Abstract
The human immunodeficiency virus Tat protein is essential for virus replication and is a candidate vaccine antigen. Macaques immunized with Tat or chemically modified Tat toxoid having the same clade B sequence developed strong antibody responses. We compared these antisera for their abilities to recognize diverse Tat sequences. An overlapping peptide array covering three clade B and two clade C Tat sequences was constructed to help identify reactive linear epitopes. Sera from Tat-immunized macaques were broadly cross-reactive with clade B and clade C sequences but recognized a clade B-specific epitope in the basic domain. Sera from Tat toxoid-immunized macaques had a more restricted pattern of recognition, reacting mainly with clade B and with only one clade B basic domain sequence, which included the rare amino acids RPPQ at positions 57 to 60. Monoclonal antibodies against the amino terminus or the domain RPPQ sequence blocked Tat uptake into T cells and neutralized Tat in a cell-based transactivation assay. Macaques immunized with Tat or Tat toxoid proteins varied in their responses to minor epitopes, but all developed a strong response to the amino terminus, and antisera were capable of neutralizing Tat in a transactivation assay.
The human immunodeficiency virus type 1 (HIV-1) Tat protein is required for virus replication and pathogenesis. Tat is produced early in the virus life cycle from a multiply spliced mRNA and is transported back into the cell nucleus, where it interacts with host factors and the TAR region of viral RNA to relieve a block of transcript elongation and increase viral gene expression (reviewed in reference 30). Extracellular Tat has distinct functions that may indirectly promote virus replication and disease (20, 21) either through receptor-mediated signal transduction (2, 47) or after internalization and transport to the nucleus (17, 18, 35). These major properties of Tat, i.e., early expression to increase viral gene transcription and indirect effects as an extracellular factor, prompted efforts to develop this protein as an HIV-1 vaccine antigen.
The Tat protein is encoded by two exons near the center of the viral genome. The first exon encodes amino acids (aa) 1 to 72, and the second exon encodes aa 73 to 101, although naturally occurring Tat sequences may be up to 113 aa long (30). A mutation in some laboratory isolates (IIIB strains) created an 86-aa version that is sufficient for virus replication in vitro and is the form of Tat studied most often. The Tat protein itself contains several functional subdomains. The amino terminus (aa 1 to 20), cysteine-rich domain (aa 21 to 40), and core region (aa 1 to 48) together constitute the minimal activation domain for transcription in vitro (30). The N-terminal portion of Tat binds cell surface CD26 with high affinity and is believed to be responsible for CD26-mediated immunosuppressive activity (26, 49, 57). The cysteine-rich domain has homology to chemokines and mediates binding to chemokine receptors (1, 2, 16). The basic domain of Tat protein (aa 45 to 56), characterized by a high content of lysines and arginines, is required for binding to short RNA transcripts containing the viral transactivation-responsive element (14, 15, 54). This basic domain is essential for importing extracellular Tat and also binds to membrane proteins, including the vascular endothelial growth factor receptor and heparan sulfate proteoglycans (54). Free peptide corresponding to the basic domain of Tat translocates through the cellular membrane and accumulates in the nucleus (42, 53). Chimeric or modified proteins that include the Tat basic domain sequence readily enter a variety of cell types (18, 45). The basic domain may also mediate toxin-like properties of Tat, including neuronal toxicity (37), and it appears to signal through cyclic nucleoside phosphodiesterase 4 to alter cyclic AMP levels (47). The function of the C terminus of Tat is largely unknown, but it seems necessary for pathogenesis in vivo, since primary isolates express Tat of ≥101 aa. The C termini of most Tat variants also contains an RGD motif that mediates Tat binding to cell surface integrins (5, 10).
An important reason for developing vaccines against Tat is to control the toxic properties of this protein. Tat suppresses mitogen-, alloantigen-, and antigen-induced lymphocyte proliferation in vitro (8, 26, 49, 52) by stimulating suppressive levels of alpha interferon (58) and by inducing apoptosis in activated lymphocytes (56). Apoptosis may be triggered directly, upon induction of caspase pathways (6, 34), or indirectly, through increased expression of CD95 or TRAIL in monocytes/macrophages (31, 56, 60). In vivo Tat may alter immunity by upregulating interleukin-10 and reducing interleukin-12 production (4, 29) or through its ability to increase chemokine receptor expression (28, 46, 51, 55). Patients infected with HIV-1 often develop antibody responses to Tat protein that may be correlated with clinical status (38, 39, 59). Patient sera (39) and sera from immunized mice (11) or macaques (48) were used in rough mapping of Tat epitopes, but the breadth of response for different viral sequences has not been reported.
The potential value of Tat as a vaccine antigen is controversial. Published reports of complete (12) or partial (22, 36) protection against virus challenge in macaques contrast with studies showing no protection effects (3, 48). Generalizations are elusive, partly because each group used different animal models, antigens, and vaccination protocols and also because there are no standardized assays for Tat immune responses. Further, previous studies did not define the mechanisms for neutralizing extracellular Tat or epitopes that elicit neutralizing antibodies.
Owing to the potential toxic and immunosuppressive effects of Tat, modified inactive forms of the protein (Tat toxoid) were produced by carboxymethylation of cysteine residues (33). This material was safe and immunogenic in human clinical trials (25, 32) and was tested by us in macaques, where we saw a similar reduction in disease after vaccinating with unmodified or carboxymethylated Tat (36). The main goal of the present study was to compare antisera from animals immunized with Tat or chemically modified Tat toxoid, in order to define common epitopes that might account for disease attenuation in vaccinated animals. In addition, we characterized the mechanism for Tat neutralization and studied the breadth of the antibody responses to Tat sequences from clade B and clade C viruses.
MATERIALS AND METHODS
Macaque antisera.
The polyclonal antisera were obtained from healthy rhesus macaques that had been immunized with Tat toxoid or Tat as described previously (37). Briefly, animals were immunized three times by intramuscular injection with polyphosphazene adjuvant (Adjumer) and twice by intramuscular injection of protein in incomplete Freund's adjuvant. Antigen doses ranged from 10 to 60 μg. Sera were collected 8 to 12 days after the last immunization and stored at −130°C until used.
Tat sequence analysis.
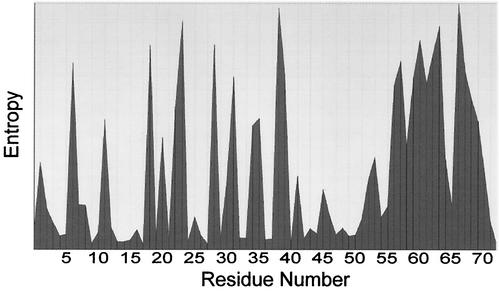
One thousand three hundred sixty Tat first-exon sequences were obtained from the Los Alamos database (www.hiv-web.lanl.gov). The sequences included roughly 50% clade B, 19% clade C, 13% clade A, 4% clade O, 2% clade D, and 1% clade G. The remainder included clades J, K, M, H, and F and 11% of sequences that were unassigned. We used only first-exon sequences to avoid problems with the variable lengths of Tat proteins. The sequences were aligned and analyzed by using the BioEdit biological sequence alignment editor (27) (Fig. 1). We compared aligned sequences by using an entropy plot that reflects the amount of variability through each column in the alignment. For entropy plotting, the sequences are treated as a matrix of characters. Entropy in a column position is independent of the total information possible at a given position and depends only upon the frequencies of characters that appear in that column. Entropy is then calculated and gives a measure of uncertainty at each position relative to other positions (27).
FIG. 1.
Variability of the Tat protein sequence. A total of 1,360 sequences derived from the Los Alamos database were used for sequence analysis with the BioEdit biological sequence alignment editor (27). The entropy plot reflects the degree of variability at each position in the full-length Tat protein.
Peptide array.
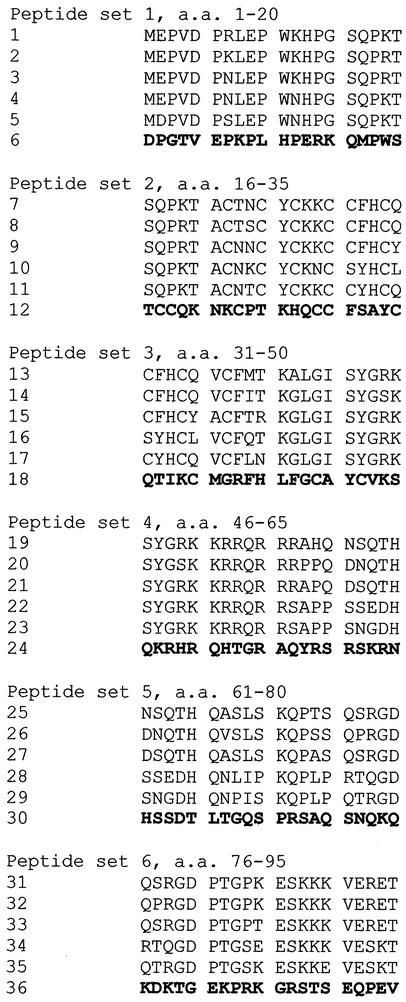
We selected three representative clade B Tat sequences, a clade C consensus sequence, and an authentic clade C sequence (B.-.NL43E9, B.AU.MBCD36, B.US.SF2, C.BW.96BW17, and Consensus C [Los Alamos HIV database]) for detecting serum antibody responses. For each complete (101- or 102-aa) sequence, we obtained synthetic peptides covering the entire protein. The peptides were 20 aa long and overlapped by 5 aa at the amino terminus and 5 aa at the carboxyl terminus. In addition, we designed a set of scrambled peptide controls. The scrambled peptides have the same amino acid composition as a consensus clade B sequence but are randomized such that no sequence of 3 aa or longer in the scrambled peptide matches any of the other five corresponding peptide sequences. Peptides were synthesized by using 9-fluorenylmethoxy carbonyl chemistry with HATU/DIEA activation at the Biopolymer Core Facility, Department of Microbiology and Immunology, University of Maryland School of Medicine. All peptides were purified by high-pressure liquid chromatography. The integrity of each preparation was confirmed by electrospray ionization mass spectrometry, and all were at least 80% the correct peptide sequence. We grouped the peptides according to their position in the protein sequence and aligned the five Tat peptides and one scrambled peptide to generate the array used for serology studies (Fig. 2).
FIG. 2.
Sequences of 36 peptides used to construct the array. Peptides are organized into six groups, with six peptides in each group. Peptides overlap by 5 aa at both ends. The first three peptides (from the top of each group) are derived from clade B sequences, and the next two are derived from clade C. The sixth peptide in each group is a scrambled (control) peptide.
ELISA.
The peptides were dissolved in water at 4 mg/ml and stored at −20°C until used; all peptides were soluble at this concentration. Peptides were adsorbed to enzyme-linked immunosorbent assay (ELISA) plates (Costar, Cambridge, Mass.) by overnight incubation at 4°C in 100 mM carbonate buffer (pH 9.5) at a concentration of 10 μg per ml. The plates were subsequently washed and treated with 50 mM Tris HCl (pH 7.8)-0.15 M NaCl-0.1% Tween 20. Serum samples were diluted 1:100 in a buffer containing 50 mM Tris HCl (pH 7.8), 0.15 M NaCl, 0.05% Tween 20, 0.5% Triton X-100, and 1% bovine serum albumin (BSA) (Sigma, St. Louis, Mo.). Wells were filled with samples (in duplicate) and incubated for 2 h at room temperature with shaking. After four washes with 50 mM Tris HCl (pH 7.8)-0.15 M NaCl-0.05% Tween 20, an anti-monkey immunoglobulin (IgG) alkaline phosphatase conjugate (affinity purified; Sigma) diluted 1:10,000 in 50 mM Tris HCl (pH 7.8)-0.15 M NaCl-0.05% Tween 20-1% BSA was added and left for 1 h at room temperature. After four washes, the substrate solution was added and plates were incubated at 37°C for 30 min.
As a control for nonspecific binding, we measured the optical density for each well and subtracted the value for scrambled peptide. Sera from immunized animals (1:100 dilution) did not react significantly with any of the scrambled peptides, indicating that the assay conditions were suitable for detecting specific binding to Tat peptides. Preimmune sera (1:100 dilution) did not react with the array.
Generation of monoclonal antibodies to the Tat protein.
BALB/c mice were injected twice with 50 μg of Tat toxoid, first with complete and then with incomplete Freund's adjuvant. Four weeks later, mice were boosted and polyethylene glycol 4000-mediated fusion was performed as described previously (19). Hybridomas were screened by ELISA, positive clones were retested by Western blotting and then cloned, and the monoclonal IgG was purified by protein G affinity chromatography. Hybridoma TR1 reacts with an epitope in aa 1 to 15. Hybridoma 9A11 reacts with the sequence from aa 46 to 60 of the HIV Tat protein sequence (46SYGSKKRRQRRRPPQ60) that is found in the B.US.YU2, B.AU.MBCD36, and B.US.JRCSF sequences (Los Alamos database).
Expression of glutathione S-transferase-Tat 89.6 fusion protein.
aa 1 to 86 of simian/HIV 89.6 Tat were expressed as a glutathione S-transferase fusion protein from the pGEX-5x-2 vector (Amersham Pharmacia, Piscataway, N.J.). The fusion protein was purified using Glutathione Sepharose 4B (Amersham Pharmacia). The purity of the preparation was confirmed by polyacrylamide gel electrophoresis and by Western blotting with monoclonal antibody TR1, which recognizes the amino-terminal peptide of Tat.
Tat internalization assay.
Jurkat cells (5 × 106 per ml) were incubated with recombinant Tat (86 aa) (Advanced Biosciences Laboratories, Kensington, Md.) at 1 μg/ml in RPMI supplemented with 0.1% ultrapure BSA (Panvera, Madison, Wis.) for 2 h at 37°C. The entire culture fluid was removed, a small fraction was saved to measure soluble Tat levels, and the fluid was transferred to fresh Jurkat cells for a second 20-min incubation. The procedure was repeated for a third serial overlay. In some cases Tat was preincubated with monoclonal antibody 9A11 or TR1 or IgG controls at the ratio of 1 μg of Tat to 50 μg of IgG (for a molar ratio of approximately 1:5). After incubation, the cells were washed three times, and then cytoplasmic and nuclear extracts were prepared with the NE-PER kit (Pierce, Rockford, Ill.). Protein concentrations were determined with Coomassie Plus Protein Assay Reagent (Pierce), and Tat was detected in lysates by Western blotting. Briefly, 12.5 μg of each nuclear extract and 50 μg of each cytoplasmic extract were separated by protein gel electrophoresis and transferred to a polyvinylidene difluoride membrane. TR1 monoclonal antibody and subsequently goat anti-mouse alkaline phosphatase conjugate (Sigma) were used to detect Tat on the blots. Membranes were incubated with the enhanced chemiluminescence substrate Lumi-Phos WB (Pierce) and exposed to CL-Xposure film (Pierce). Three nanograms of Tat was used as a positive control.
We also used this assay to test whether Tat-specific monoclonal or polyclonal antibodies could block Tat internalization. Tat was preincubated with protein G-purified monkey IgG, purified control IgG, or monoclonal antibodies for 30 min at room temperature. The mixtures were added to Jurkat cells, and the Western blot assay for nuclear accumulation of Tat was done as described above. All samples were within 8% as judged by the band intensity of an internal loading control.
Tat transactivation assay.
We used a CD4+ HeLa cell line (kindly provided by Barbara Felber and George Pavlakis) containing an HIV-1 provirus that does not express Tat and does not release virus particles (44). Cells were seeded into a 96-well plate at 40,000 cells per well and incubated overnight. The attached cells were washed three times with warmed serum-free RPMI and then overlaid with RPMI-0.1% ultrapure BSA (Panvera) containing Tat protein for 90 min. The Tat solutions were removed and replaced with complete Dulbecco’s modified Eagle medium. Culture fluids were collected 96 h later, and cell-free virus was detected with a commercial antigen capture ELISA for p24 capsid antigen (R&D Systems). Data are expressed as the mean optical density ± the standard deviation from quadruplicate samples.
We also used this assay to measure the ability of monoclonal antibodies or polyclonal antibodies to neutralize the Tat activity. Tat was preincubated with protein G-purified monkey IgG, purified control IgG, or monoclonal antibodies for 30 min at room temperature. The Tat-antibody solution was added to indicator cells, left for 90 min, and then removed and replaced with Dulbecco’s modified Eagle medium containing 10% heat-inactivated fetal bovine serum.
RESULTS
Amino acid sequence variation in Tat from different viral isolates.
The Tat protein has often been described as having a conserved sequence (20, 21). Our study and work of others (11, 23) support a different interpretation. Analysis of the first exon sequences (aa 1 to 72) from 1,360 Tat sequences in the Los Alamos database revealed significant variability among Tat amino acid sequences (Fig. 1), although there were some islands of sequence conservation. The basic region, consisting of the sequence SYGSKKRRQRRR for aa 45 to 56, is generally conserved, as are all seven cysteine residues that are required for transactivation activity (30) and pathogenic effects (9). In addition, the amino-terminal sequence is relatively conserved. Other regions of Tat showed substantial variation in this entropy plot.
A peptide microarray for detecting Tat antibodies.
Overlapping peptides were synthesized to match three authentic clade B sequences, a clade C consensus sequence, an authentic clade C sequence, and a scrambled peptide control as described in Materials and Methods. Sequences were selected to represent the most common Tat variants. This peptide microarray was tested with Tat-specific monoclonal antibodies TR1 and 9A11 (described below) and with hyperimmune mouse serum raised by immunization with Tat toxoid. Although complete validation of the assay requires a larger collection of monoclonal antibodies and well-characterized antisera, the results cited above were highly reproducible in terms of both the level of antibody binding and the pattern of binding to different sequence variants.
Spectrum of antibodies induced by immunization with unmodified Tat protein.
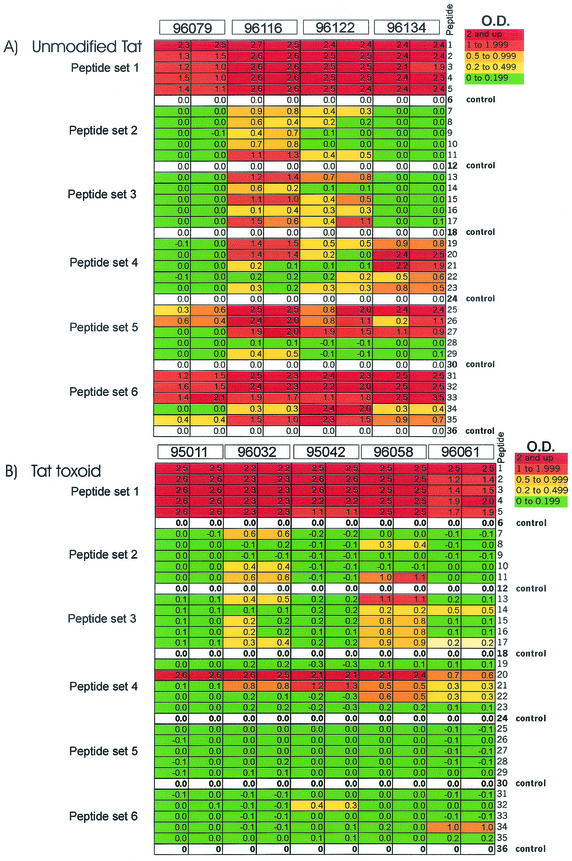
Macaques immunized with Tat developed robust serum antibody responses, with endpoint titers ranging from 1:5,000 to 1:64,000 in ELISA (36). Sera from all immunized macaques reacted strongly with N-terminal peptides (set 1) and did not discriminate B and C clade sequences (Fig. 3). Although there is some variation in the amino-terminal sequences for Tat proteins, there are sufficient conserved residues (including the amino-terminal sequence MEPVD, the sequence LEPW at aa 8 to 11, and the sequence HPGSQP at aa 13 to 18) to account for the cross-reactions. Two of four animals reacted with peptides from sets 2 and 3, and two of four macaques reacted with peptides from set 4, although not all sera reacted with all sequences, indicating the recognition of nonconserved epitopes. All Tat-immunized animals recognized peptides in set 5 (Fig. 3A), although the reaction was restricted to clade B sequences, indicating the presence of a clade-specific epitope. Recognition of the C-terminal portion (aa 76 to 95) was strong in all animals and was not restricted to either clade.
FIG.3.
Sera from Tat-immunized macaques recognize multiple epitopes in clade B and clade C Tat sequences. The reactivities of individual sera of immunized monkeys with peptides in ELISA are presented according to the optical densities in duplicate measurements. Individual values are color coded (indicated in the top right corner, with red showing the strongest reaction). For each peptide set, optical densities of the wells containing the control (scrambled) peptide were subtracted from the optical densities of the wells containing the test peptides. The subtraction was performed for each animal serum individually. (A) Sera from Tat-immunized macaques; (B) sera from animals immunized with Tat toxoid.
Overall, epitopes in peptide sets 1 (aa 1 to 20) and 6 (aa 76 to 95) were recognized by sera from all animals immunized with Tat. The epitopes appear to be conserved, because there were no consistent differences among individual sequences or across clades. Peptide sets 2 and 3 were recognized by half of the sera. Recognition of peptide set 4 was inconsistent, suggesting the presence of a nonconserved epitope, and recognition of peptide set 5 showed clear evidence for clade B-specific recognition by sera raised against the clade B Tat protein.
Spectrum of antibodies induced by Tat toxoid immunization.
Animals immunized with Tat toxoid also developed strong Tat-binding antibodies, with titers similar to those in the group immunized with Tat. However, Tat toxoid elicited a more restricted antibody response, suggesting that carboxymethylation reduced the immunogenicity for some but not all Tat epitopes (Fig. 3B).
Sera from all Tat toxoid-immunized macaques recognized conserved epitopes in the amino-terminal sequence. There was weak recognition of peptides in sets 2 and 3. Peptide 20 from set 4 was recognized strongly by all animals immunized with Tat toxoid, but the epitope appeared to be highly variable and was present only in peptide 20 and not in other peptides from the same set. We mapped the epitope in peptide 20 (aa 46 to 65) by using additional synthetic peptides. Sera from some of the immunized animals preferentially reacted with peptides consisting of aa 46 to 65 and 46 to 60 (Table 1). Three animals (96061, 96079, and 96122) had weak responses to this region. For five animals (96032, 95042, 96058, 96116, and 96134), the response to the truncated peptide including aa 46 to 60 was strong, and the response to the peptide including aa 41 to 56 was less. Antisera from these macaques recognize an epitope that includes aa 57 to 60. For animal 95011, the response to aa 46 to 60 was substantially less than the response to aa 46 to 65. In this animal the epitope may be more complex and potentially shifted to the C terminus compared with the other sera.
TABLE 1.
Polyclonal sera from macaques immunized with unmodified (Tat) or carboxymethylated Tat (Tat Tx) recognize epitopes that include the amino acid sequence 57RPPQ60
| Peptide | Reaction (optical density)a of serum from animal:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 95011 (Tat Tx) | 96032 (Tat Tx) | 95042 (Tat Tx) | 96058 (Tat Tx) | 96061 (Tat Tx) | 96079 (Tat) | 96116 (Tat) | 96122 (Tat) | 96134 (Tat) | |
| aa 46-65 (SYGSKKRRQRRRPPQDNQTH) | 2.412, 2.452 | 2.474, 2.457 | 1.558, 1.624 | 1.25, 1.267 | 0.254, 0.257 | 0.053, 0.052 | 1.322, 1.336 | 0.234, 0.259 | 2.025, 2.007 |
| aa 46-60 (SYGSKKRRQRRRPPQ) | 0.867, 0.865 | 1.678, 1.664 | 1.169, 1.098 | 1.142, 1.138 | 0.331, 0.317 | 0.196, 0.182 | 1.052, 1.012 | 0.723, 0.743 | 2.279, 2.318 |
| aa 41-56 (KALGISYGSKKRRQRR) | 0.059, 0.062 | 0.519, 0.533 | 0.145, 0.145 | 0.79, 0.796 | 0.124, 0.105 | 0.036, 0.032 | 0.78, 0.76 | 0.699, 0.648 | 0.071, 0.145 |
Results of two determinations are shown.
Overall, Tat toxoid immunization elicited antibody responses to a limited set of linear epitopes in the Tat molecule (Fig. 3B). We did not find a significant antibody response to the fifth and the sixth peptide sets, representing C-terminal parts of the 86-aa Tat sequence, despite the fact that the endpoint titers were similar to those in animals immunized with unmodified Tat antigen (36).
Antibodies discriminate amino acid sequences in Tat aa 57 to 60.
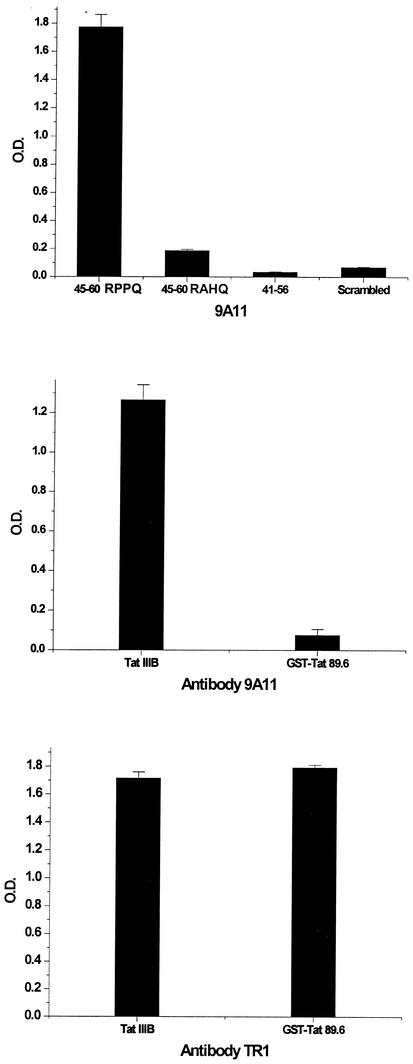
We used the peptide consisting of aa 46 to 60 to show the presence of antibodies to the Tat basic domain. In order to define the epitope, we generated a monoclonal antibody against this region. BALB/c mice were immunized with Tat toxoid, and we identified several monoclonal antibodies reacting with the Tat protein. One of the antibodies demonstrated strong reactivity with the basic peptide 46SYGSKKRRQRRRPPQ60. This antibody reacted poorly with the peptide consisting of aa 41 to 56 (41KALGISYGSKKRRQRR56), showing that the epitope includes all or part of aa 57 to 60. The antibody bound only weakly with the sequence 46SYGSKKRRQRRRAHQ60, confirming that the 56RPPQ60 sequence is within the epitope. The same pattern of reactivity was found with polyclonal sera from immunized mice (not shown). Monoclonal antibody 9A11 had much better binding to the homologous Tat protein (containing the 56RPPQ60 sequence) than to a related Tat sequence from HIV-1 89.6, which has a 56RAHQ60 sequence (Fig. 4). The sequence from aa 57 to 60 is part of a variable epitope located next to the highly conserved basic region sequence 41KALGISYGSKKRRQRR56.
FIG. 4.
A monoclonal antibody to the basic region recognizes a variable epitope. The monoclonal antibody 9A11 recognizes only peptides (top panel) or Tat proteins (middle panel) containing the 57RPPQ60 sequence (present in IIIB and not present in 89.6). The amino terminus antibody TR1 recognizes an epitope conserved in both proteins (bottom panel). O.D., optical density. Error bars indicate standard deviations.
Tat internalization in Jurkat cells.
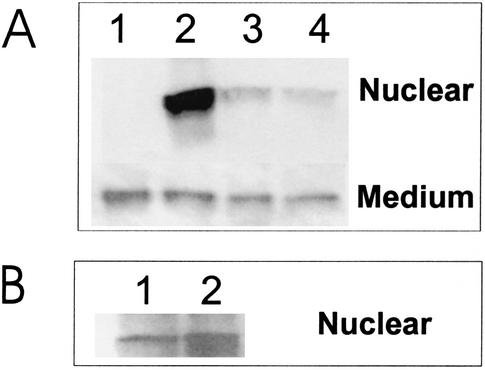
We tested individual Tat preparations to measure the rate and extent of nuclear accumulation in Jurkat cells as part of developing assays for antibody neutralization of Tat protein. Tat levels in the nucleus and medium were measured by a Western blotting assay (Fig. 5A). There was little change in the concentration of extracellular Tat throughout the study, showing that only a small percentage of the Tat preparation was taken up by Jurkat cells. High levels of nuclear Tat were seen only in the first incubation, indicating that just a fraction of the starting material could enter the cells and travel to the nucleus. On the basis of band intensity in Western blots, we roughly estimated that the fraction of Tat competent to enter the cell nucleus was <5% of the starting material. As a control, Tat was incubated at 37°C for 90 min and then added to Jurkat cells. The control incubation had little effect on Tat uptake into Jurkat cells (Fig. 5B). The protein appears to be relatively stable in tissue culture medium and retained a similar, albeit low, capacity for penetrating Jurkat cells.
FIG. 5.
A small fraction of soluble Tat is competent for internalization in Jurkat cells. A solution of 1 μg of Tat per ml in RPMI plus 0.1% ultrapure BSA was mixed with Jurkat cells, and then the cells were removed by centrifugation, the same Tat solution was added to fresh Jurkat cells, and the procedure was repeated for a third serial passage. (A) A Western blot assay showed that the majority of Tat uptake occurred in the first cell exposure (lane 2) and that there was minimal uptake in the second (lane 3) or third (lane 4) passage. The concentration of soluble Tat was only slightly decreased from starting levels (lane 1, before cell addition). (B) To show that Tat was stable in solution, we used either fresh Tat (lane 1) or Tat incubated in medium for 90 min at 37°C (lane 2) before addition to Jurkat cells.
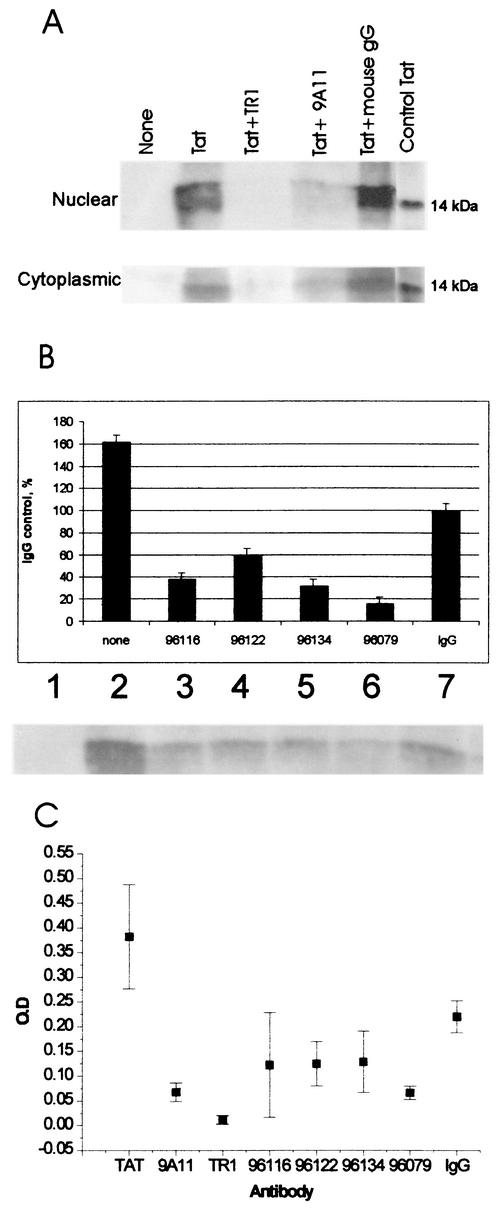
We also found that sera from immunized macaques or mouse monoclonal antibodies blocked Tat uptake by Jurkat cells. Incubation of recombinant Tat with monoclonal anti-Tat antibodies directed against aa 1 to 15 (TR1) or against aa 57 to 60 (9A11), but not incubation with a control antibody, inhibited the accumulation of Tat in nuclear and cytoplasmic fractions of Jurkat cells (Fig. 6A). Substantial inhibition of uptake was also seen with purified immune monkey IgG (Fig. 6B), although high concentrations of control monkey IgG also partly blocked uptake.
FIG. 6.
Antibody neutralization of Tat uptake and transactivation activities. (A) The mouse monoclonal antibodies 9A11 and TR1 blocked Tat uptake into Jurkat cells. A Western blot assay showed a sharp reduction in nuclear and cytoplasmic Tat by 9A11 and TR1 but not by an irrelevant control mouse monoclonal antibody. (B) A similar result was observed with protein G-purified IgG from Tat-immunized macaques. Tat internalization was measured by Western blotting of Jurkat nuclear extracts; we show a typical blot and the data collectedwith a phosphorimager. Uptake levels were normalized to the value observed in the presence of nonimmune, control macaque Ig (100% in lane 7). In the absence of IgG (lane 2), uptake was above the control level. Purified IgG from four immunized macaques all blocked Tat uptake and nuclear internalization. Error bars indicate standard deviations. (C) These same monoclonal antibodies and IgG neutralized Tat in the transactivation assay. The data are represented as the optical density (O.D.) from the ELISA for p24 capsid antigen at 96 h after Tat addition. The monoclonal antibodies 9A11 and TR1 had the strongest neutralizing effect and were comparable to IgG for macaque 96079. Macaques 96116, 96122, and 96134 showed a lesser but distinct neutralization of Tat transactivation compared to the nonimmune IgG control.
Tat transactivation in HeLa cells.
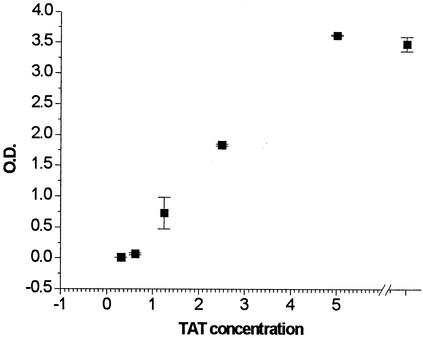
We had reported previously that some antisera from Tat-immunized macaques neutralized Tat activity in a chloramphenicol acetyltransferase assay for transactivation (36). Here, we tested whether monoclonal antibodies or purified IgG could neutralize Tat activity in CD4+ HeLa cells containing a defective provirus. We first determined that there was a normal dose-response curve for soluble Tat protein and that we could measure transactivation with Tat concentrations of around 1 μg/ml (Fig. 7). For antibody neutralization studies it is important to keep the antigen levels as low as possible.
FIG. 7.
Tat transactivation of virus production has a normal dose response in CD4+ HeLa cells carrying Tat-defective provirus. Tat was added at 0.3 to 10 μg/ml in RPMI plus 0.1% ultrapure BSA. The incubation and culture were as described in Materials and Methods. At 96 h after Tat addition, culture fluids were tested for virus levels by using a capture ELISA for p24 capsid antigen. Data are represented as optical density (O.D.) in the p24 ELISA for each Tat concentration. Error bars indicate standard deviations.
We then tested monoclonal antibodies 9A11 and TR1 and purified IgG from four macaques that were immunized with Tat protein for the ability to block transactivation. Monoclonal antibodies 9A11 and TR1, which were shown previously to block uptake, strongly neutralized the transactivation activity of Tat protein (Fig. 6C), giving a sevenfold reduction in optical density for the p24 antigen capture ELISA. Purified IgG from macaque 96079 also strongly neutralized Tat activity. A lesser neutralizing effect was seen with sera from macaques 96116, 96122, and 96134. We noted the problem of some neutralizing activity in purified, nonimmune IgG. The effect of nonimmune IgG was observed with macaque sera but was not seen with control mouse monoclonal antibodies (not shown). We were unable to perform this assay with sera from Tat toxoid-immunized macaques, as our samples were exhausted in previous studies.
DISCUSSION
We compared the Tat-specific antibody responses in macaques immunized with Tat or Tat toxoid. In a previous study, both animal groups showed substantial Tat-binding antibody titers and disease was attenuated in both groups (36), although no animals were protected from infection. There were no statistically significant differences in disease among animals immunized with Tat or Tat toxoid in terms of viral RNA levels or CD4 cell counts, although the Tat-immunized group tended to have lower viral RNA levels at set point compared with the Tat toxoid-immunized animals (36). Our goal in these immunization studies was to characterize responses to Tat that affect viral pathogenesis and might help to resolve the controversy over whether Tat is acting exclusively in nuclei of infected cells or is also taken up by uninfected bystander cells to modify their function and promote disease progression. Ultimately, Tat would be only one component of a vaccine, with the objective of augmenting the protective responses against HIV structural antigens.
In order to characterize the antibodies elicited by immunization, we developed an array of overlapping peptides representing the first 86 aa from three clade B Tat sequences, the clade C consensus sequence, and one individual clade C Tat sequence. We interrogated the array with antisera from four Tat-immunized and five Tat toxoid-immunized macaques; all macaques exhibited Tat-binding antibody titers of between 5,000 and 64,000. Sera from Tat-immunized macaques reacted with several regions of Tat, and there was little discrimination among clade B and clade C sequences. The antibody response to Tat toxoid was more restricted, with substantial recognition of only two Tat regions (amino terminus and basic domain), and the antibodies to the basic domain recognized only one of the clade B sequences. Thus, antibodies raised against Tat recognized epitopes that were generally conserved among clade B and clade C Tat sequences, while antibodies present after Tat toxoid immunization recognized only two main epitopes, and one of these (including aa 57 to 60) was present in only one of the clade B sequences and in neither of the clade C sequences. The same 57RPPQ60 sequence was present in both the Tat and Tat toxoid antigens. Importantly, the chemical modifications in Tat toxoid occurred in the cysteine-rich domain (aa 21 to 40), yet they affected antibody responses to distant sequences at aa 57 to 60.
The epitope 57RPPQ60 is recognized by sera from immunized macaques and monoclonal antibodies from immunized mice. A monoclonal antibody against this sequence reacted strongly with a homologous Tat preparation and with peptides containing the RPPQ sequence. The same antibody did not react with a Tat preparation or peptides having the sequence RAHQ at this position. The sequences RAHQ and RAPQ are common at positions 57 to 60 for clade B Tat proteins, while clade C viruses tend to have the sequence SAPQ at this same site. Recognition of the 57RPPQ60 epitope may be important for clinical studies of Tat immunity. This sequence is infrequent among clade B isolates and, to our knowledge, has never been reported to be present in a clade C isolate. Should this region prove to be important for Tat immunity, if may be beneficial to develop new immunogens with more common sequences in this region.
Monoclonal anti-Tat antibodies directed against aa 1 to 15 (TR1) or against aa 57 to 60 (9A11) inhibited the accumulation of extracellular Tat in the cytoplasm and nuclei of Jurkat cells (Fig. 6A). Surprisingly, the amino-terminus-specific antibody TR1 showed the strongest inhibition of Tat uptake. These data show a requirement for the amino terminus in Tat internalization and argue against the idea that basic sequences are sufficient for internalization of the intact Tat molecule (18, 43, 50) even though they function as protein transduction domains in chimeric proteins.
We observed that <5% of the Tat preparation used here was competent to enter cells. Even though we add Tat at a concentration of 1 μg/ml to measure uptake or transactivation, the active fraction is present at less than 5% or 50 ng/ml, a concentration comparable to what is used commonly to measure biological activity of cytokines and chemokines. The low specific activity of Tat preparations necessitated the addition of large amounts of Tat in transactivation assays. The presence of large amounts of inactive protein reduces the ability of monoclonal antibodies or polyclonal IgG to block uptake or to neutralize the transactivation activity. We know that nonimmune IgG from macaques can partly neutralize Tat activities, and this limits the amount of IgG that can be added to these assays. This effect might be due to the presence of “natural” low-affinity anti-Tat antibodies in some nonimmune sera (40, 41).
The mechanisms for Tat neutralization in this study were indistinguishable from those for the inhibition of Tat uptake. Other groups reported activities of Tat that may not require internalization, but we have not yet tested for antibody neutralization in a broad panel of Tat bioassays. However, for neutralization of Tat uptake and viral transactivation, it was sufficient to have antibodies against the amino terminus of the Tat protein. Apparently, the basic region, or transduction domain, that mediates uptake of many other proteins (18, 43, 45) is not sufficient for Tat uptake, and portions of the amino terminus may also be required. This result, combined with the observation that only a small fraction of Tat molecules are competent for internalization, argues that a specific conformer of Tat carries the highest activity. Such a conclusion would not be apparent from the published structural studies, where Tat is reported to have little secondary or tertiary structure (7, 24). Judging from our experience, it is likely that these published structures represent the inactive forms of Tat and that we have not yet seen the active structure that is capable of penetrating cells and entering the nucleus.
The HIV-1 Tat and Tat toxoid proteins are highly immunogenic in macaques (36) and humans (25). Modified forms of Tat, including Tat toxoid, were designed to avoid potential toxic effects of the protein (9, 13), but we now report that these modifications restrict the pattern of antibody responses and elicit type-specific antibodies in macaques that do not recognize all Tat sequences equally. In order to study the response to Tat during infection or to develop broadly cross-reacting Tat vaccines, it is important to use sequences commonly present in the target population or to develop a mixture of antigens that overcomes the problem of sequence specificity. Whether Tat sequence variation reflects immune selection by antibodies and the consequent evolution of virus escape variants is a question that will be addressed in our ongoing clinical studies.
Acknowledgments
The work was supported by Public Health Service grant AI49805 from the National Institute of Allergy and Infectious Diseases.
We are grateful to Maria Salvato, Robert R. Redfield, and Robert C. Gallo for comments on the manuscript and to Brian Foley for suggesting the use of entropy plots.
REFERENCES
- 1.Albini, A., R. Benelli, D. Giunciuglio, T. Cai, G. Mariani, S. Ferrini, and D. M. Noonan. 1998. Identification of a novel domain of HIV tat involved in monocyte chemotaxis. J. Biol. Chem. 273:15895-15900. [DOI] [PubMed] [Google Scholar]
- 2.Albini, A., S. Ferrini, R. Benelli, S. Sforzini, D. Giunciuglio, M. G. Aluigi, A. E. Proudfoot, S. Alouani, T. N. Wells, G. Mariani, R. L. Rabin, J. M. Farber, and D. M. Noonan. 1998. HIV-1 Tat protein mimicry of chemokines. Proc. Natl. Acad. Sci. USA 95:13153-13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen, T. M., L. Mortara, B. R. Mothe, M. Liebl, P. Jing, B. Calore, M. Piekarczyk, R. Ruddersdorf, D. H. O'Connor, X. Wang, C. Wang, D. B. Allison, J. D. Altman, A. Sette, R. C. Desrosiers, G. Sutter, and D. I. Watkins. 2002. Tat-vaccinated macaques do not control simian immunodeficiency virus SIVmac239 replication. J. Virol. 76:4108-4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badou, A., Y. Bennasser, M. Moreau, C. Leclerc, M. Benkirane, and E. Bahraoui. 2000. Tat protein of human immunodeficiency virus type 1 induces interleukin-10 in human peripheral blood monocytes: implication of protein kinase C-dependent pathway. J. Virol. 74:10551-10562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barillari, G., R. Gendelman, R. C. Gallo, and B. Ensoli. 1993. The Tat protein of human immunodeficiency virus type 1, a growth factor for AIDS Kaposi sarcoma and cytokine-activated vascular cells, induces adhesion of the same cell types by using integrin receptors recognizing the RGD amino acid sequence. Proc. Natl. Acad. Sci. USA 90:7941-7945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartz, S. R., and M. Emerman. 1999. Human immunodeficiency virus type 1 Tat induces apoptosis and increases sensitivity to apoptotic signals by up-regulating FLICE/caspase-8. J. Virol. 73:1956-1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bayer, P., M. Kraft, A. Ejchart, M. Westendorp, R. Frank, and P. Rosch. 1995. Structural studies of HIV-1 Tat protein. J. Mol. Biol. 247:529-535. [DOI] [PubMed] [Google Scholar]
- 8.Benjouad, A., K. Mabrouk, M. Moulard, J. C. Gluckman, H. Rochat, J. Van Rietschoten, and J. M. Sabatier. 1993. Cytotoxic effect on lymphocytes of Tat from human immunodeficiency virus (HIV-1). FEBS Lett. 319:119-124. [DOI] [PubMed] [Google Scholar]
- 9.Boykins, R. A., R. Mahieux, U. T. Shankavaram, Y. S. Gho, S. F. Lee, I. K. Hewlett, L. M. Wahl, H. K. Kleinman, J. N. Brady, K. M. Yamada, and S. Dhawan. 1999. Cutting edge: a short polypeptide domain of HIV-1-Tat protein mediates pathogenesis. J. Immunol. 163:15-20. [PubMed] [Google Scholar]
- 10.Brake, D. A., C. Debouck, and G. Biesecker. 1990. Identification of an Arg-Gly-Asp (RGD) cell adhesion site in human immunodeficiency virus type 1 transactivation protein, tat. J. Cell Biol. 111:1275-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brake, D. A., J. Goudsmit, W. J. Krone, P. Schammel, N. Appleby, R. H. Meloen, and C. Debouck. 1990. Characterization of murine monoclonal antibodies to the Tat protein from human immunodeficiency virus type 1. J. Virol. 64:962-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cafaro, A., A. Caputo, C. Fracasso, M. T. Maggiorella, D. Goletti, S. Baroncelli, M. Pace, L. Sernicola, M. L. Koanga-Mogtomo, M. Betti, A. Borsetti, R. Belli, L. Akerblom, F. Corrias, S. Butto, J. Heeney, P. Verani, F. Titti, and B. Ensoli. 1999. Control of SHIV-89.6P-infection of cynomolgus monkeys by HIV-1 Tat protein vaccine. Nat. Med. 5:643-650. [DOI] [PubMed] [Google Scholar]
- 13.Cohen, S. S., C. Li, L. Ding, Y. Cao, A. B. Pardee, E. M. Shevach, and D. I. Cohen. 1999. Pronounced acute immunosuppression in vivo mediated by HIV Tat challenge. Proc. Natl. Acad. Sci. USA 96:10842-10847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cordingley, M. G., R. L. LaFemina, P. L. Callahan, J. H. Condra, V. V. Sardana, D. J. Graham, T. M. Nguyen, K. LeGrow, L. Gotlib, A. J. Schlabach, et al. 1990. Sequence-specific interaction of Tat protein and Tat peptides with the transactivation-responsive sequence element of human immunodeficiency virus type 1 in vitro. Proc. Natl. Acad. Sci. USA 87:8985-8989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delling, U., S. Roy, M. Sumner-Smith, R. Barnett, L. Reid, C. A. Rosen, and N. Sonenberg. 1991. The number of positively charged amino acids in the basic domain of Tat is critical for trans-activation and complex formation with TAR RNA. Proc. Natl. Acad. Sci. USA 88:6234-6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Paulis, A., R. De Palma, L. Di Gioia, M. Carfora, N. Prevete, G. Tosi, R. S. Accolla, and G. Marone. 2000. Tat protein is an HIV-1-encoded beta-chemokine homolog that promotes migration and up-regulates CCR3 expression on human Fc epsilon RI+ cells. J. Immunol. 165:7171-7179. [DOI] [PubMed] [Google Scholar]
- 17.Ensoli, B., L. Buonaguro, G. Barillari, V. Fiorelli, R. Gendelman, R. A. Morgan, P. Wingfield, and R. C. Gallo. 1993. Release, uptake, and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation. J. Virol. 67:277-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fawell, S., J. Seery, Y. Daikh, C. Moore, L. L. Chen, B. Pepinsky, and J. Barsoum. 1994. Tat-mediated delivery of heterologous proteins into cells. Proc. Natl. Acad. Sci. USA 91:664-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galfre, G., and C. Milstein. 1981. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 73:3-46. [DOI] [PubMed] [Google Scholar]
- 20.Gallo, R. C. 1999. Tat as one key to HIV-induced immune pathogenesis and Tat (correction of Pat) toxoid as an important component of a vaccine. Proc. Natl. Acad. Sci. USA 96:8324-8326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldstein, G. 1996. HIV-1 Tat protein as a potential AIDS vaccine. Nat. Med. 2:960-964. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein, G., K. Manson, G. Tribbick, and R. Smith. 2000. Minimization of chronic plasma viremia in rhesus macaques immunized with synthetic HIV-1 Tat peptides and infected with a chimeric simian/human immunodeficiency virus (SHIV33). Vaccine 18:2789-2795. [DOI] [PubMed] [Google Scholar]
- 23.Goldstein, G., G. Tribbick, and K. Manson. 2001. Two B cell epitopes of HIV-1 Tat protein have limited antigenic polymorphism in geographically diverse HIV-1 strains. Vaccine 19:1738-1746. [DOI] [PubMed] [Google Scholar]
- 24.Gregoire, C., J. M. Peloponese, Jr., D. Esquieu, S. Opi, G. Campbell, M. Solomiac, E. Lebrun, J. Lebreton, and E. P. Loret. 2001. Homonuclear (1)H-NMR assignment and structural characterization of human immunodeficiency virus type 1 Tat Mal protein. Biopolymers 62:324-335. [DOI] [PubMed] [Google Scholar]
- 25.Gringeri, A., E. Santagostino, M. Muca-Perja, P. M. Mannucci, J. F. Zagury, B. Bizzini, A. Lachgar, M. Carcagno, J. Rappaport, M. Criscuolo, W. Blattner, A. Burny, R. C. Gallo, and D. Zagury. 1998. Safety and immunogenicity of HIV-1 Tat toxoid in immunocompromised HIV-1-infected patients. J. Hum. Virol. 1:293-298. [PubMed] [Google Scholar]
- 26.Gutheil, W. G., M. Subramanyam, G. R. Flentke, D. G. Sanford, E. Munoz, B. T. Huber, and W. W. Bachovchin. 1994. Human immunodeficiency virus 1 Tat binds to dipeptidyl aminopeptidase IV (CD26): a possible mechanism for Tat's immunosuppressive activity. Proc. Natl. Acad. Sci. USA 91:6594-6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hall, T. A. 1999. BioEdit: a user friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41:95-98. [Google Scholar]
- 28.Huang, L., I. Bosch, W. Hofmann, J. Sodroski, and A. B. Pardee. 1998. Tat protein induces human immunodeficiency virus type 1 (HIV-1) coreceptors and promotes infection with both macrophage-tropic and T-lymphotropic HIV-1 strains. J. Virol. 72:8952-8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ito, M., T. Ishida, L. He, F. Tanabe, Y. Rongge, Y. Miyakawa, and H. Terunuma. 1998. HIV type 1 Tat protein inhibits interleukin 12 production by human peripheral blood mononuclear cells. AIDS Res. Hum. Retroviruses 14:845-849. [DOI] [PubMed] [Google Scholar]
- 30.Jeang, K. T., H. Xiao, and E. A. Rich. 1999. Multifaceted activities of the HIV-1 transactivator of transcription, Tat. J. Biol. Chem. 274:28837-28840. [DOI] [PubMed] [Google Scholar]
- 31.Katsikis, P. D., M. E. Garcia-Ojeda, J. F. Torres-Roca, D. R. Greenwald, and L. A. Herzenberg. 1997. HIV type 1 Tat protein enhances activation- but not Fas (CD95)-induced peripheral blood T cell apoptosis in healthy individuals. Int. Immunol. 9:835-841. [DOI] [PubMed] [Google Scholar]
- 32.Lambert, J. 1998. Tat toxoid: its potential role as an HIV vaccine. J. Hum. Virol. 1:249-250. [PubMed] [Google Scholar]
- 33.Le Buanec, H., and B. Bizzini. 2000. Procedures for preparing biologically inactive, but immunogenic HIV-1 Tat protein (Tat toxoid) for human use. Biomed. Pharmacother. 54:41-44. [DOI] [PubMed] [Google Scholar]
- 34.Macho, A., M. A. Calzado, L. Jimenez-Reina, E. Ceballos, J. Leon, and E. Munoz. 1999. Susceptibility of HIV-1-TAT transfected cells to undergo apoptosis. Biochemical mechanisms. Oncogene 18:7543-7551. [DOI] [PubMed] [Google Scholar]
- 35.Mann, D. A., and A. D. Frankel. 1991. Endocytosis and targeting of exogenous HIV-1 Tat protein. EMBO J. 10:1733-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pauza, C. D., P. Trivedi, M. Wallace, T. J. Ruckwardt, H. Le Buanec, W. Lu, B. Bizzini, A. Burny, D. Zagury, and R. C. Gallo. 2000. Vaccination with tat toxoid attenuates disease in simian/HIV-challenged macaques. Proc. Natl. Acad. Sci. USA 97:3515-3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Philippon, V., C. Vellutini, D. Gambarelli, G. Harkiss, G. Arbuthnott, D. Metzger, R. Roubin, and P. Filippi. 1994. The basic domain of the lentiviral Tat protein is responsible for damages in mouse brain: involvement of cytokines. Virology 205:519-529. [DOI] [PubMed] [Google Scholar]
- 38.Re, M. C., G. Furlini, M. Vignoli, E. Ramazzotti, G. Roderigo, V. De Rosa, G. Zauli, S. Lolli, S. Capitani, and M. La Placa. 1995. Effect of antibody to HIV-1 Tat protein on viral replication in vitro and progression of HIV-1 disease in vivo. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 10:408-416. [DOI] [PubMed] [Google Scholar]
- 39.Re, M. C., M. Vignoli, G. Furlini, D. Gibellini, V. Colangeli, F. Vitone, and M. La Placa. 2001. Antibodies against full-length Tat protein and some low-molecular-weight Tat-peptides correlate with low or undetectable viral load in HIV-1 seropositive patients. J. Clin. Virol. 21:81-89. [DOI] [PubMed] [Google Scholar]
- 40.Rodman, T. C., J. D. Lutton, S. Jiang, H. B. Al-Kouatly, and R. Winston. 2001. Circulating natural IgM antibodies and their corresponding human cord blood cell-derived Mabs specifically combat the Tat protein of HIV. Exp. Hematol. 29:1004-1009. [DOI] [PubMed] [Google Scholar]
- 41.Rodman, T. C., S. E. To, H. Hashish, and K. Manchester. 1993. Epitopes for natural antibodies of human immunodeficiency virus (HIV)-negative (normal) and HIV-positive sera are coincident with two key functional sequences of HIV Tat protein. Proc. Natl. Acad. Sci. USA 90:7719-7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruben, S., A. Perkins, R. Purcell, K. Joung, R. Sia, R. Burghoff, W. A. Haseltine, and C. A. Rosen. 1989. Structural and functional characterization of human immunodeficiency virus Tat protein. J. Virol. 63:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rusnati, M., G. Tulipano, C. Urbinati, E. Tanghetti, R. Giuliani, M. Giacca, M. Ciomei, A. Corallini, and M. Presta. 1998. The basic domain in HIV-1 Tat protein as a target for polysulfonated heparin-mimicking extracellular Tat antagonists. J. Biol. Chem. 273:16027-16037. [DOI] [PubMed] [Google Scholar]
- 44.Sadaie, M. R., T. Benter, and F. Wong-Staal. 1988. Site-directed mutagenesis of two trans-regulatory genes (tat-III,trs) of HIV-1. Science 239:910-913. [DOI] [PubMed] [Google Scholar]
- 45.Schwarze, S. R., A. Ho, A. Vocero-Akbani, and S. F. Dowdy. 1999. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science 285:1569-1572. [DOI] [PubMed] [Google Scholar]
- 46.Secchiero, P., D. Zella, S. Capitani, R. C. Gallo, and G. Zauli. 1999. Extracellular HIV-1 tat protein up-regulates the expression of surface CXC-chemokine receptor 4 in resting CD4+ T cells. J. Immunol. 162:2427-2431. [PubMed] [Google Scholar]
- 47.Secchiero, P., D. Zella, S. Curreli, P. Mirandola, S. Capitani, R. C. Gallo, and G. Zauli. 2000. Pivotal role of cyclic nucleoside phosphodiesterase 4 in Tat-mediated CD4+ T cell hyperactivation and HIV type 1 replication. Proc. Natl. Acad. Sci. USA 97:14620-14625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Silvera, P., M. W. Richardson, J. Greenhouse, J. Yalley-Ogunro, N. Shaw, J. Mirchandani, K. Khalili, J. F. Zagury, M. G. Lewis, and J. Rappaport. 2002. Outcome of simian-human immunodeficiency virus strain 89.6p challenge following vaccination of rhesus macaques with human immunodeficiency virus Tat protein. J. Virol. 76:3800-3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Subramanyam, M., W. G. Gutheil, W. W. Bachovchin, and B. T. Huber. 1993. Mechanism of HIV-1 Tat induced inhibition of antigen-specific T cell responsiveness. J. Immunol. 150:2544-2553. [PubMed] [Google Scholar]
- 50.Tyagi, M., M. Rusnati, M. Presta, and M. Giacca. 2001. Internalization of HIV-1 tat requires cell surface heparan sulfate proteoglycans. J. Biol. Chem. 276:3254-3261. [DOI] [PubMed] [Google Scholar]
- 51.van Baalen, C. A., O. Pontesilli, R. C. Huisman, A. M. Geretti, M. R. Klein, F. de Wolf, F. Miedema, R. A. Gruters, and A. D. Osterhaus. 1997. Human immunodeficiency virus type 1 Rev- and Tat-specific cytotoxic T lymphocyte frequencies inversely correlate with rapid progression to AIDS. J. Gen Virol. 78:1913-1918. [DOI] [PubMed] [Google Scholar]
- 52.Viscidi, R. P., K. Mayur, H. M. Lederman, and A. D. Frankel. 1989. Inhibition of antigen-induced lymphocyte proliferation by Tat protein from HIV-1. Science 246:1606-1608. [DOI] [PubMed] [Google Scholar]
- 53.Vives, E., P. Brodin, and B. Lebleu. 1997. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J. Biol. Chem. 272:16010-16017. [DOI] [PubMed] [Google Scholar]
- 54.Weeks, K. M., and D. M. Crothers. 1991. RNA recognition by Tat-derived peptides: interaction in the major groove? Cell 66:577-588. [DOI] [PubMed] [Google Scholar]
- 55.Weiss, J. M., A. Nath, E. O. Major, and J. W. Berman. 1999. HIV-1 Tat induces monocyte chemoattractant protein-1-mediated monocyte transmigration across a model of the human blood-brain barrier and up-regulates CCR5 expression on human monocytes. J. Immunol. 163:2953-2959. [PubMed] [Google Scholar]
- 56.Westendorp, M. O., R. Frank, C. Ochsenbauer, K. Stricker, J. Dhein, H. Walczak, K. M. Debatin, and P. H. Krammer. 1995. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature 375:497-500. [DOI] [PubMed] [Google Scholar]
- 57.Wrenger, S., T. Hoffmann, J. Faust, C. Mrestani-Klaus, W. Brandt, K. Neubert, M. Kraft, S. Olek, R. Frank, S. Ansorge, and D. Reinhold. 1997. The N-terminal structure of HIV-1 Tat is required for suppression of CD26-dependent T cell growth. J. Biol. Chem. 272:30283-30288. [DOI] [PubMed] [Google Scholar]
- 58.Zagury, D., A. Lachgar, V. Chams, L. S. Fall, J. Bernard, J. F. Zagury, B. Bizzini, A. Gringeri, E. Santagostino, J. Rappaport, M. Feldman, A. Burny, and R. C. Gallo. 1998. Interferon alpha and Tat involvement in the immunosuppression of uninfected T cells and C-C chemokine decline in AIDS. Proc. Natl. Acad. Sci. USA 95:3851-3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zagury, J. F., A. Sill, W. Blattner, A. Lachgar, H. Le Buanec, M. Richardson, J. Rappaport, H. Hendel, B. Bizzini, A. Gringeri, M. Carcagno, M. Criscuolo, A. Burny, R. C. Gallo, and D. Zagury. 1998. Antibodies to the HIV-1 Tat protein correlated with nonprogression to AIDS: a rationale for the use of Tat toxoid as an HIV-1 vaccine. J. Hum. Virol. 1:282-292. [PubMed] [Google Scholar]
- 60.Zhang, M., X. Li, X. Pang, L. Ding, O. Wood, K. Clouse, I. Hewlett, and A. I. Dayton. 2001. Identification of a potential HIV-induced source of bystander-mediated apoptosis in T cells: upregulation of trail in primary human macrophages by HIV-1 tat. J. Biomed. Sci. 8:290-296. [DOI] [PubMed] [Google Scholar]