAREs are found predominantly in intracellular vesicles in addition to a variable amount of plasma membrane staining. In ∼10% of the cells, large intracellular vacuolar structures can be observed (arrows). After 1 d, the monolayer is confluent and uninterrupted circumferential tight junctions are established. A substantial portion of all SNAREs has relocalized to the plasma membrane in a polarized manner. Syntaxins 2 and 11 as well as SNAP-23 are found at both the basolateral and the apical plasma membrane in addition to some remaining intracellular labeling. Syntaxin 3 is absent from the basolateral domain but localizes to the apical domain in addition to intracellular lysosomes, as established previously (Low et al., 1996; Delgrossi et al., 1997). Syntaxin 4, in turn, is absent from the apical domain but has partially relocalized to the basolateral domain. During the course of the experiment, until d 7, the cells grow somewhat in height and form a straight apical surface. All of the SNAREs continue to move to their final destination at their specific plasma membrane domains; however, even after 7 d some intracellular staining remains in each case, as observed previously (Low et al., 1996, 1998b).
Figure 3.
All plasma membrane t-SNAREs relocalize from intracellular compartments to their final plasma membrane domains during development of MDCK cells into a polarized monolayer. MDCK cells stably expressing syntaxins 2, 3, 4, and 11 and SNAP-23 were seeded at high density onto polycarbonate filters. At the indicated times, the cells were fixed and stained with affinity-purified antibodies against the respective SNAREs (green) as well as an antibody against the tight junction protein ZO-1 (red). Nuclei were stained with propidium iodide (red). Confocal optical sections through the monolayers are shown with the apical side on top. Once the cells have established contacts, the tight junctions can be seen as red dots at the junction between the apical and basolateral plasma membranes. At the earliest time, large intracellular vacuoles are occasionally detected (arrows), most frequently with syntaxin 3 and SNAP-23. While the distribution of all studied SNAREs is predominantly intracellular at 2 h, it shifts to a predominantly plasma membrane localization during the course of 7 d. Note that starting at d 1, syntaxin 3 is always excluded from the basolateral plasma membrane, whereas syntaxin 4 is always excluded from the apical plasma membrane. Bar, 5 μm.
This change in subcellular localization of the machinery that normally mediates vesicle fusion at the plasma membrane suggests that these membrane traffic pathways are fundamentally altered in epithelial cells during the course of the establishment of cellular polarity.
Sustained Inhibition of Epithelial Polarity Causes Intracellular Accumulation of Apical and Basolateral t-SNAREs into Distinct Compartments
To study the nature of the intracellular location of plasma membrane t-SNAREs in detail, we sought to arrest MDCK cells in a nonpolarized state. The formation of a polarized epithelial layer can be prevented experimentally by the inhibition of E-cadherin–mediated interactions between neighboring cells (Birchmeier et al., 1996; Bracke et al., 1996; Gumbiner, 1996). Inhibition of calcium-dependent homotypic E-cadherin binding by withdrawal of high calcium concentrations in the medium keeps MDCK cells in a nonpolarized state. It has been observed that, when grown in low-calcium medium (LCM), MDCK cells form large intracellular vacuoles that bear ultrastructural resemblance to the apical plasma membrane, including the presence of microvilli and an associated actin cytoskeleton. This compartment was termed “vacuolar apical compartment” or VAC (Vega-Salas et al., 1987). Similar vacuoles are found in a variety of carcinomas (Remy, 1986; Kern et al., 1987; Vega-Salas et al., 1993).
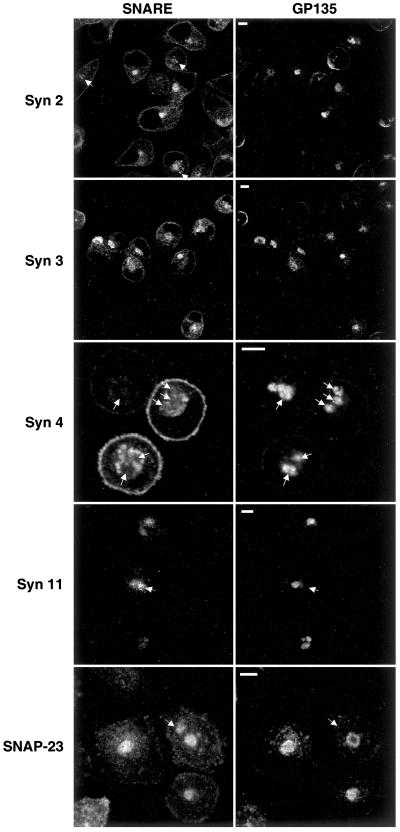
We studied the subcellular localization of plasma membrane SNAREs in MDCK cells grown under these conditions. A high percentage (>50%) of the cells display one or more large vacuolar compartments that are positive for the endogenous apical marker protein gp135 and are indistinguishable in appearance from previously described VACs (Figure 4). Plasma membrane t-SNAREs that normally localize to the apical domain (syntaxins 2, 3, and 11 and SNAP-23) colocalize with gp135 in these VACs. In contrast, the normally exclusively basolateral syntaxin 4 is excluded from gp135-positive VACs. Instead, in addition to small vesicles, in the majority of cells syntaxin 4 is found in larger structures that resemble VACs but exclude gp135. The SNAREs that are normally localized to both apical and basolateral plasma membrane domains (syntaxins 2 and 11 and SNAP-23) can be found in large gp135-negative structures (arrows) in addition to gp135-positive VACs. These results suggest that at least two distinct intracellular organelles exist in nonpolarized MDCK cells to which plasma membrane t-SNAREs are targeted.
Figure 4.
In nonpolarized MDCK cells, apical t-SNAREs localize to the VAC, whereas basolateral SNAREs are excluded from it. MDCK cells stably expressing syntaxins 2, 3, 4, and 11 and SNAP-23 were seeded onto glass coverslips in regular medium. After 2 h, the cells were switched to LCM and incubated for 16 h. Cells were fixed, permeabilized, and stained for the individual SNAREs (left column) and with an antibody against an endogenous apical plasma membrane protein, gp135 (right column). gp135 localizes to the VAC. Syntaxins 2, 3, and 11 and SNAP-23 colocalize with gp135 in VACs. In contrast, syntaxin 4 is excluded from VACs (arrows indicate VACs for better orientation). Syntaxins 2 and 11 and SNAP-23 are also found in large intracellular compartments that exclude gp135 (arrows) in addition to gp135-positive VACs. Bars, 5 μm.
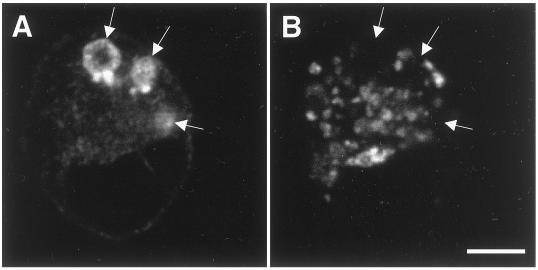
To verify the results obtained with exogenously expressed syntaxin 3 in MDCK cells, we investigated whether endogenous syntaxin 3 would also localize to VACs in a different cell line. The human colon carcinoma cell line Caco-2 was grown in LCM as described above and stained for endogenously expressed syntaxin 3 and the microvillar protein villin. While syntaxin 3 and villin are localized at the apical plasma membrane in fully polarized Caco-2 cells (Delgrossi et al., 1997; Galli et al., 1998; Riento et al., 1998; our unpublished results), they are strongly enriched in VACs in nonpolarized cells (Figure 5). This result indicates that the localization of syntaxin 3 in VACs is a general phenomenon of nonpolarized epithelial cells and not an artifact of syntaxin overexpression.
Figure 5.
Endogenous syntaxin 3 localizes to VACs in Caco-2 cells. The human colon carcinoma cell line Caco-2 was grown under low-calcium conditions as described in Figure 4. Endogenously expressed syntaxin 3 (A) and the microvillar protein villin (B) were labeled with the appropriate antibodies. Syntaxin 3 is strongly enriched in VACs that are also positive for villin, indicating that VACs are lined by microvilli (arrows). Bar, 5 μm.
We and others found previously that syntaxin 3 partially localizes to lysosomes in addition to the apical plasma membrane in fully polarized MDCK and Caco-2 cells (Low et al., 1996; Delgrossi et al., 1997). To investigate whether VACs containing syntaxin 3 may represent an enlarged type of lysosomes in cells grown under low-calcium conditions, MDCK cells were colabeled for syntaxin 3 and the late endosomal/lysosomal protein LAMP-2. Figure 6 shows that VACs and lysosomes are clearly distinct. This result indicates that VACs are not connected to the late endosomal/lysosomal system and therefore are not degradative compartments. Moreover, none of the other plasma membrane SNAREs colocalized with LAMP-2 in MDCK cells grown in LCM (our unpublished results).
Figure 6.
VACs are not related to lysosomes. MDCK cells expressing syntaxin 3 were grown in LCM for 16 h, fixed, permeabilized, and stained for syntaxin 3 (A) and the late endosomal/lysosomal protein LAMP-2 (B). Note that large vacuoles stain for syntaxin 3 but not LAMP-2 (arrows). Bar, 5 μm.
Syntaxin 4 Is a Marker for a Novel Intracellular Organelle in Nonpolarized MDCK Cells
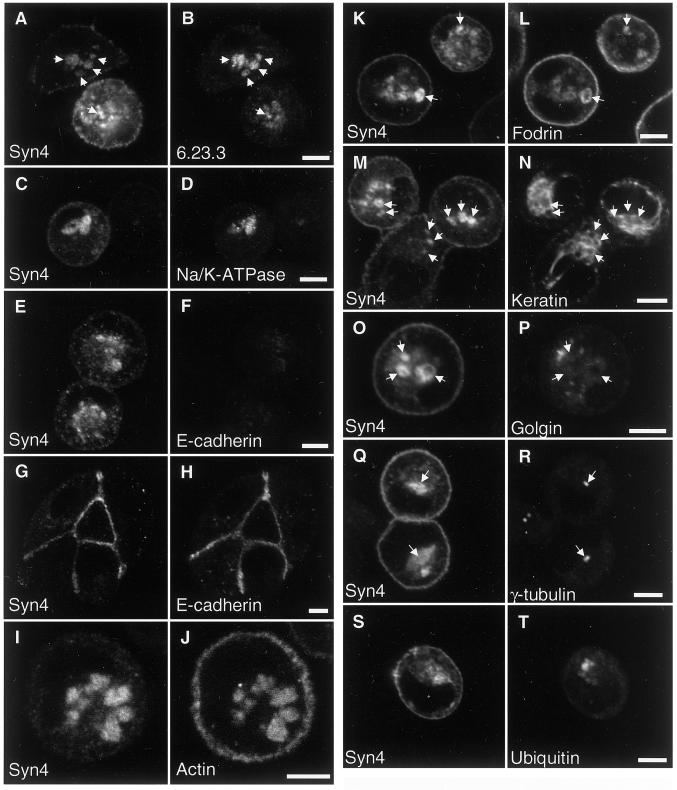
VACs have been described previously in nonpolarized MDCK cells, and our finding that they contain apical-specific t-SNAREs suggest that they receive apical-specific membrane traffic. Our finding of syntaxin 4–positive intracellular organelles suggests that another class of intracellular plasma membrane–like organelles exists that may be the basolateral equivalent of the VAC. Because such an organelle has not been described before, we sought to investigate its composition and relationship to other organelles in more detail by the colabeling studies described below. Figure 7, A–D, shows that the basolateral syntaxin 4–positive compartment also contains two proteins that are normally specifically localized at the basolateral plasma membrane domain in polarized MDCK cells: an endogenous 58-kDa basolateral plasma membrane protein (6.23.3) and the Na/K-ATPase. Both proteins appear to be even more concentrated in the intracellular compartments than syntaxin 4, which is also present at the plasma membrane. These data indicate that the syntaxin 4–positive compartment has a protein composition similar to that of the basolateral plasma membrane of polarized cells.
Figure 7.
Syntaxin 4 is a marker for a novel intracellular organelle in nonpolarized MDCK cells. MDCK cells expressing syntaxin 4 were grown in LCM (A–F and I–T) or regular medium (G and H) for 16 h, fixed, permeabilized, and stained for syntaxin 4 (left columns). Cells were colabeled for the following antigens: (B) antigen 6.23.3, a 58-kDa basolateral plasma membrane protein; (D) Na/K-ATPase; (F and H) E-cadherin; (J) F-actin (staining with fluorescent phalloidin); (L) fodrin; (N) cytokeratin (antibody against pan-cytokeratin); (P) the Golgi protein Golgin-97; (R) γ-tubulin to stain the MTOCs; and (T) ubiquitin. Note that cells in E and F were grown in LCM and show very low E-cadherin signals, in contrast to cells in G and H, which were grown in regular medium. Micrographs for panels F and H were recorded at identical imaging settings for comparison of signal intensities. Note also that cells in S and T were grown in the presence of the proteasome inhibitor acetyl-leucyl-leucyl-norleucinal (ALLN) to allow the accumulation of aggresomes. Arrows at identical locations between panels are drawn for better orientation. Bars, 5 μm.
It is important to note that the degree of intracellular localization of SNAREs or any of the plasma membrane marker proteins studied here in nonpolarized MDCK cells is relatively heterogeneous. We typically observed a range of individual cells displaying varying degrees of retention ranging from complete absence of plasma membrane markers from the plasma membrane to almost complete absence of these markers in intracellular organelles (see Figures 4–9). This heterogeneity was seen in all MDCK clones investigated, indicating that it is not due to clonal variation.
Figure 9.
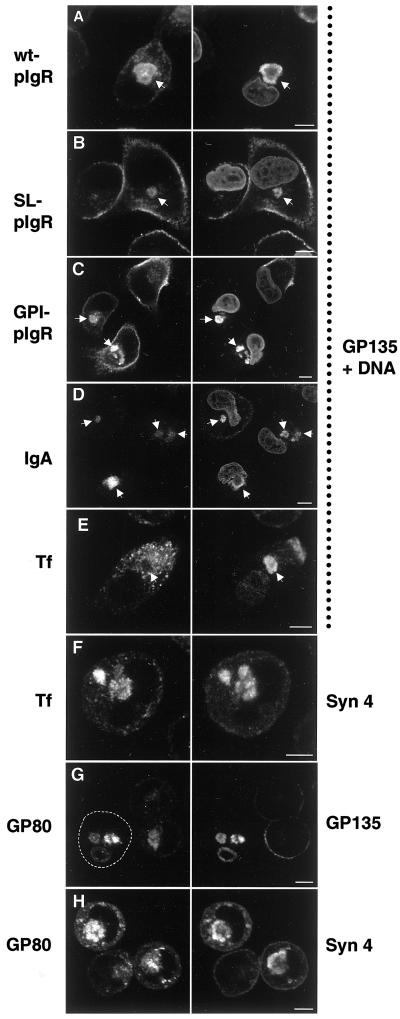
The “apical” and “basolateral” intracellular compartments receive membrane traffic from cognate polarized trafficking pathways. MDCK cells or MDCK cells stably expressing the wild-type pIgR (wt-pIgR), signalless pIgR (SL-pIgR), or a GPI-anchored mutant form of pIgR (GPI-pIgR) were grown in LCM for 16 h. IgA, 50 μg/ml polymeric IgA was added during the 16-h incubation of cells expressing wild-type pIgR. Tf, 1 μg/ml iron-loaded caninetransferrin was added to MDCK cells during the incubation. The cells were fixed, permeabilized, and stained with an antibody against the endogenous apical plasma membrane protein gp135 to label VACs and propidium iodide as a nuclear stain (right column, as indicated). The pIgR was detected with the use of an antibody against the ectodomain (left column, top three panels). IgA, transferrin, syntaxin 4, and gp80/clusterin were detected with the use of specific antibodies. Note that wt-pIgR, SL-pIgR, and GPI-pIgR are transported to the VAC (arrows). Also, IgA added to the medium of wt-pIgR–expressing cells is transported to the VAC (arrows), indicating that wt-pIgR is first transported to the plasma membrane after biosynthesis, where it can bind IgA, internalize it, and transport it into the VAC. In contrast, internalized transferrin does not reach the VAC (arrow). Instead, it is deposited into a syntaxin 4–positive compartment (F). The endogenous soluble protein gp80/clusterin, which is normally secreted apically and basolaterally (∼2:1 ratio) in polarized MDCK cells, is transported to both VACs (G) and syntaxin 4–positive organelles (H) in nonpolarized cells, suggesting that it reaches these compartments directly after biosynthesis. The panels in the left column show the same fields as those in the right column. Arrows are drawn for better orientation. The outline of one cell in G is drawn for clarity. Bars, 5 μm.
Next, we investigated whether E-cadherin might be accumulated in the syntaxin 4–positive compartment. In control cultures, E-cadherin colocalizes with syntaxin 4 at the cell–cell contacts (Figure 7, G and H). In contrast, E-cadherin expression is strongly down-regulated in cells grown in LCM (Figure 7, E and F). The remaining minor amounts of E-cadherin partially colocalize with syntaxin 4 in intracellular organelles in addition to spreading in a diffuse staining pattern, but they are not detectable at sites of cell–cell contact. Therefore, in contrast to the antigen 6.23.3 and the Na/K-ATPase, the normally lateral plasma membrane protein E-cadherin is not only redirected from the surface but its expression is also down-regulated.
Both apical and basolateral plasma membrane domains of polarized epithelial cells are generally associated with an actin cytoskeleton. Phalloidin staining shows that in nonpolarized MDCK cells, in addition to the plasma membrane, both syntaxin 3–positive vacuoles (our unpublished results) and syntaxin 4–positive vacuoles (Figure 7, I and J) display an associated actin cytoskeleton. This result distinguishes VACs and the intracellular syntaxin 4–positive compartment from endosomes or other intracellular organelles that do not typically contain a prominent actin cytoskeleton.
α-Fodrin (nonerythroid spectrin) is a component of the actin-associated cytoskeleton that normally underlies the lateral plasma membrane in polarized epithelial cells and has been implicated in the sorting of basolateral membrane proteins, such as the Na/K-ATPase, by selective retention at the basolateral surface (Nelson and Hammerton, 1989; Nelson et al., 1990; Hammerton et al., 1991). We found that in MDCK cells grown in LCM fodrin significantly colocalizes with syntaxin 4 in intracellular organelles in addition to plasma membrane staining and diffuse cytoplasmic staining (Figure 7, K and L). This suggests that intracellular syntaxin 4–positive organelles possess not only the machinery for fusion of incoming transport vesicles (syntaxin 4) but also the cytoskeletal components required for selective retention of basolateral membrane proteins and explains the accumulation of Na/K-ATPase in these organelles (Figure 7, C and D).
Cytokeratin intermediate filaments are normally closely attached to the basolateral plasma membrane domain of polarized epithelial cells by anchoring to desmosomes and hemidesmosomes. Using a pan-keratin antibody, we found that intermediate filaments are largely retracted from the plasma membrane in MDCK cells grown in LCM. They are concentrated in the center of the cell, where they often appear to be closely associated with syntaxin 4–positive intracellular organelles (Figure 7, M and N).
The syntaxin 4–positive organelles often localize in a perinuclear position in MDCK cells grown in LCM. Because this is typically also the localization of the Golgi apparatus within nonpolarized cells, we double labeled cells for syntaxin 4 and the Golgi protein Golgin-97. Figure 7, O and P, shows that both proteins localize quite distinctly with no significant overlap, excluding the possibility that the syntaxin 4–positive organelles represent a distended Golgi apparatus in MDCK cells grown in LCM.
Many cellular organelles tend to cluster around the microtubule organizing center (MTOC), which is typically found in a perinuclear position in nonpolarized cells and indicates that these organelles are clustered there by microtubule-mediated transport. Costaining of MDCK cells grown in LCM for syntaxin 4 and γ-tubulin (Figure 7, Q and R) shows that although the syntaxin 4–positive organelles can be located very close to the MTOC, they show a more dispersed distribution, suggesting that they are not necessarily being actively recruited toward the MTOC.
Recently, a novel organelle, termed an “aggresome,” has been discovered in cells that either express excessive amounts of misfolded proteins or whose proteasome degradation machinery is inhibited (Johnston et al., 1998; Wigley et al., 1999). Aggresomes are pericentriolar cytoplasmic inclusions containing misfolded, ubiquitinated protein ensheathed in a cage of intermediate filament and closely associated with the centrosome. Because aggresomes share common features with our syntaxin 4 organelles morphologically, we investigated whether these two organelles could be related to or identical to each other. When MDCK cells grown in LCM were stained for syntaxin 4 and ubiquitin, only a diffuse cytoplasmic signal could be detected for ubiquitin, which was clearly different from the signal in large syntaxin 4–positive organelles (our unpublished results). Next, we treated the cells with the proteasome inhibitor acetyl-leucyl-leucyl-norleucinal to induce the formation of aggresomes. Under these conditions, ubiquitin-positive aggresomes can clearly be identified that do not significantly overlap with syntaxin 4–positive organelles (Figure 7, S and T), demonstrating that they are distinct.
Together, these data suggest that although “apical” and “basolateral” t-SNAREs are localized intracellularly in nonpolarized epithelial cells, they are nevertheless sorted to distinct compartments. These intracellular compartments resemble the respective plasma membrane domains due to the presence of apical or basolateral t-SNAREs as well as other plasma membrane marker proteins and an actin-based cytoskeleton.
Intracellular Plasma Membrane Organelles Can Be Observed under Normal Calcium Conditions
To exclude the possibility that the observed generation of apical and basolateral intracellular organelles in MDCK cells grown in LCM may be caused by a decrease in the intracellular calcium concentration or any other irrelevant effect, we seeded MDCK cells sparsely in medium containing serum and a normal concentration of calcium. After 16 h, the cells were stained for syntaxin 4 and gp135. Under these conditions, a mixture of patches of confluent cells and smaller aggregates down to single cells is observed. Consistently, cells that are located at the edge of a cell patch tend to display intracellular syntaxin 4–positive organelles, whereas syntaxin 4 is restricted to the basolateral plasma membrane in cells that are completely surrounded by other cells (Figure 8A). Single cells very frequently show intracellular syntaxin 4–positive organelles under these conditions, whereas gp135-positive VACs are relatively sparse (Figure 8, C and D). Omission of serum increases the frequency of VACs, in agreement with a previous report (Vega-Salas et al., 1993), but it has no other apparent effect on syntaxin 4–positive organelles (Figure 8, E and F). Altogether, these results demonstrate that the occurrence of intracellular apical and basolateral organelles is not a function of the calcium concentration per se but rather depends on the degree of cell–cell interactions.
Figure 8.
Intracellular syntaxin 4–positive organelles can be observed under normal extracellular calcium conditions in cells that are not within a monolayer. MDCK cells expressing syntaxin 4 were grown for 16 h in medium containing a normal calcium concentration. The medium for A–D contained 5% FCS, whereas the medium for E and F was serum-free. Cells were costained for syntaxin 4 (left column) and gp135 (right column). Syntaxin 4 frequently localizes to intracellular organelles in single cells (C) as well as in cells at the periphery of a colony (A, arrow) regardless of the presence of serum. In contrast, syntaxin 4 is mostly restricted to the plasma membrane in cells in the midst of a colony. Prominent intracellular gp135-positive VACs are frequent in cells grown in the absence of serum (E and F, arrows) but rare when serum is included. Bars, 5 μm.
Protein Sorting Is Preserved in Nonpolarized MDCK Cells
The presence of normally apical and basolateral plasma membrane t-SNAREs in cognate intracellular compartments in nonpolarized cells suggests that these SNAREs function in the membrane fusion of vesicles from incoming transport pathways that are equivalent to the plasma membrane–directed transport pathways in polarized cells. We investigated how several proteins whose trafficking in polarized MDCK cells is well characterized are targeted in nonpolarized cells.
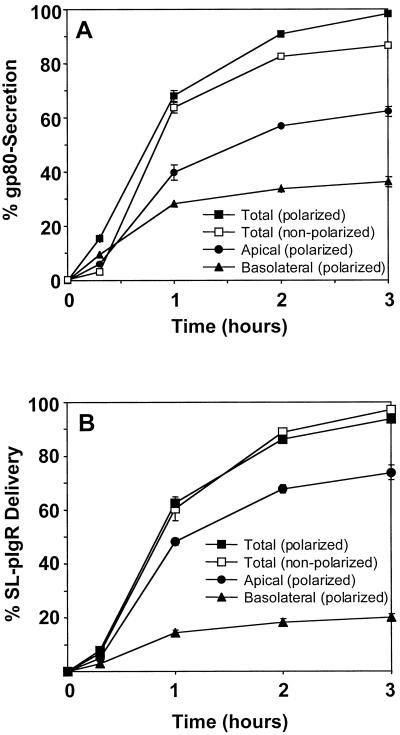
The soluble secretory protein gp80/clusterin is endogenously expressed in MDCK cells and is normally secreted apically and basolaterally (∼2:1 ratio) in polarized MDCK cells (Urban et al., 1987). Figure 9, G and H, shows that at steady state, 16 h after plating in LCM, large amounts of gp80 are retained inside individual cells and localize to both gp135-positive VACs and syntaxin 4–positive basolateral organelles. The protein must have reached these organelles on a direct route after biosynthesis, because otherwise it would be secreted and lost from the cells. To estimate what proportion of gp80 is secreted versus accumulated, we performed pulse-chase experiments and compared polarized cells grown on polycarbonate filters with nonpolarized cells grown in LCM. After 3 h, the secretion of newly synthesized gp80 from polarized cells was complete, with 62% secreted apically and 36% secreted basolaterally (Figure 10A). In contrast, 13% of gp80 remained intracellular after 3 h on a whole-population basis in nonpolarized cells. Considering that only approximately half of the cells display clearly identifiable apical and basolateral intracellular compartments under the culture conditions, we estimate that in individual cells approximately one-fourth of the synthesized gp80 is diverted into apical and basolateral intracellular organelles, and the remainder is secreted.
Figure 10.
Quantitation of surface transport of the secreted protein gp80/clusterin in polarized and nonpolarized MDCK cells. MDCK cells (A) or MDCK cells stably expressing SL-pIgR (B) were grown as either a polarized monolayer on polycarbonate filters in regular medium or as nonpolarized cells on glass coverslips in LCM. Proteins were pulse-labeled with [35S]cysteine. (A) The secretion of radiolabeled gp80 was monitored by collecting apical or basolateral medium (polarized) or total medium (nonpolarized) at different times and quantitation by SDS-PAGE and phosphorimaging and is represented as a percentage of total radiolabeled gp80 (intracellular plus secreted). Apically and basolaterally secreted gp80 was added to yield total secretion of gp80 from polarized cells. Note that after 3 h, gp80 secretion was nearly complete in polarized cells, whereas ∼15% of gp80 remained intracellular in nonpolarized cells. (B) The surface delivery of newly synthesized SL-pIgR was monitored by the addition of a low concentration of V8 protease to the chase medium, which efficiently released the extracytoplasmic domain of pIgR into the medium. Quantitation was as described above. Note that the kinetics of SL-pIgR delivery in polarized and nonpolarized cells is nearly identical, indicating that the majority of SL-pIgR is delivered directly to the plasma membrane. Experiments were done in triplicate, and error bars represent SDs. Error bars were omitted when they were smaller than the symbol.
Next, we investigated trafficking of the pIgR. In polarized MDCK cells, pIgR is first transported to the basolateral plasma membrane domain and is subsequently transcytosed to the apical plasma membrane and released into the apical medium after proteolytic cleavage (Mostov et al., 1995). We asked whether pIgR would be “transcytosed” into the VAC in nonpolarized MDCK cells. Figure 9A shows that after 16 h of incubation in LCM, a large amount of pIgR accumulates in the VAC. To support the interpretation that this is equivalent to the situation in polarized cells and follows an indirect route via the plasma membrane, we incubated nonpolarized MDCK cells expressing pIgR in the presence of polymeric IgA, the ligand of pIgR. If at least a fraction of the pIgR is targeted first to the plasma membrane before it reaches the VAC, we would expect it to have the ability to transport IgA from the medium into the VAC. Figure 9D shows that this is the case. After incubation for 16 h, the majority of the internalized IgA is found in gp135-positive VACs, demonstrating the specificity of this cognate transcytotic pathway in nonpolarized MDCK cells.
Two mutant forms of the pIgR have been generated previously that are deficient in direct TGN-to-basolateral plasma membrane transport in polarized MDCK cells and are instead targeted directly to the apical domain. Signalless pIgR (SL-pIgR) is a transmembrane protein in which the basolateral targeting signal in the cytoplasmic domain of pIgR has been deleted (Casanova et al., 1991). In GPI-anchored pIgR (GPI-pIgR), the entire cytoplasmic domain has been deleted (Mostov et al., 1986), resulting in the attachment of a GPI anchor (S.H.L., K.E.M., and T.W., unpublished data). We have shown previously that trafficking to the apical plasma membrane of SL-pIgR and GPI-pIgR involves syntaxin 3 and SNAP-23 (Low et al., 1998a). As shown in Figure 9, B and C, both proteins are transported to the gp135-positive VAC in nonpolarized MDCK cells. To assess whether the VAC is reached directly after biosynthesis or indirectly after initial delivery to the plasma membrane, we measured the surface delivery of SL-pIgR quantitatively by pulse-chase analysis. We made use of the previous finding that the extracytoplasmic domain of pIgR contains a cleavage site that allows the rapid release of this domain into the medium in the presence of low amounts of Staphylococcus aureus V8 endoprotease. Figure 10B shows that the surface delivery of SL-pIgR in filter-grown polarized cells is complete after 3 h, with an apical:basolateral ratio of ∼4:1. In contrast to the observed diminished secretion of gp80 (see above), the kinetics of SL-pIgR surface delivery was nearly identical between polarized and nonpolarized cells. This indicates that almost all of the SL-pIgR that is found in the VAC at steady state has reached this organelle indirectly via the plasma membrane. This is supported by the finding that both SL-pIgR and GPI-pIgR are able to transport IgA from the medium into the VACs (our unpublished results).
In contrast to IgA, transferrin is normally endocytosed from the basolateral plasma membrane and recycled back to the same domain in polarized MDCK cells. Only a very small percentage, if any, of internalized transferrin is transcytosed to the apical plasma membrane (Odorizzi and Trowbridge, 1997; Leung et al., 1998). If the VAC is indeed a cognate compartment to the apical plasma membrane, we expect that transferrin added to the medium would have no access to this compartment. Figure 9E shows that this is the case. After 16 h of incubation, transferrin is internalized in nonpolarized MDCK cells but remains excluded from the gp135-positive VAC. Instead, internalized transferrin significantly colocalizes with syntaxin 4 in large intracellular organelles (Figure 9F). This result shows that transferrin is endocytosed and—instead of being recycled to the basolateral plasma membrane in polarized cells—at least a fraction of it is transported to the intracellular syntaxin 4 compartment. The majority of the additional small punctate transferrin staining does not coincide with syntaxin 4 but is typical for endosomes that transferrin normally travels through. These results indicate that the intracellular syntaxin 4 compartment is distinct from typical endosomes but receives endocytic traffic characteristic of the basolateral plasma membrane.
Together, these results strongly suggest that the intracellular apical and basolateral compartments in nonpolarized MDCK cells are cognate compartments to the apical and basolateral plasma membrane in polarized cells. They are equipped with a set of t-SNAREs that correspond to the respective domains of polarized cells and receive membrane traffic from equivalent biosynthetic and endocytic pathways. The overall conclusion from these findings is that protein sorting is still preserved after MDCK cells have lost their cell polarity and that apical and basolateral proteins are not simply randomly mixed together.
DISCUSSION
In the present paper, we show that t-SNAREs that are normally localized at the plasma membrane in polarized epithelial cells distribute to intracellular compartments when cell polarity is lost or not yet established. Syntaxin 11 was identified as a new additional plasma membrane SNARE in polarized MDCK cells. This increases the number of epithelial plasma membrane syntaxins to four and raises the question of whether they all serve separate membrane traffic pathways. Only syntaxins 3 and 4 show a polarized distribution at the apical and basolateral plasma membrane domain, respectively; syntaxins 2 and 11 and SNAP-23 localize to both domains. SNAP-23 may be a common binding partner of all plasma membrane syntaxins because it can bind to syntaxins 1, 2, 3, 4, and 11 (Ravichandran et al., 1996; Valdez et al., 1999). To date, the only functional information on the involvement of syntaxin homologues in plasma membrane traffic in polarized cells is available for syntaxin 3, which plays a role in transport from the TGN and from apical endosomes to the apical plasma membrane (Low et al., 1998a; Lafont et al., 1999), and syntaxin 4, which was found to be involved in the biosynthetic pathway leading to the basolateral plasma membrane (Lafont et al., 1999). The role of the nonpolarized syntaxins 2 and 11 remains unclear, but they may serve nonpolarized pathways directed toward both domains. Syntaxin 11 has been reported to localize to endosomal and TGN-related compartments when it is exogenously expressed in nonpolarized NRK or HeLa cells (Advani et al., 1998; Valdez et al., 1999). We show that in nonpolarized MDCK cells, syntaxin 11 is also localized to punctate intracellular vesicles with very little, if any, detectable plasma membrane staining. However, as the cells form a polarized monolayer, the majority of syntaxin 11 relocalizes to both the apical and basolateral plasma membrane domains. This suggests that, at least in polarized epithelial cells, syntaxin 11 functions primarily as a plasma membrane t-SNARE. The tissue distribution of syntaxin 11 (Advani et al., 1998; Tang et al., 1998; Valdez et al., 1999) suggests that it may be predominantly expressed in epithelial cells, which is supported by our finding that it is expressed in several epithelial cell lines. This emphasizes the importance of studying the localization and function of SNAREs in fully differentiated cells, such as polarized epithelial cells.
We found that all of the plasma membrane t-SNAREs relocalize to varying degrees to intracellular compartments in MDCK cells when the formation of a polarized cell monolayer is prevented either temporarily during the course of the establishment of a monolayer or after a sustained inhibition of cell contacts in LCM. t-SNAREs are an integral part of the machinery accomplishing the final step of each membrane trafficking pathway. Therefore, this surprising result strongly suggests that membrane trafficking pathways that are normally directed to the plasma membrane in polarized epithelial cells undergo a fundamental shift toward intracellular compartments upon loss of cell polarity. This may have profound implications for our understanding of the pathogenesis of diseases involving a loss of epithelial polarity, e.g., the mistargeting of basement membrane proteins, proteases, integrins, etc., that play a role in the pathogenesis of invasive and metastatic carcinomas (Birchmeier et al., 1996), or the mistargeting of ion transporters, growth hormone receptors, etc., in noncancerous epithelial diseases such as polycystic kidney disease (Murcia et al., 1998; Sullivan et al., 1998). Also, during tubule formation, e.g., in kidney development, epithelial cells temporarily lose their cellular polarity while cell rearrangements occur (Pollack et al., 1998).
Is it possible that the observed intracellular localization of SNAREs in nonpolarized MDCK cells is an artifact caused by heterologous SNARE expression or calcium deficiency? Most experiments presented here made use of canine MDCK cells that stably express rat (syntaxins 2, 3, and 4) or human (syntaxin 11 and SNAP-23) t-SNAREs. The following considerations argue against the idea that heterologously expressed SNAREs would be targeted differently from endogenous SNAREs. First, the expression levels are generally comparable to the endogenous levels (Figure 1) (Low et al., 1996, 1998b). Second, the comparison of either different clones with varying SNARE expression levels or individual cells in a mixed clonal population fails to reveal a correlation between expression level and subcellular localization in both polarized and nonpolarized cells. Third, the localization of syntaxins (Low et al., 1996) and SNAP-23 (Low et al., 1998b) in transfected MDCK cells could be confirmed with endogenously expressed proteins in other cell lines or tissues (Gaisano et al., 1996; Delgrossi et al., 1997; Fujita et al., 1998; Galli et al., 1998; Riento et al., 1998). Fourth, in this study, we have shown that endogenously expressed syntaxin 3 in Caco-2 cells localizes to VACs just as in transfected MDCK cells (Figure 5). Therefore, we consider it unlikely that SNARE expression levels as used in this study have adverse effects on SNARE targeting in MDCK cells. The possibility that cellular calcium depletion per se may cause the generation of intracellular plasma membrane organelles independent of epithelial cell polarity is unlikely for the following reasons. First, VACs have been observed by others in mammary carcinoma cells grown in medium containing a regular calcium concentration (Vega-Salas et al., 1993). Second, VAC-like organelles are frequently found in a variety of carcinomas in situ (Remy, 1986; Vega-Salas et al., 1993) and in intestinal epithelial cells in the genetic disorder microvillus inclusion disease (Ameen and Salas, 2000). Third, the intracellular calcium concentration has been measured previously in MDCK cells grown in high- or low-calcium medium and was found to be not significantly different (Vega-Salas et al., 1987). Fourth, we observed VACs and syntaxin 4–positive basolateral organelles in MDCK cells that are grown either in regular-calcium medium for brief periods (Figure 3) or in single cells or cells at the edge of cell patches after sparse seeding and growth for 16 h (Figure 8).
The VAC has been described and characterized previously in nonpolarized MDCK cells and other epithelial cell lines as well as in carcinomas (Vega-Salas et al., 1987, 1988, 1993; Gilbert and Rodriguez-Boulan, 1991; Brignoni et al., 1993). In contrast, to our knowledge, the basolateral compartment that we identified here is a novel organelle that has not been described previously, perhaps because of the lack of availability of a marker protein such as syntaxin 4. Our data show that the syntaxin 4 compartment contains other, normally basolateral, plasma membrane proteins such as the Na/K-ATPase and the antigen 6.23.3. One basolateral marker protein, E-cadherin, was not strongly accumulated in the syntaxin 4–positive organelle but was instead down-regulated in nonpolarized cells, similar to a recent finding (Stewart et al., 2000). The small amount of E-cadherin that was still present in the cells, however, did partially localize to the syntaxin 4–positive organelle. In addition, this organelle possesses a prominent membrane cytoskeleton containing actin and fodrin that is typical for the basolateral plasma membrane in polarized cells. This organelle excludes apical plasma membrane markers, including syntaxin 3. We showed that this novel organelle does not overlap with the morphologically similar Golgi apparatus or the aggresome. The absence of the lysosomal protein LAMP-2 as well as ubiquitin makes it highly unlikely that the syntaxin 4–positive organelles, or VACs, are degradative compartments.
The presence of normally apical or basolateral plasma membrane t-SNAREs on intracellular organelles in nonpolarized cells suggests that plasma membrane proteins and secretory proteins are targeted to these vacuoles. Because apical and basolateral SNAREs are found in two separate organelles, this suggests that protein sorting is still preserved in nonpolarized MDCK cells. Our finding that internalized IgA reaches only the VAC but that transferrin reaches the syntaxin 4–positive organelle demonstrates that both compartments receive endocytic traffic and that trafficking into these organelles is specific. Both compartments also receive direct biosynthetic traffic because the soluble secretory protein gp80 accumulates in them. By pulse-chase analysis, however, we found that only a fraction of gp80 (estimated 25%) is deposited into VACs and syntaxin 4 organelles, whereas the majority is secreted. This fits with the finding that even in nonpolarized cells, variable amounts of plasma membrane SNAREs are typically located at the plasma membrane in addition to intracellular organelles. In contrast to the soluble marker gp80, the sorting of integral membrane proteins into apical and basolateral intracellular organelles appears to be more efficient. At steady state, gp135 is often very strongly enriched in VACs, whereas Na/K-ATPase and the 6.23.3 antigen are strongly enriched in basolateral organelles. Our data indicate that this high efficiency is mostly due to sorting after endocytosis of these proteins. Pulse-chase analysis of the SL-pIgR shows that nearly all of the newly synthesized protein is initially targeted to the surface. Because SL-pIgR is able to internalize IgA into VACs, and because we find SL-pIgR enriched in VACs at steady state, the majority of the protein must be internalized and transported to VACs after its initial plasma membrane delivery. This is the first direct evidence that trafficking into VACs follows mostly an indirect route via the plasma membrane. It had been suggested previously that trafficking of the influenza hemagglutinin into VACs occurs directly from the TGN (Brignoni et al., 1995), but the possibility of an indirect pathway could not be experimentally excluded.
Together, our results suggests that apical/basolateral sorting is preserved in nonpolarized epithelial cells and leads to specific intracellular organelles. It has been found previously that nonpolarized, fibroblastic cells also have the capability to sort apical and basolateral plasma membrane proteins (presumably in the TGN) and transport them on separate routes to the identical plasma membrane (Müsch et al., 1996; Yoshimori et al., 1996). A major difference between nonpolarized cells of epithelial and nonepithelial origin, therefore, may be that in the former plasma membrane proteins are eventually retained inside the cell rather than displayed at the surface.
What can be the possible function of intracellular apical and basolateral plasma membranes? These compartments are observed in epithelial cells that have lost their cellular polarity temporarily (e.g., sparsely seeded cells that have not yet established cell contacts) or permanently (e.g., when cell contacts are inhibited or in tumor cells). It is likely that many plasma membrane or secreted proteins are still synthesized under these conditions. We speculate that there may be two reasons for the intracellular sequestration of plasma membrane proteins by nonpolarized epithelial cells. The phenomenon may be a cellular survival mechanism to relocalize (normally apical and basolaterally separated) ion channels and transporters to intracellular compartments that would prevent potential ATP depletion caused by futile cycles of ion transport in and out of the cell. Also, excessive intracellular ion accumulation or depletion would be prevented. This view is supported by our finding that the majority of Na/K-ATPase relocalizes to syntaxin 4–positive organelles. The phenomenon may also be an organismal protection mechanism, because it would prevent the unwanted surface display of inappropriate proteins, e.g., proteases and cell–cell- or cell–matrix interacting proteins that may promote tumor invasion and metastasis. Interestingly, a variety of hydrolytic enzymes that are normally expressed at the apical plasma membrane of Caco-2 cells have been found in VACs after microtubule disruption (Gilbert and Rodriguez-Boulan, 1991). The finding that neither the apical nor the basolateral vacuole appears to be a degradative, lysosomal compartment indicates that proteins may be stored in them for later use once cell contacts have been reestablished. This is supported by the observation that VACs can be rapidly exocytosed as a whole from MDCK cells after reestablishment of cell–cell contacts (Vega-Salas et al., 1988) or after increasing the intracellular cAMP concentration (Brignoni et al., 1993).
In conclusion, we have shown that upon loss of cell polarity, epithelial cells relocalize plasma membrane t-SNAREs and redirect membrane trafficking pathways to intracellular cognate apical and basolateral compartments. This is likely to be a general phenomenon in epithelia and may play a fundamental role in the pathogenesis of epithelial diseases that involve a breakdown of cell polarity.
ACKNOWLEDGMENTS
We greatly appreciate the generous gifts of antibodies and/or cDNAs from Drs. E. Rodriguez-Boulan, G. Ojakian, B. Gumbiner, K. Matlin, C. Koch-Brandt, and M. Bennett. T.W. and S.H.L. are grateful to Drs. Gary Herman (Department of Pediatrics, University of California, San Francisco) and Robert Kim (Department of Ophthalmology, University of California, San Francisco) for their support. This work was supported by National Institutes of Health grants RO1AI25144 and DAMD17-97-7249 to K.E.M., by fellowships from the Alexander von Humboldt Foundation to T.W., and from the Irvington Institute for Immunology to T.W. and S.H.L.
REFERENCES
- Advani RJ, Bae HR, Bock JB, Chao DS, Doung YC, Prekeris R, Yoo JS, Scheller RH. Seven novel mammalian SNARE proteins localize to distinct membrane compartments. J Biol Chem. 1998;273:10317–10324. doi: 10.1074/jbc.273.17.10317. [DOI] [PubMed] [Google Scholar]
- Ameen NA, Salas PJI. Microvillus inclusion disease: a genetic defect affecting apical membrane protein traffic in intestinal epithelium. Traffic. 2000;1:76–83. doi: 10.1034/j.1600-0854.2000.010111.x. [DOI] [PubMed] [Google Scholar]
- Apodaca G, Katz KA, Mostov KE. Receptor-mediated transcytosis of IgA in MDCK cells via apical endosome. J Cell Biol. 1994;125:67–86. doi: 10.1083/jcb.125.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcarova-Stander J, Pfeiffer SE, Fuller SD, Simons K. Development of cell surface polarity in the epithelial Madin-Darby canine kidney (MDCK) cell line. EMBO J. 1984;3:2687–2694. doi: 10.1002/j.1460-2075.1984.tb02194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MK, Garcia-Arraras JE, Elferink LA, Peterson K, Fleming AM, Hazuka CD, Scheller RH. The syntaxin family of vesicular transport receptors. Cell. 1993;74:863–873. doi: 10.1016/0092-8674(93)90466-4. [DOI] [PubMed] [Google Scholar]
- Birchmeier W, Behrens J, Weidner KM, Hulsken J, Birchmeier C. Epithelial differentiation and the control of metastasis in carcinomas. Curr Top Microbiol Immunol. 1996;213/1:117–135. doi: 10.1007/978-3-642-61109-4_6. [DOI] [PubMed] [Google Scholar]
- Bracke ME, Van Roy FM, Mareel MM. The E-cadherin/catenin complex in invasion and metastasis. Curr Top Microbiol Immunol. 1996;213/2:123–161. doi: 10.1007/978-3-642-61107-0_9. [DOI] [PubMed] [Google Scholar]
- Breitfeld P, Casanova JE, Harris JM, Simister NE, Mostov KE. Expression and analysis of the polymeric immunoglobulin receptor. Methods Cell Biol. 1989;32:329–337. doi: 10.1016/s0091-679x(08)61178-4. [DOI] [PubMed] [Google Scholar]
- Brignoni M, Pignataro OP, Rodriguez ML, Alvarez A, Vega-Salas DE, Rodriguez-Boulan E, Salas PJ. Cyclic AMP modulates the rate of ‘constitutive’ exocytosis of apical membrane proteins in Madin-Darby canine kidney cells. J Cell Sci. 1995;108:1931–1943. doi: 10.1242/jcs.108.5.1931. [DOI] [PubMed] [Google Scholar]
- Brignoni M, Podesta EJ, Mele P, Rodriguez ML, Vega-Salas DE, Salas PJ. Exocytosis of vacuolar apical compartment (VAC) in Madin-Darby canine kidney epithelial cells: cAMP is involved as second messenger. Exp Cell Res. 1993;205:171–178. doi: 10.1006/excr.1993.1072. [DOI] [PubMed] [Google Scholar]
- Casanova JE, Apodaca G, Mostov KE. An autonomous signal for basolateral sorting in the cytoplasmic domain of the polymeric immunoglobulin receptor. Cell. 1991;66:65–75. doi: 10.1016/0092-8674(91)90139-p. [DOI] [PubMed] [Google Scholar]
- Christoforidis S, McBride HM, Burgoyne RD, Zerial M. The Rab5 effector EEA1 is a core component of endosome docking. Nature. 1999;397:621–625. doi: 10.1038/17618. [DOI] [PubMed] [Google Scholar]
- Delgrossi MH, Breuza L, Mirre C, Chavrier P, Le Bivic A. Human syntaxin 3 is localized apically in human intestinal cells. J Cell Sci. 1997;110:2207–2214. doi: 10.1242/jcs.110.18.2207. [DOI] [PubMed] [Google Scholar]
- Drubin DG, Nelson WJ. Origins of cell polarity. Cell. 1996;84:335–344. doi: 10.1016/s0092-8674(00)81278-7. [DOI] [PubMed] [Google Scholar]
- Fish EM, Molitoris BA. Alterations in epithelial polarity and the pathogenesis of disease states. N Engl J Med. 1994;330:1580–1588. doi: 10.1056/NEJM199406023302207. [DOI] [PubMed] [Google Scholar]
- Fujita H, Tuma PL, Finnegan CM, Locco L, Hubbard AL. Endogenous syntaxins 2, 3 and 4 exhibit distinct but overlapping patterns of expression at the hepatocyte plasma membrane. Biochem J. 1998;329:527–538. doi: 10.1042/bj3290527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaisano HY, Ghai M, Malkus PN, Sheu L, Bouquillon A, Bennett MK, Trimble WS. Distinct cellular locations and protein-protein interactions of the syntaxin family of proteins in rat pancreatic acinar cells. Mol Biol Cell. 1996;7:2019–2027. doi: 10.1091/mbc.7.12.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli T, Zahraoui A, Vaidyanathan VV, Raposo G, Tian JM, Karin M, Niemann H, Louvard D. A novel tetanus neurotoxin-insensitive vesicle-associated membrane protein in SNARE complexes of the apical plasma membrane of epithelial cells. Mol Biol Cell. 1998;9:1437–1448. doi: 10.1091/mbc.9.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert T, Rodriguez-Boulan E. Induction of vacuolar apical compartments in the Caco-2 intestinal epithelial cell line. J Cell Sci. 1991;100:451–458. doi: 10.1242/jcs.100.3.451. [DOI] [PubMed] [Google Scholar]
- Gonzalez L, Jr, Scheller RH. Regulation of membrane trafficking: structural insights from a Rab/effector complex. Cell. 1999;96:755–758. doi: 10.1016/s0092-8674(00)80585-1. [DOI] [PubMed] [Google Scholar]
- Gumbiner B, Simons K. A functional assay for proteins involved in establishing an epithelial occluding barrier: identification of a uvomorulin-like polypeptide. J Cell Biol. 1986;102:457–468. doi: 10.1083/jcb.102.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- Hammerton RW, Krzeminski KA, Mays RW, Ryan TA, Wollner DA, Nelson WJ. Mechanism for regulating cell surface distribution of Na+,K(+)-ATPase in polarized epithelial cells. Science. 1991;254:847–850. doi: 10.1126/science.1658934. [DOI] [PubMed] [Google Scholar]
- Hanson PI, Heuser JE, Jahn R. Neurotransmitter release: four years of SNARE complexes. Curr Opin Neurobiol. 1997;7:310–315. doi: 10.1016/s0959-4388(97)80057-8. [DOI] [PubMed] [Google Scholar]
- Hay JC, Scheller RH. SNAREs and NSF in targeted membrane fusion. Curr Opin Cell Biol. 1997;9:505–512. doi: 10.1016/s0955-0674(97)80026-9. [DOI] [PubMed] [Google Scholar]
- Johnston JA, Ward CL, Kopito RR. Aggresomes: a cellular response to misfolded proteins. J Cell Biol. 1998;143:1883–1898. doi: 10.1083/jcb.143.7.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern HF, Roher HD, von Bulow M, Kloppel G. Fine structure of three major grades of malignancy of human pancreatic adenocarcinoma. Pancreas. 1987;2:2–13. doi: 10.1097/00006676-198701000-00002. [DOI] [PubMed] [Google Scholar]
- Lafont F, Verkade P, Galli T, Wimmer C, Louvard D, Simons K. Raft association of SNAP receptors acting in apical trafficking in Madin-Darby canine kidney cells. Proc Natl Acad Sci USA. 1999;96:3734–3738. doi: 10.1073/pnas.96.7.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gall AH, Yeaman C, Muesch A, Rodriguez-Boulan E. Epithelial cell polarity: new perspectives. Semin Nephrol. 1995;15:272–284. [PubMed] [Google Scholar]
- Leung SM, Chen D, DasGupta BR, Whiteheart SW, Apodaca G. SNAP-23 requirement for transferrin recycling in streptolysin-O-permeabilized Madin-Darby canine kidney cells. J Biol Chem. 1998;273:17732–17741. doi: 10.1074/jbc.273.28.17732. [DOI] [PubMed] [Google Scholar]
- Louvard D, Kedinger M, Hauri HP. The differentiating intestinal epithelial cell: establishment and maintenance of functions through interactions between cellular structures. Annu Rev Cell Biol. 1992;8:157–195. doi: 10.1146/annurev.cb.08.110192.001105. [DOI] [PubMed] [Google Scholar]
- Low SH, Chapin SJ, Weimbs T, Kömüves LG, Bennett MK, Mostov KE. Differential localization of syntaxin isoforms in polarized MDCK cells. Mol Biol Cell. 1996;7:2007–2018. doi: 10.1091/mbc.7.12.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low SH, Chapin SJ, Wimmer C, Whiteheart SW, Kömüves LK, Mostov KE, Weimbs T. The SNARE machinery is involved in apical plasma membrane trafficking in MDCK cells. J Cell Biol. 1998a;141:1503–1513. doi: 10.1083/jcb.141.7.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low SH, Roche PA, Anderson HA, van IJzendoorn SCD, Zhang M, Mostov KE, Weimbs T. Targeting of SNAP-23 and SNAP-25 in polarized epithelial cells. J Biol Chem. 1998b;273:3422–3430. doi: 10.1074/jbc.273.6.3422. [DOI] [PubMed] [Google Scholar]
- Mostov KE, Altschuler Y, Chapin SJ, Enrich C, Low SH, Luton F, Richman-Eisenstat J, Singer KL, Tang K, Weimbs T. Regulation of protein traffic in polarized epithelial cells: the polymeric immunoglobulin receptor model. Cold Spring Harbor Symp Quant Biol. 1995;60:775–781. doi: 10.1101/sqb.1995.060.01.083. [DOI] [PubMed] [Google Scholar]
- Mostov KE, de Bruyn Kops A, Deitcher DL. Deletion of the cytoplasmic domain of the polymeric immunoglobulin receptor prevents basolateral localization and endocytosis. Cell. 1986;47:359–364. doi: 10.1016/0092-8674(86)90592-1. [DOI] [PubMed] [Google Scholar]
- Mostov KE, Deitcher DL. Polymeric immunoglobulin receptor expressed in MDCK cells transcytoses IgA. Cell. 1986;46:613–621. doi: 10.1016/0092-8674(86)90887-1. [DOI] [PubMed] [Google Scholar]
- Murcia NS, Woychik RP, Avner ED. The molecular biology of polycystic kidney disease. Pediatr Nephrol. 1998;12:721–726. doi: 10.1007/s004670050534. [DOI] [PubMed] [Google Scholar]
- Müsch A, Xu H, Shields D, Rodriguez-Boulan E. Transport of vesicular stomatitis virus G protein to the cell surface is signal mediated in polarized and nonpolarized cells. J Cell Biol. 1996;133:543–558. doi: 10.1083/jcb.133.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabi IR, Rodriguez-Boulan E. Increased LAMP-2 polylactosamine glycosylation is associated with its slower Golgi transit during establishment of a polarized MDCK epithelial monolayer. Mol Biol Cell. 1993;4:627–635. doi: 10.1091/mbc.4.6.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson WJ, Hammerton RW. A membrane-cytoskeletal complex containing Na+,K+-ATPase, ankyrin, and fodrin in Madin-Darby canine kidney (MDCK) cells: implications for the biogenesis of epithelial cell polarity. J Cell Biol. 1989;108:893–902. doi: 10.1083/jcb.108.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson WJ, Hammerton RW, Wang AZ, Shore EM. Involvement of the membrane-cytoskeleton in development of epithelial cell polarity. Semin Cell Biol. 1990;1:359–371. [PubMed] [Google Scholar]
- Nichols BJ, Pelham HR. SNAREs and membrane fusion in the Golgi apparatus. Biochim Biophys Acta. 1998;1404:9–31. doi: 10.1016/s0167-4889(98)00044-5. [DOI] [PubMed] [Google Scholar]
- Odorizzi G, Trowbridge IS. Structural requirements for basolateral sorting of the human transferrin receptor in the biosynthetic and endocytic pathways of Madin-Darby canine kidney cells. J Cell Biol. 1997;137:1255–1264. doi: 10.1083/jcb.137.6.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojakian GK, Schwimmer R. The polarized distribution of an apical cell surface glycoprotein is maintained by interactions with the cytoskeleton of Madin-Darby canine kidney cells. J Cell Biol. 1988;107:2377–2387. doi: 10.1083/jcb.107.6.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer SR. Transport-vesicle targeting: tethers before SNAREs. Nat Cell Biology. 1999;1:E17–E22. doi: 10.1038/8967. [DOI] [PubMed] [Google Scholar]
- Pollack AL, Runyan RB, Mostov KE. Morphogenetic mechanisms of epithelial tubulogenesis: MDCK cell polarity is transiently rearranged without loss of cell-cell contact during scatter factor/hepatocyte growth factor-induced tubulogenesis. Dev Biol. 1998;204:64–79. doi: 10.1006/dbio.1998.9091. [DOI] [PubMed] [Google Scholar]
- Ravichandran V, Chawla A, Roche PA. Identification of a novel syntaxin- and synaptobrevin/VAMP-binding protein, SNAP-23, expressed in non-neuronal tissues. J Biol Chem. 1996;271:13300–13303. doi: 10.1074/jbc.271.23.13300. [DOI] [PubMed] [Google Scholar]
- Remy L. The intracellular lumen: origin, role and implications of a cytoplasmic neostructure. Biol Cell. 1986;56:97–105. doi: 10.1111/j.1768-322x.1986.tb00446.x. [DOI] [PubMed] [Google Scholar]
- Riento K, Galli T, Jansson S, Ehnholm C, Lehtonen E, Olkkonen VM. Interaction of munc-18-2 with syntaxin 3 controls the association of apical SNAREs in epithelial cells. J Cell Sci. 1998;111:2681–2688. doi: 10.1242/jcs.111.17.2681. [DOI] [PubMed] [Google Scholar]
- Rothman JE, Warren G. Implications of the SNARE hypothesis for intracellular membrane topology and dynamics. Curr Biol. 1994;4:220–233. doi: 10.1016/s0960-9822(00)00051-8. [DOI] [PubMed] [Google Scholar]
- Simons K, Dupree P, Fiedler K, Huber LA, Kobayashi T, Kurzchalia T, Olkkonen V, Pimplikar S, Parton R, Dotti C. Biogenesis of cell-surface polarity in epithelial cells and neurons. Cold Spring Harbor Symp Quant Biol. 1992;57:611–619. doi: 10.1101/sqb.1992.057.01.067. [DOI] [PubMed] [Google Scholar]
- Sorokin L, Ekblom P. Development of tubular and glomerular cells of the kidney. Kidney Int. 1992;41:657–664. doi: 10.1038/ki.1992.101. [DOI] [PubMed] [Google Scholar]
- St-Denis JF, Cabaniols JP, Cushman SW, Roche PA. SNAP-23 participates in SNARE complex assembly in rat adipose cells. Biochem J. 1999;338:709–715. [PMC free article] [PubMed] [Google Scholar]
- Stewart DB, Barth AI, Nelson WJ. Differential regulation of endogenous cadherin expression in Madin-Darby canine kidney cells by cell-cell adhesion and activation of beta-catenin signaling. J Biol Chem. 2000;275:20707–20716. doi: 10.1074/jbc.M000467200. [DOI] [PubMed] [Google Scholar]
- Sullivan LP, Wallace DP, Grantham JJ. Epithelial transport in polycystic kidney disease. Physiol Rev. 1998;78:1165–1191. doi: 10.1152/physrev.1998.78.4.1165. [DOI] [PubMed] [Google Scholar]
- Tang BL, Low DY, Hong W. Syntaxin 11: a member of the syntaxin family without a carboxyl terminal transmembrane domain. Biochem Biophys Res Commun. 1998;245:627–632. doi: 10.1006/bbrc.1998.8490. [DOI] [PubMed] [Google Scholar]
- Urban J, Parczyk K, Leutz A, Kayne M, Kondor-Koch C. Constitutive apical secretion of an 80-kD sulfated glycoprotein complex in the polarized epithelial Madin-Darby canine kidney cell line. J Cell Biol. 1987;105:2735–2743. doi: 10.1083/jcb.105.6.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez AC, Cabaniols J-P, Brown MJ, Roche PA. Syntaxin 11 is associated with SNAP-23 on late endosomes and the trans-Golgi network. J Cell Sci. 1999;112:845–854. doi: 10.1242/jcs.112.6.845. [DOI] [PubMed] [Google Scholar]
- Vega-Salas DE, Salas PJ, Rodriguez-Boulan E. Modulation of the expression of an apical plasma membrane protein of Madin-Darby canine kidney epithelial cells: cell-cell interactions control the appearance of a novel intracellular storage compartment. J Cell Biol. 1987;104:1249–1259. doi: 10.1083/jcb.104.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega-Salas DE, Salas PJ, Rodriguez-Boulan E. Exocytosis of vacuolar apical compartment (VAC): a cell-cell contact controlled mechanism for the establishment of the apical plasma membrane domain in epithelial cells. J Cell Biol. 1988;107:1717–1728. doi: 10.1083/jcb.107.5.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega-Salas DE, San Martino JA, Salas PJ, Baldi A. Vacuolar apical compartment (VAC) in breast carcinoma cell lines (MCF-7 and T47D): failure of the cell-cell regulated exocytosis mechanism of apical membrane. Differentiation. 1993;54:131–141. doi: 10.1111/j.1432-0436.1993.tb00716.x. [DOI] [PubMed] [Google Scholar]
- Wang G, Witkin JW, Hao G, Bankaitis VA, Scherer PE, Baldini G. Syndet is a novel SNAP-25 related protein expressed in many tissues. J Cell Sci. 1997;110:505–513. doi: 10.1242/jcs.110.4.505. [DOI] [PubMed] [Google Scholar]
- Weimbs T, Low SH, Chapin SJ, Mostov KE. Apical targeting in polarized epithelial cells: there's more afloat than rafts. Trends Cell Biol. 1997a;7:393–399. doi: 10.1016/S0962-8924(97)01130-6. [DOI] [PubMed] [Google Scholar]
- Weimbs T, Low SH, Chapin SJ, Mostov KE, Bucher P, Hofmann K. A conserved domain is present in different families of vesicular fusion proteins: a new superfamily. Proc Natl Acad Sci USA. 1997b;94:3046–3051. doi: 10.1073/pnas.94.7.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimbs T, Mostov KE, Low SH, Hofmann K. A model for structural similarity between different SNARE complexes based on sequence relationships. Trends Cell Biol. 1998;8:260–262. doi: 10.1016/s0962-8924(98)01285-9. [DOI] [PubMed] [Google Scholar]
- Wigley WC, Fabunmi RP, Lee MG, Marino CR, Muallem S, DeMartino GN, Thomas PJ. Dynamic association of proteasomal machinery with the centrosome. J Cell Biol. 1999;145:481–490. doi: 10.1083/jcb.145.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman C, Grindstaff KK, Nelson WJ. New perspectives on mechanisms involved in generating epithelial cell polarity. Physiol Rev. 1999;79:73–98. doi: 10.1152/physrev.1999.79.1.73. [DOI] [PubMed] [Google Scholar]
- Yoshimori T, Keller P, Roth MG, Simons K. Different biosynthetic transport routes to the plasma membrane in BHK and CHO cells. J Cell Biol. 1996;133:247–256. doi: 10.1083/jcb.133.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]