Abstract
Human immunodeficiency virus type 1 (HIV-1) Nef is a key pathogenic factor necessary for the development of AIDS. One important function of Nef is to reduce cell surface levels of major histocompatibility complex class I (MHC-I) molecules, thereby protecting HIV-infected cells from recognition by cytotoxic T lymphocytes. The mechanism of MHC-I downmodulation by Nef has not been clearly elucidated, and its reported effect on MHC-I steady-state levels ranges widely, from 2-fold in HeLa cells to 200-fold in HIV-infected primary T cells. Here, we directly compared downmodulation of HLA-A2 in HIV-infected HeLa cells to that in T cells. We found that similar amounts of Nef protein resulted in a much more dramatic downmodulation of HLA-A2 in T cells than in HeLa cells. A comparison of Nef's effects on HLA-A2 endocytosis, recycling, and transport rates indicated that the most prominent effect of Nef on HLA-A2 in T cells was to inhibit transport to the cell surface. The phosphatidylinositol 3-kinase inhibitor, LY294002, previously reported to inhibit Nef-mediated MHC-I downmodulation in astrocytic cells, did not directly affect Nef's ability to block transport of MHC-I to the cell surface in T cells.
The expression of human immunodeficiency virus (HIV) Nef in the context of HIV infection is important for the development of AIDS (23). Its functions include downmodulation of cell surface molecules (e.g., CD4 and major histocompatibility complex class I [MHC-I]), disruption of cell signaling, and increased viral infectivity (10). Downmodulation of MHC-I by Nef has been shown to protect infected cells from cytotoxic T lymphocyte (CTL) killing (7, 47), and evidence has been accumulating that MHC-I downmodulation has an important role in vivo (15, 30).
Despite multiple studies, the mechanism by which Nef downmodulates MHC-I has remained unclear. In T cells stably expressing Nef, total cellular MHC-I is synthesized and transported to the medial Golgi apparatus normally (37). Multiple reports indicate that it then accumulates in the trans-Golgi network of Nef-expressing HeLa, fibroblast, or A7 melanoma cells (17, 25, 35). In astrocytic cells, MHC-I partially localizes to the region of the Golgi apparatus (39). There is currently debate as to whether MHC-I accumulates in these intracellular organelles because Nef disrupts normal anterograde transport to the cell surface (24, 39) or because Nef promotes endocytosis of MHC-I from the plasma membrane and retrograde transport to intracellular organelles (17, 25, 35, 37).
Expression of Nef clearly results in some acceleration of MHC-I endocytosis at the plasma membrane (24, 37). However, acceleration of endocytosis does not appear to be the entire answer. In HeLa cells, the rate of Nef-dependent MHC-I endocytosis is much slower than that of an MHC-I molecule modified to include a canonical endocytosis signal in its cytoplasmic tail (24). Additionally, Nef-dependent accumulation of MHC-I inside HeLa cells is not blocked by dominant-negative dynamin (39), a protein required for most types of endocytic processes (8, 20, 43). Moreover, Nef-mediated reduction in steady-state MHC-I cell surface levels is not affected by dominant-negative dynamin (24). (Albeit, MHC-I might be endocytosed via a recently discovered ARF-6-dependent mechanism [4, 36] and data are conflicting as to whether dominant-negative dynamin affects this pathway [1, 9].) Finally, there is direct evidence that Nef can inhibit transport to the cell surface in astrocytic cells (39). Thus, it is possible that there are two pathways contributing to Nef-mediated MHC-I downmodulation or that Nef behaves in a cell type-specific manner. We and others (24, 25, 37) have found that downmodulation of the MHC-I HLA-A2 allotype in HeLa cells (and other adherent cell lines) ranges from 2- to 4-fold, whereas downmodulation of HLA-A2 in T cells can range up to 200-fold (7). It is not clear whether these apparent cell type differences result from qualitative or quantitative differences in Nef function.
To further characterize the two pathways contributing to Nef-mediated MHC-I downmodulation, and to characterize any cell type-specific differences, we directly compared the relative effects of Nef on HLA-A2 expression in HeLa cells and T cells expressing equal amounts of Nef protein. We found that T cells were significantly more active at supporting Nef-mediated HLA-A2 downmodulation than HeLa cells, regardless of whether Nef was expressed in the context of HIV infection or alone. An examination of the mechanism of Nef-mediated HLA-A2 downmodulation in T cells revealed that the main effect of Nef was to inhibit HLA-A2 transport to the cell surface.
MATERIALS AND METHODS
Antibodies.
The monoclonal antibody BB7.2, which specifically recognizes the HLA-A2 allotype of MHC-I (31), was purified, using a Hitrap protein A FF column (Pharmacia) and following the manufacturer's protocol, from ascites or HB-82 hybridoma (American Type Culture Collection) supernatant. The antibody directed at placental alkaline phosphatase (PLAP) was obtained from Biomeda. The secondary antibodies used in this study were goat anti-mouse immunoglobulin G-phycoerythrin (PE; Caltag) for HLA-A2 stains and goat anti-rabbit-fluorescein isothiocyanate (Caltag) for PLAP stains. HIV-1 Nef antiserum for Western blotting was obtained from Ronald Swanstrom (AIDS Research and Reference Reagent Repository, Division of AIDS, National Institute of Allergy and Infection Diseases, National Institutes of Health) (38).
Cell lines and culture conditions.
HeLa cell lines were maintained in Dulbecco modified Eagle medium (DMEM) supplemented with 2 mM glutamine, 10% fetal bovine serum (FBS), penicillin, and streptomycin. The HeLa-A2 cell line has been described previously (12). CEM-SS lines were maintained in RPMI medium supplemented with 2 mM glutamine, 10% FBS, penicillin, and streptomycin. To create a stable CEM-SS T-cell line expressing HLA-A2, CEM-SS T cells were transduced with a murine stem cell virus retroviral construct that contained HLA-A2 (19, 32) and, using neomycin, were selected for expression. HLA-A2+ peripheral blood mononuclear cells were isolated from a buffy coat supplied by the Lansing Red Cross and were stimulated and infected as previously described (7).
HIV genome mutant construction and HIV transduction protocol.
Viruses were prepared from HIV-1 molecular clones and used to infect T cells as previously described (7) except that they were pseudotyped with pCMV VSV-G (Nancy Hopkins, Massachusetts Institute of Technology) to allow infection of HeLa cells. Briefly, 106 T cells or 100,000 HeLa cells were centrifuged at 2,500 rpm in a Sorvall tabletop centrifuge for 2 h with 1 ml of high-titer HIV supernatant prepared from transfected 293 cells (7), 4 μg of Polybrene/ml, and 10 mM HEPES. NL-PIenv− was constructed as follows. The SalI-NotI fragment of NL-PIrevHXB (7), containing the env open reading frame, was subcloned into pBluescript II KS. The NdeI site was filled in, disrupting the env open reading frame, and then the SalI-NotI fragment was religated back into NL-PI, creating NL-PIenv−. In this virus, PLAP is inserted into the Nef open reading frame and Nef expression is restored with a downstream internal ribosome entry site, as previously described (5, 7). The nef− version of this virus was created by making a frameshift mutation at the XhoI site in nef (7). NL-PIenv− achieved amounts of HLA-A2 downmodulation similar to those of another virus used in these studies, HXB-EP, which has PLAP inserted into the envelope open reading frame and expresses levels of Nef equivalent to those expressed by unmodified HIV molecular clones (references 5 and 7 and data not shown).
Adenovirus transduction.
A replication-defective adenoviral vector expressing HIV-1 Nef (adeno-Nef) and lacking a segment of the E3 region (including E319K) was constructed as previously described by the University of Michigan Vector Core Facility (39). The control virus (control-adeno) is identical to adeno-Nef except that it lacks the Nef open reading frame. Adenovirus transductions were performed as follows. HeLa cells or T cells were incubated with adenovirus in DMEM supplemented with 2% FBS. After 2 h, an equal volume of fresh DMEM plus 10% FBS was added. Cells were then incubated for 24 to 48 h prior to harvest. Multiplicity of infection (MOI) was based on 293 cell infectivity.
Analysis by fluorescence-activated cell sorting (FACS).
To assess the level of cell surface class I molecules or PLAP, cells were incubated with primary antibody for 20 min at 4°C in FACS buffer (phosphate-buffered saline, 2% FBS, 1% HEPES, 1% sodium azide), washed, incubated with the appropriate secondary antibody (see above), washed again, and analyzed (using CELLQuest software) on a Becton Dickinson FACScan apparatus.
Western blot analysis of Nef expression levels.
Cells were lysed in NP-40 lysis buffer (1% NP-40, 50 mM Tris [pH 7.3], 5 mM MgCl2, 1 mM phenylmethylsulfonyl fluoride), and equal amounts of protein were loaded onto a sodium dodecyl sulfate (SDS)-15% polyacrylamide gel. Samples were electroblotted onto Immobilon, blocked in 5% milk in TBS buffer (10 mM Tris [pH 8.0], 140 mM NaCl), and probed with HIV-1 Nef antiserum (see above). Blots were developed using Supersignal (Pierce) according to the manufacturer's instructions. Equal protein loading was confirmed by staining the polyacrylamide gel with Coomassie brilliant blue after transfer.
Endocytosis assays.
To assess the amount of HLA-A2 internalization, HeLa cells and T cells were treated with control-adeno or adeno-Nef at the indicated MOI. Approximately 24 h later, cells were harvested and incubated with BB7.2 anti-HLA-A2 monoclonal antibody for 20 min at 4°C, washed, and placed at 37°C and 5% CO2 for the indicated time periods in culture medium lacking serum. The cells were then washed and incubated with goat anti-mouse PE in FACS buffer containing 0.2% bovine serum albumin instead of serum. Using CELLQuest software, the cells were analyzed on a Becton Dickinson FACScan apparatus.
Recycling assays.
Recycling assays were performed as described previously (24), except that washes were performed at room temperature to avoid inhibiting recycling with cold temperature (4). Briefly, cells were treated with control-adeno or adeno-Nef at the indicated MOI. Approximately 24 h later, the cells were incubated with 150 μg of cycloheximide (Sigma)/ml for 2 to 3 h in DMEM (HeLa cells) or RPMI medium (T cells) plus 10% serum. Then cells were harvested, and an aliquot was removed and placed on ice. The remainder of the samples were stripped of stainable HLA-A2 by washing in 50 mM glycine-100 mM NaCl (at pH 3.0 for HeLa cells or pH 3.4 for T cells) at room temperature. The stripped cells were then washed in phosphate-buffered saline and incubated at 37°C and 5% CO2 in medium without serum for the indicated period of time. (Samples were incubated without serum to avoid substitution of bovine β2-microglobulin present in serum for the human β2-microglobulin removed with the stripping protocol.) Cells were then stained for HLA-A2 as described above.
Intracellular transport assays.
For the flow cytometric transport assay, cells were treated as described above for the recycling assay, except that samples were prepared in duplicate treatments with or without cycloheximide. To determine the amount of newly synthesized HLA-A2 appearing at each time point, the mean fluorescence intensity (MFI) of the cycloheximide-treated cells was subtracted from the MFI of the untreated cells at each time point. In addition, the background remaining after stripping at time zero was subtracted from each sample. To test the role of phosphatidylinositol (PI) 3-kinase, the indicated amount of LY294002 (Upstate Biotechnology) was added to cells at the same time as cycloheximide and was left in throughout the experiment.
The biochemical transport assay protocol used was similar to that reported previously (28). Briefly, CEM and CEM-A2 T cells were infected with VSV-G pseudotyped NLPIenv− with or without Nef expression, as previously described (7). At 48 h later, infected cells (6 × 106/time point) were pulse labeled for 30 min with 0.2 mCi of 35S-Pro-Mix (Amersham Pharmacia)/ml. The labeled cells were washed and incubated for the indicated chase period and surface labeled with 0.5 mg of sulfo-NHS-biotin (Pierce)/ml for 30 min at room temperature. The reaction was quenched in 25 mM lysine. The cells were then washed in 25 mM lysine and lysed in NP-40 lysis buffer (1% NP-40, 5 mM MgCl2, 50 mM Tris [pH 7.3], plus protease inhibitor cocktail [Boehringer Mannheim]). The lysates were immunoprecipitated with monoclonal antibody BB7.2 as previously described (45), except that the immune complexes were washed with radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris [pH 8], 150 mM NaCl, 1% NP-40, 0.5% deoxycholate, 0.1% SDS, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride). The precipitated sample was eluted from the beads by boiling for 5 min in 100 μl of 10% SDS. One-third was analyzed directly to assess total HLA-A2. The remaining two-thirds was diluted 20-fold in RIPA buffer and reprecipitated using avidin agarose beads (Calbiochem) for 24 h as previously described (28). Total and cell surface HLA-A2 was then analyzed by SDS-polyacrylamide gel electrophoresis (PAGE).
RESULTS
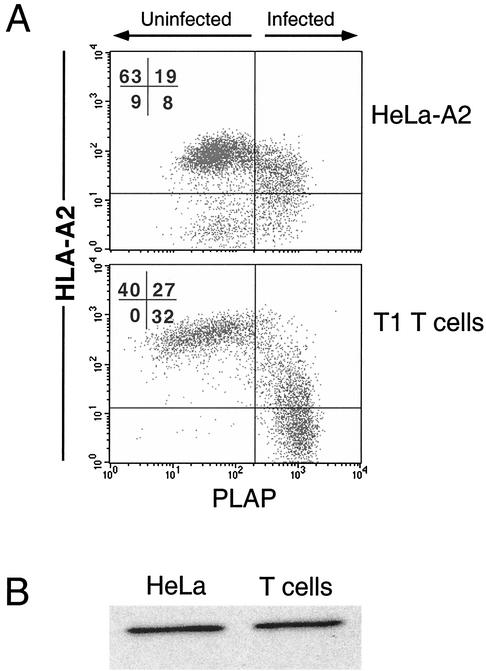
To determine whether HeLa cells and T cells (two cell types commonly used to study Nef-mediated downmodulation) differed in their sensitivity to Nef, we examined the effects of Nef on HeLa cells stably expressing HLA-A2 (HeLa-A2) and T1 T cells that expressed endogenous HLA-A2. These cells were infected with a version of NL4-3 (NL-PIenv−) that had been pseudotyped with VSV-G protein to allow transduction of HeLa cells (which are CD4−). NL-PIenv− is an HIV molecular clone that expresses the marker gene encoding PLAP, allowing specific identification of HIV-infected cells (5, 7). Infection of HeLa cells with this virus resulted in a 2-fold downmodulation of HLA-A2 (MFI decreased from 71 to 34), whereas HIV infection of T1 T cells resulted in a 10-fold downmodulation (MFI decreased from 400 to 60) (Fig. 1A). Western blot analysis comparing Nef expression levels in cell lysates normalized for infection rate revealed that there was no significant difference that would explain this result (Fig. 1B). There was no detectable downmodulation of HLA-A2 in cells treated with a control virus that lacked Nef expression (reference 7 and data not shown).
FIG. 1.
HIV-infected T cells downmodulate MHC-I more efficiently than HIV-infected HeLa cells. (A) HeLa-A2 cells or T1 T cells were transduced with an HIV (NL-PIenv−) pseudotyped with VSV-G protein as described in Materials and Methods. At 48 h, the cells were harvested and stained for HLA-A2 and PLAP. Control uninfected cells fell to the left of the vertical line, and control HLA-A2-negative cells fell below the horizontal line. (B) Western blot analysis of Nef expression in HeLa and T1 cells was performed on the indicated cell lines normalized for total protein and infection rate. Results shown are typical of at least three separate experiments.
To rule out the possibility that differences in Nef activity were due to another HIV factor that might behave differently in HeLa versus T cells, we assessed downmodulation of HLA-A2 by Nef expressed alone in a replication-defective adenoviral vector. It has previously been determined that expression of Nef in this context results in specific downmodulation of wild-type HLA-A2 but not HLA-A2 mutants that do not respond to Nef (12, 39, 45). Additionally, Nef mutants known to be defective at HLA-A2 downmodulation behaved appropriately when expressed in the adenoviral vector system (12, 39, 45).
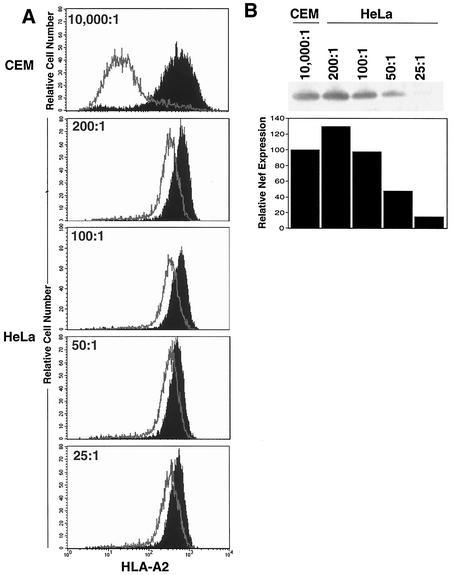
In agreement with the data shown in Fig. 1, when CEM T cells stably expressing HLA-A2 (CEM-A2) and HeLa-A2 cells were transduced with adeno-Nef, T cells responded much more robustly. In T cells, the HLA-A2 MFI decreased from 699 to 61 (11-fold downmodulation), whereas HeLa cell HLA-A2 MFI decreased from 635 to 343 at best (MOI = 200:1 Fig. 2A). This was not due to differences in Nef expression based on 293 cell infectivity; because at the highest Nef level tested, there was more Nef protein expressed in the HeLa cell lysates than in the T-cell lysates (Fig. 2B). Thus, it seems that T cells and HeLa cells responded differently to similar amounts of Nef protein. In both cell types, there was no detectable downmodulation by the control adenovirus, which differed only in that it lacked the nef open reading frame (data not shown).
FIG. 2.
Nef expressed in an adenoviral vector downmodulates MHC-I more efficiently in T cells than in HeLa cells. HeLa-A2 and CEM-A2 cells were transduced with the indicated amount of adeno-Nef or control-adeno as described in Materials and Methods. (A) After 48 h, the cells were stained with the HLA-A2-specific monoclonal antibody BB7.2 to assess HLA-A2 expression levels by using flow cytometry. Nef reduced the expression of HLA-A2 by 11-fold in T cells and 1.2- to 2.7-fold in HeLa cells. (B) Western blot analysis assessing Nef expression levels revealed that HeLa cells treated with adeno-Nef at an estimated MOI of 100:1 (as determined in 293 cells) had Nef expression similar to that of CEM T cells treated at an MOI of 10,000:1. Results shown are typical of at least five separate experiments.
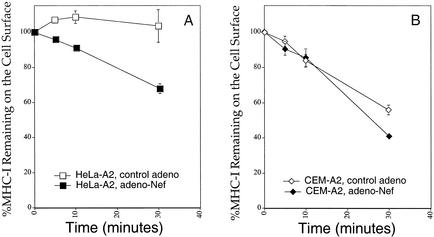
To further explore the cell type-specific effect of Nef shown in Fig. 1 and 2, we examined the rate of Nef-mediated HLA-A2 endocytosis, recycling, or intracellular transport in the two cell types. To assess endocytosis rates, HeLa-A2 cells and CEM-A2 cells were transduced with adeno-Nef or control-adeno, adjusted so that there were equal amounts of Nef in the HeLa cells and the CEM T cells (MOI = 10,000:1 for CEM and 100:1 for HeLa). At 24 h later, the cells were stained with antibodies directed at HLA-A2 and incubated at 37°C for the indicated time to allow internalization. They were then stained with a secondary antibody conjugated to PE and assayed by flow cytometry. In agreement with prior reports (24), there was a significant Nef-dependent stimulation of endocytosis in HeLa cells (Fig. 3A). In HeLa cells without Nef, there was internalization of 0 to 10% of HLA-A2 in 30 min, and this increased to 35% when Nef was expressed. As reported previously, this effect of Nef was relatively small compared to the rate of internalization of an HLA-A2 molecule with a canonical endocytosis signal (YSQL) in its cytoplasmic tail. Approximately 70% of HLA-A2 molecules with a YSQL signal are removed from the cell surface in 10 min (24).
FIG. 3.
Nef has a small effect on endocytosis rates in HeLa cells and a lesser effect on endocytosis rates in T cells. The endocytosis rates of MHC-I in CEM-A2 (A) or HeLa-A2 (B) cells 24 h after treatment with adeno-Nef or control-adeno were measured as described in Materials and Methods. Shown are the means ± standard deviations for one experiment performed in triplicate. Results shown are typical of at least two separate experiments. At the MOIs of adenovirus used in these experiments (100:1 for HeLa and 10,000:1 for CEM T cells), Nef was expressed at similar levels (Fig. 2).
In contrast, HLA-A2 in T cells is continuously endocytosed and recycled even without Nef expression (21, 27, 41, 42, 44, 46). Indeed, we observed that without Nef, approximately 45% of HLA-A2 in CEM-A2 cells was internalized in 30 min (Fig. 3B). Nef expression increased the amount of HLA-A2 internalized during this time period by only about 15% to a total of 60% internalized in 30 min (Fig. 3B). Based on these results, it seems unlikely that this small Nef-dependent effect on endocytosis can explain the dramatic effects of Nef on steady-state HLA-A2 surface expression in T cells. In addition, these data do not explain why T cells respond to Nef more robustly than HeLa cells.
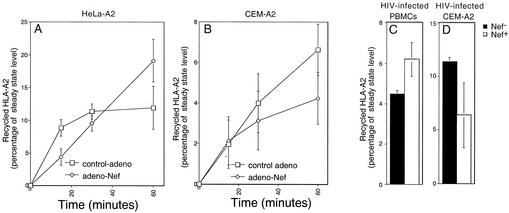
To determine whether the major effect of Nef on HLA-A2 surface levels in T cells was due to effects on recycling, we performed recycling assays, as previously described (24), using T cells or HeLa cells treated with adeno-Nef and expressing similar amounts of Nef protein. To measure the amount of HLA-A2 that was recycled under these conditions, surface HLA-A2 complexes on the plasma membrane were denatured by washing cells with an acidic buffer. By disrupting the noncovalent association between the HLA-A2 heavy and light chains, this treatment destroys the ability of HLA-A2 to be recognized by the anti-HLA-A2 monoclonal antibody BB7.2. The treated cells were then incubated at 37°C, and the appearance of recycled HLA-A2 on the cell surface was measured by flow cytometry over time. To ensure that only the recycled HLA-A2 molecules were measured, protein synthesis was inhibited by pretreatment for 2 to 3 h with cycloheximide (150 μg/ml). Using this assay, we tested the effect of Nef on recycling in adeno-Nef-transduced HeLa cells (Fig. 4A), adeno-Nef-transduced CEM-A2 cells (Fig. 4B), HIV-infected primary T cells (Fig. 4C), and HIV-infected CEM-A2 cells (Fig. 4D). In agreement with prior experiments performed with HeLa cells (24), we did not observe significant inhibition of recycling by Nef within the error of the assay system.
FIG. 4.
Nef does not significantly inhibit recycling of MHC-I to the cell surface in HeLa cells or T cells. The rates of MHC-I recycling in HeLa-A2 (A) or CEM-A2 (B) cells 24 h after treatment with adeno-Nef or control-adeno were measured as described in Materials and Methods. Shown are the means ± standard deviations for three separate experiments. At the MOIs of adenovirus used in these experiments (50:1 for HeLa and 10,000:1 for CEM T cells), there was about half as much Nef expressed in HeLa cell lysates as in T-cell lysates normalized for total protein (Fig. 2B). Additional experiments did not detect a significant Nef-dependent inhibition of HLA-A2 recycling in HIV-infected primary T cells (C) or HIV-infected CEM T cells (D) after a 60-min incubation.
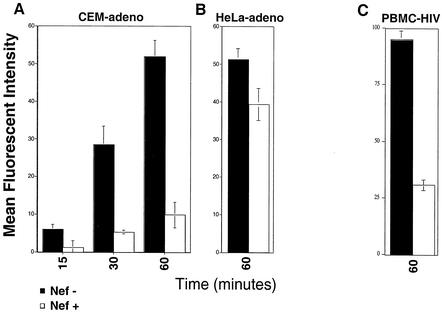
To examine the relative effect of Nef on HLA-A2 intracellular transport, the appearance of newly synthesized HLA-A2 on the cell surface, with or without Nef expression, was quantified over time. This was accomplished by stripping surface HLA-A2 as described above and measuring its reappearance on the cell surface in the absence of protein synthesis inhibitors. To correct for HLA-A2 appearing on the cell surface as a result of recycling from internal compartments, a duplicate set of cycloheximide-treated samples was run in parallel. The amount of newly synthesized material transported to the cell surface was then calculated for each time point by subtracting the amount of HLA-A2 that had appeared on the cell surface as a result of recycling.
When this assay was performed using T cells treated with adeno-Nef or control-adeno, we observed a dramatic (up to eightfold) reduction of newly synthesized HLA-A2 appearing on the surface of cells expressing Nef (Fig. 5A). This large effect of Nef on transport of newly synthesized HLA-A2 to the cell surface contrasts with the relatively small effect of Nef on endocytosis and recycling rates in T cells. Thus, it is likely that the dramatic effect of Nef on the appearance of newly synthesized HLA-A2 at the plasma membrane results primarily from a transport block rather than from extremely rapid endocytosis of newly synthesized HLA-A2 as soon as it appears on the cell surface.
FIG. 5.
Nef blocks the transport of newly synthesized MHC-I to the cell surface in T cells. (A) Transport of HLA-A2 to the cell surface in T cells transduced with a Nef-expressing or control adenovirus at an MOI of 10,000:1. Shown here is a summary graph of the MFI at each time point corrected for recycling and background expression as described in Materials and Methods. The means ± standard deviations for two separate experiments are shown. (B) Transport of HLA-A2 to the cell surface in HeLa cells was measured as described for panel A, except that only the 60-min time point was measured. At the MOI of adenovirus used in these experiments (100:1), Nef expression was comparable to that in CEM T cells transduced at an MOI of 10,000:1 (Fig. 2). These results, from assays performed in duplicate, are typical of at least three experiments. (C) Transport of HLA-A2 to the cell surface in primary T cells infected with HXB-EP with or without Nef expression (5). Shown here is the average MFI ± standard deviation of newly synthesized HLA-A2 in PLAP+ (HIV-infected) cells after 60 min of incubation with or without Nef expression.
The effect of similar Nef levels on HLA-A2 transport to the cell surface was much less dramatic in HeLa cells. In these cells, we observed a relatively small (1.3-fold) reduction of HLA-A2 transport at similar Nef levels (Fig. 5B). (At Nef expression levels that are higher than those which are typically required for HLA-A2 downmodulation in T cells, a greater effect of Nef on HLA-A2 transport was measured in HeLa cells [2.9-fold at an MOI of 500:1; data not shown]). Based on these data, Nef is more active in T cells than HeLa cells because it is better able to disrupt HLA-A2 transport to the plasma membrane in T cells.
To confirm the physiologic significance of these data, we asked whether there were defects in transport of HLA-A2 to the cell surface of primary T cells. This question was addressed by preparing HLA-A2+ primary T cells and transducing them with HXB-EP with or without Nef expression, as previously described (7). Then, the capacity of the infected cell subset (approximately 10% of the cells as assessed by PLAP expression) to transport HLA-A2 to the cell surface was measured, using the protocol outlined above, by two-color flow cytometry. We found that there was a dramatic, Nef-dependent block in transport of HLA-A2 to the surface in the PLAP+ Nef-expressing cells but not in the PLAP+ nef− cells (Fig. 5C). These data strongly suggest that HIV disrupts the trafficking of HLA-A2 in T cells primarily by preventing transport of newly synthesized molecules to the cell surface.
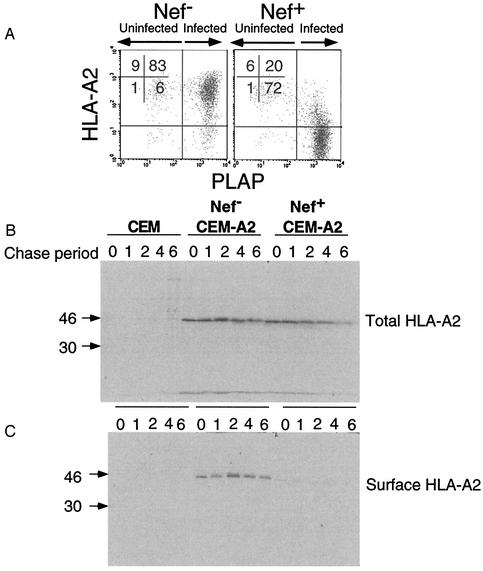
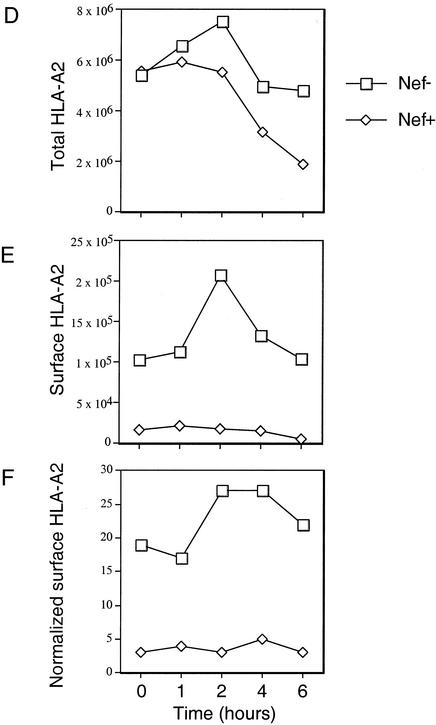
To confirm these results, we used a biochemical system that more directly distinguished newly synthesized molecules from recycling molecules. Briefly, CEM-A2 cells were infected with NL-PIenv− with or without nef expression. After 48 h, most of the CEM-A2 cells treated in this manner are generally infected and downmodulate HLA-A2 approximately 50-fold (Fig. 6A). The infected cells were pulse labeled and then surface labeled with biotin as described in Materials and Methods. They were then lysed, and HLA-A2 was immunoprecipitated using monoclonal antibody BB7.2. Two-thirds of the immunoprecipitate was reprecipitated with avidin beads to specifically pull down only the molecules that were on the cell surface. The results of this experiment, shown in Fig. 6B to E, indicate that Nef reduced the amount of newly synthesized HLA-A2 appearing on the cell surface by eightfold. As was previously reported, Nef also induced a comparatively small (approximately two- to threefold) effect on the stability of the HLA-A2 protein (37, 45).
FIG. 6.
Nef blocks the transport of newly synthesized MHC-I to the cell surface in T cells. (A) CEM and CEM-A2 T cells were infected with NL-PIenv− with or without nef expression, harvested after 48 h, and stained for HLA-A2 and PLAP. As shown, almost all the cells are infected and Nef-expressing cells had approximately 50-fold less HLA-A2 expression. (B and C) HIV-infected CEM and CEM-A2 T cells were pulse labeled with [35S]methionine and cysteine and were then surface labeled with biotin as described in Materials and Methods. Lysates were made, and HLA-A2 was immunoprecipitated with monoclonal antibody BB7.2. (B) One-third of the immunoprecipitate was analyzed by SDS-PAGE to assess total HLA-A2 expression. (C) Two-thirds of the immunoprecipitate was reprecipitated with avidin beads to isolate cell surface HLA-A2 prior to analysis by SDS-PAGE. (D to F) The amount of total HLA-A2 (panel D) or surface HLA-A2 (panel E) or the percentage of recovered surface HLA-A2 relative to total HLA-A2 (panel F) was determined from the gels shown in panels B and C by using a phosphorimager. Results obtained were similar in three independent experiments.
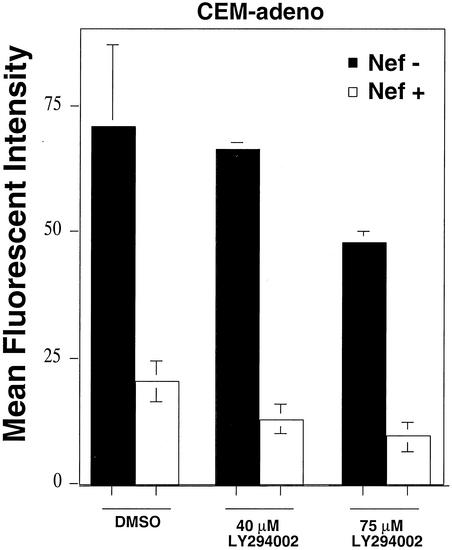
It was previously reported that the phosphatidylinositol (PI) 3-kinase inhibitor, LY294002, inhibits Nef's ability to block intracellular transport in astrocytic cells but not Nef's effects on CD4, supporting the idea that Nef affects MHC-I and CD4 by different mechanisms (39). To determine whether LY294002 differentially affected Nef-mediated HLA-A2 downmodulation in HeLa cells and T cells, we transduced these cells with adeno-Nef or control-adeno and treated them with 40 or 75 μM LY294002 overnight. Under these conditions, LY294002 inhibited downmodulation of HLA-A2 in astrocytic cells and T cells but not in HeLa cells (data not shown). However, we noted greater toxicity of the inhibitor for T cells than for the other cell types. In addition, the inhibitor reduced baseline HLA-A2 levels in T cells. Therefore, to confirm the effect of the inhibitor, we asked whether it had an effect when added for the shorter (4 h) time period of incubation required for the flow cytometric transport assay. Under these conditions, Nef-expressing T cells treated with the inhibitor remained defective for HLA-A2 transport to the cell surface (Fig. 7). These data appear to rule out the simple model that Nef links MHC-I to PI 3-kinase to disrupt MHC-I trafficking and suggest that the effect of the inhibitor over longer time periods is indirect—perhaps affecting Nef localization or a posttranslational modification of Nef.
FIG. 7.
LY294002 does not inhibit Nef's ability to disrupt transport of HLA-A2 to the cell surface in CEM-A2 T cells. T cells were transduced with a Nef-expressing or control adenovirus at an MOI of 10,000:1. Transport of newly synthesized HLA-A2 to the cell surface was measured flow cytometrically, with or without the addition of 40 or 75 μM LY294002 as described in Materials and Methods. Shown here is a summary graph of the MFI at each time point corrected for recycling and background expression as described in Materials and Methods. The means ± standard deviations are shown. Results were similar in two independent experiments. DMSO, dimethyl sulfoxide.
DISCUSSION
Multiple studies have now demonstrated that HIV Nef downmodulates MHC-I and that this process affects CTL recognition in vitro (7, 47) and HIV pathogenicity in vivo (15, 30). However, the molecular mechanism has remained unclear. In addition, the biological relevance of MHC-I downmodulation has remained controversial. This is due, in part, to the wide range of efficacy reported in the literature. These differences might result from the need for allotype-specific antibodies to distinguish the subset of MHC-I molecules affected by Nef (6, 7, 25) and the need for high Nef levels in some cell types (25, 26). As shown here, a direct comparison reveals that similar amounts of Nef caused a much more dramatic downmodulation in T cells than in some adherent cell lines, such as HeLa cells. In T cells, Nef levels equivalent to those achieved by natural HIV genomes are sufficient to promote dramatic (up to 200-fold) effects on HLA-A2 levels in primary T cells (7).
Nef has been reported to affect endocytosis rates in T-cell lines stably expressing Nef and to block transport of newly synthesized HLA-A2 to the cell surface in astrocytic cells transiently transduced with adeno-Nef (37, 39). However, it was not known whether both of these pathways were active in all cell types or in HIV-infected cells. Furthermore, if both pathways were active, it was not known whether one might be more important than the other. To further explore these questions, we examined Nef's effects on HLA-A2 endocytosis, recycling, and transport to the cell surface in T cells. We found that the effects of Nef on HLA-A2 transport to the cell surface were much more dramatic than its effects on endocytosis or recycling. In addition, the differential effect of Nef on HLA-A2 transport to the cell surface explained the differences in degree of downmodulation in HeLa cells versus T cells. Moreover, we were able to demonstrate that Nef blocked the transport of HLA-A2 to the cell surface in HIV-infected T cells.
The precise molecular mechanism for the greater efficacy of Nef in T cells requires further study. It is possible that HeLa cells transfected to express high levels of HLA-A2 have a relative deficiency in a required cellular factor. Alternatively, HLA-A2 and/or Nef might be processed differently in T cells. A greater understanding of why these cells behave differently is likely to provide insight into the mechanism of Nef-mediated MHC-I downmodulation and normal MHC-I biology.
To downmodulate MHC-I, Nef physically associates with the cytoplasmic tail of MHC-I (45) and might then act as an adaptor molecule, linking MHC-I with other cellular proteins that bind Nef. For example, there are a number of proteins involved in intracellular trafficking that can physically interact with Nef and are candidate factors that can affect MHC-I trafficking (e.g., PACS-1 [35] and β-COP [2, 34]). Other trafficking proteins that have been reported to associate with Nef (vacuolar ATPase and adaptin complexes [AP-1 and AP-2]) might not be necessary for MHC-I downmodulation, because amino acids in Nef required for association of Nef with these molecules are not needed for MHC-I downmodulation (16, 17, 29). Further research is needed to determine what cellular factors are important for Nef-mediated MHC-I downmodulation in T cells.
The mechanism of Nef-mediated MHC-I downmodulation contrasts with that of Nef-mediated CD4 and CD28 downmodulation. Nef is thought to reduce cell surface expression of CD4 (and CD28) by binding to their cytoplasmic tails and linking them to the endocytic apparatus via associations with adaptins and/or vacuolar ATPase (3, 11, 13, 14, 16, 18, 22, 33, 40). It will be important for future studies to elucidate how HIV Nef is able to differentially associate with these cellular factors and use distinct mechanisms to affect the trafficking of CD4, MHC-I, and CD28.
The data presented here put the relative effect of Nef on MHC-I endocytosis and intracellular transport in perspective by using a cell type that is a natural target of HIV infection. The results of these studies highlight the need to understand the effect of Nef on inhibition of MHC-I intracellular transport to the cell surface—a new function for this complex protein.
Acknowledgments
We thank Tracey Filzen for excellent technical assistance, the University of Michigan Gene Vector Core facility for supplying recombinant adenovirus, and the Michigan Flow Cytometry Core for assistance in developing cell lines. We are grateful to members of the Collins laboratory for helpful suggestions and critical reading of the manuscript.
This publication was made possible by NIH grant RO1 AI46998, a grant from the Pew Charitable Trusts, and the University of Michigan Biological Scholars program. M.R.K. was supported by a University of Michigan Genetics Training Grant.
REFERENCES
- 1.Altschuler, Y., S. Liu, L. Katz, K. Tang, S. Hardy, F. Brodsky, G. Apodaca, and K. Mostov. 1999. ADP-ribosylation factor 6 and endocytosis at the apical surface of Madin-Darby canine kidney cells. J. Cell Biol. 147:7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benichou, S., M. Bomsel, M. Bodeus, H. Durand, M. Doute, F. Letourneur, J. Camonis, and R. Benarous. 1994. Physical interaction of the HIV-1 Nef protein with β-COP, a component of non-clathrin-coated vesicles essential for membrane traffic. J. Biol. Chem. 269:30073-30076. [PubMed] [Google Scholar]
- 3.Bresnahan, P., W. Yonemoto, S. Ferrell, D. Williams-Herman, R. Geleziunas, and W. Greene. 1998. A dileucine motif in HIV-1 Nef acts as an internalization signal for CD4 downregulation and binds the AP-1 clathrin adaptor. Cur. Biol. 8:1235-1238. [DOI] [PubMed] [Google Scholar]
- 4.Caplan, S., N. Naslavsky, L. M. Hartnell, R. Lodge, R. S. Polishchuk, J. G. Donaldson, and J. S. Bonifacino. 2002. A tubular EHD1-containing compartment involved in the recycling of major histocompatibility complex class I molecules to the plasma membrane. EMBO J. 21:2557-2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, B., R. Gandhi, and D. Baltimore. 1996. CD4 down-modulation during infection of human T cells with human immunodeficiency virus type 1 involves independent activities of vpu, env, and nef. J. Virol. 70:6044-6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen, G. B., R. T. Gandhi, D. M. Davis, O. Mandelboim, B. K. Chen, J. L. Strominger, and D. Baltimore. 1999. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity 10:661-671. [DOI] [PubMed] [Google Scholar]
- 7.Collins, K., B. Chen, S. Kalams, B. Walker, and D. Baltimore. 1998. HIV-1 Nef protein protects infected primary human cells from killing by cytotoxic T lymphocytes. Nature 391:397-401. [DOI] [PubMed] [Google Scholar]
- 8.Damke, H., T. Baba, D. Warnock, and S. Schmid. 1994. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J. Cell Biol. 127:915-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delaney, K. A., M. M. Murph, L. M. Brown, and H. Radhakrishna. 2002. Transfer of M2 muscarinic acetylcholine receptors to clathrin-derived early endosomes following clathrin-independent endocytosis. J. Biol. Chem. 277:33439-33446. [DOI] [PubMed] [Google Scholar]
- 10.Doms, R. W., and D. Trono. 2000. The plasma membrane as a combat zone in the HIV battlefield. Genes Dev. 14:2677-2688. [DOI] [PubMed] [Google Scholar]
- 11.Erdtmann, L., K. Janvier, G. Raposo, H. M. Craig, P. Benaroch, C. Berlioz-Torrent, J. C. Guatelli, R. Benarous, and S. Benichou. 2000. Two independent regions of HIV-1 Nef are required for connection with the endocytic pathway through binding to the mu 1 chain of AP1 complex. Traffic 1:871-883. [DOI] [PubMed] [Google Scholar]
- 12.Fleis, R., T. Filzen, and K. Collins. 2002. Species-specific effects of HIV-1 Nef-mediated MHC-I downmodulation. Virology 303:120-129. [DOI] [PubMed] [Google Scholar]
- 13.Foti, M., A. Mangasarian, V. Piguet, D. P. Lew, K. H. Krause, D. Trono, and J. L. Carpentier. 1997. Nef-mediated clathrin-coated pit formation. J. Cell Biol. 139:37-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geyer, M., H. Yu, R. Mandic, T. Linnemann, Y. H. Zheng, O. T. Fackler, and B. M. Peterlin. 2002. Subunit H of the V-ATPase binds to the medium chain of AP-2 and connects Nef to the endocytic machinery. J. Biol. Chem. 277:28521-28529. [DOI] [PubMed] [Google Scholar]
- 15.Glushakova, S., J. Münch, S. Carl, T. C. Greenough, J. L. Sullivan, L. Margolis, and F. Kirchhoff. 2001. CD4 down-modulation by human immunodeficiency virus type 1 Nef correlates with the efficiency of viral replication and with CD4+ T-cell depletion in human lymphoid tissue ex vivo. J. Virol. 75:10113-10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenberg, M., L. DeTulleo, I. Rapoport, J. Skowronski, and T. Kirchhausen. 1998. A dileucine motif in HIV-1 Nef is essential for sorting into clathrin-coated pits and for downregulation of CD4. Curr. Biol. 8:1239-1242. [DOI] [PubMed] [Google Scholar]
- 17.Greenberg, M., A. Iafrate, and J. Skowronski. 1998. The SH3 domain-binding surface and an acidic motif in HIV-1 Nef regulate trafficking of class I MHC complexes. EMBO J. 17:2777-2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenberg, M. E., S. Bronson, M. Lock, M. Neumann, G. N. Pavlakis, and J. Skowronski. 1997. Co-localization of HIV-1 Nef with the AP-2 adaptor protein complex correlates with Nef-induced CD4 down-regulation. EMBO J. 16:6964-6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hawley, R., F. Lieu, A. Fong, and T. Hawley. 1994. Versatile retroviral vectors for potential use in gene therapy. Gene Ther. 1:136-138. [PubMed] [Google Scholar]
- 20.Henley, J. R., E. W. Krueger, B. J. Oswald, and M. A. McNiven. 1998. Dynamin-mediated internalization of caveolae. J. Cell Biol. 141:85-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hochman, J. H., H. Jiang, L. Matyus, M. Edidin, and B. Pernis. 1991. Endocytosis and dissociation of class I MHC molecules labeled with fluorescent beta-2 microglobulin. J. Immunol. 146:1862-1867. [PubMed] [Google Scholar]
- 22.Janvier, K., H. Craig, S. Le Gall, R. Benarous, J. Guatelli, O. Schwartz, and S. Benichou. 2001. Nef-induced CD4 downregulation: a diacidic sequence in human immunodeficiency virus type 1 Nef does not function as a protein sorting motif through direct binding to β-COP. J. Virol. 75:3971-3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kestler, H., D. Ringler, K. Mori, D. Panicali, P. Sehgal, M. Daniel, and R. Desrosiers. 1991. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 65:651-652. [DOI] [PubMed] [Google Scholar]
- 24.Le Gall, S., F. Buseyne, A. Trocha, B. Walker, J. Heard, and O. Schwartz. 2000. Distinct trafficking pathways mediate Nef-induced and clathrin-dependent major histocompatibility complex class I down-regulation. J. Virol. 74:9256-9266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le Gall, S., L. Erdtmann, S. Benichou, C. Berlioz-Torrent, L. Liu, R. Benarous, J. Heard, and O. Schwartz. 1998. Nef interacts with mu subunit of clathrin adaptor complexes and reveals a cryptic sorting signal in MHC I molecules. Immunity 8:483-495. [DOI] [PubMed] [Google Scholar]
- 26.Liu, X., J. A. Schrager, G. D. Lange, and J. W. Marsh. 2001. HIV Nef-mediated cellular phenotypes are differentially expressed as a function of intracellular Nef concentrations. J. Biol. Chem. 276:32763-32770. [DOI] [PubMed] [Google Scholar]
- 27.Machy, P., A. Truneh, D. Gennaro, and S. Hoffstein. 1987. Major histocompatibility complex class I molecules internalized via coated pits in T lymphocytes. Nature 328:724-726. [DOI] [PubMed] [Google Scholar]
- 28.Mangasarian, A., F. Michelangelo, D. Aiken, D. Chin, J. Carpentier, and D. Trono. 1997. The HIV-1 Nef protein acts as a connector with sorting pathways in the Golgi and at the plasma membrane. Immunity 6:67-77. [DOI] [PubMed] [Google Scholar]
- 29.Mangasarian, A., V. Piguet, J. Wang, Y. Chen, and D. Trono. 1999. Nef-induced CD4 and major histocompatibility complex class I (MHC-I) down-regulation are governed by distinct determinants: N-terminal alpha helix and proline repeat of Nef selectively regulate MHC-I trafficking. J. Virol. 73:1964-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Münch, J., N. Stolte, D. Fuchs, C. Stahl-Hennig, and F. Kirchhoff. 2001. Efficient class I major histocompatibility complex down-regulation by simian immunodeficiency virus Nef is associated with a strong selective advantage in infected rhesus macaques. J. Virol. 75:10532-10536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parham, P., and F. M. Brodsky. 1981. Partial purification and some properties of BB7.2. A cytotoxic monoclonal antibody with specificity for HLA-A2 and a variant of HLA-A28. Hum. Immunol. 3:277-299. [DOI] [PubMed] [Google Scholar]
- 32.Pear, W. S., G. P. Nolan, M. L. Scott, and D. Baltimore. 1993. Production of high-titer helper-free retroviruses by transient transfection. Proc. Natl. Acad. Sci. USA 90:8392-8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piguet, V., Y. L. Chen, A. Mangasarian, M. Foti, J. L. Carpentier, and D. Trono. 1998. Mechanism of Nef-induced CD4 endocytosis: Nef connects CD4 with the mu chain of adaptor complexes. EMBO J. 17:2472-2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piguet, V., F. Gu, M. Foti, N. Demaurex, J. Gruenberg, J. Carpentier, and D. Trono. 1999. Nef-induced CD4 degradation: a diacidic-based motif in Nef functions as a lysosomal targeting signal through the binding of beta-COP in endosomes. Cell 97:63-73. [DOI] [PubMed] [Google Scholar]
- 35.Piguet, V., L. Wan, C. Borel, A. Mangasarian, N. Demaurex, G. Thomas, and D. Trono. 2000. HIV-1 Nef protein binds to the cellular protein PACS-1 to downregulate class I major histocompatibility complexes. Nat. Cell Biol. 2:163-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Radhakrishna, H., and J. G. Donaldson. 1997. ADP-ribosylation factor 6 regulates a novel plasma membrane recycling pathway. J. Cell Biol. 139:49-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartz, O., V. Marechal, S. Le Gall, F. Lemonnier, and J. Heard. 1996. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat. Med. 2:338-342. [DOI] [PubMed] [Google Scholar]
- 38.Shugars, D., M. Smith, D. Glueck, P. Nantermet, F. Seillier-Moiseiwitsch, and R. Swanstrom. 1993. Analysis of human immunodeficiency virus type 1 nef gene sequences present in vivo. J. Virol. 67:4639-4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swann, S. A., M. Williams, C. M. Story, K. R. Bobbitt, R. Fleis, and K. L. Collins. 2001. HIV-1 Nef blocks transport of MHC class I molecules to the cell surface via a PI 3-kinase-dependent pathway. Virology 282:267-277. [DOI] [PubMed] [Google Scholar]
- 40.Swigut, T., N. Shohdy, and J. Skowronski. 2001. Mechanism for down-regulation of CD28 by Nef. EMBO J. 20:1593-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tse, D. B., C. R. Cantor, J. McDowell, and B. Pernis. 1986. Recycling class I MHC antigens: dynamics of internalization, acidification, and ligand-degradation in murine T lymphoblasts. J. Mol. Cell. Immunol. 2:315-329. [PubMed] [Google Scholar]
- 42.Tse, D. B., and B. Pernis. 1984. Spontaneous internalization of class I major histocompatibility complex molecules in T lymphoid cells. J. Exp. Med. 159:193-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van der Bliek, A. M., T. E. Redelmeier, H. Damke, E. J. Tisdale, E. M. Meyerowitz, and S. L. Schmid. 1993. Mutations in human dynamin block an intermediate stage in coated vesicle formation. J. Cell Biol. 122:553-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weber, D. A., L. B. Buck, T. M. Delohery, N. Agostino, and B. Pernis. 1990. Class II MHC molecules are spontaneously internalized in acidic endosomes by activated B cells. J. Mol. Cell. Immunol. 4:255-266. [PubMed] [Google Scholar]
- 45.Williams, M., J. F. Roeth, M. R. Kasper, R. I. Fleis, C. G. Przybycin, and K. L. Collins. 2002. Direct binding of human immunodeficiency virus type 1 Nef to the major histocompatibility complex class I (MHC-I) cytoplasmic tail disrupts MHC-I trafficking. J Virol 76:12173-12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamane, Y., M. Perez, R. Edelson, N. Agostino, and B. Pernis. 1991. Endocytosis of the TCR/CD3 complex and the class-I major histocompatibility complex in a human T cell line. Cell. Immunol. 136:496-503. [DOI] [PubMed] [Google Scholar]
- 47.Yang, O. O., P. T. Nguyen, S. A. Kalams, T. Dorfman, H. G. Gottlinger, S. Stewart, I. S. Chen, S. Threlkeld, and B. D. Walker. 2002. Nef-mediated resistance of human immunodeficiency virus type 1 to antiviral cytotoxic T lymphocytes. J Virol. 76:1626-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]