Abstract
Treacher Collins syndrome (TCS) is an autosomal dominant disorder of craniofacial development caused by mutations in the gene TCOF1. Its gene product, treacle, consists mainly of a central repeat domain, which shows it to be structurally related to the nucleolar phosphoprotein Nopp140. Treacle remains mostly uncharacterized to date. Herein we show that it, like Nopp140, is a highly phosphorylated nucleolar protein. However, treacle fails to colocalize with Nopp140 to Cajal (coiled) bodies. As in the case of Nopp140, casein kinase 2 appears to be responsible for the unusually high degree of phosphorylation as evidenced by its coimmunoprecipitation with treacle. Based on these and other observations, treacle and Nopp140 exhibit distinct but overlapping functions. The majority of TCOF1 mutations in TCS lead to premature termination codons that could affect the cellular levels of the full-length treacle. We demonstrate however, that the cellular amount of treacle varies less than twofold among a collection of primary fibroblasts and lymphoblasts and regardless of whether the cells were derived from TCS patients or healthy individuals. Therefore, cells of TCS patients possess a mechanism to maintain wild-type levels of full-length treacle from a single allele.
INTRODUCTION
Treacher Collins syndrome (TCS) is an autosomal dominant disorder of craniofacial development with an incidence of ∼1 in 50,000 live births (Jones et al., 1975; Gorlin et al., 1990). Its major characteristics include bilaterally symmetric midface hypoplasia, downward slant of the palpebral fissures, colobomata of lower lids, microtia, and other deformities of the ear that often lead to conductive hearing loss, and cleft palate (Rovin et al., 1964; Fazen et al., 1967; Phelps et al., 1981). TCOF1, the gene mutated in TCS was recently identified by positional cloning (The Treacher Collins Syndrome Collaborative Group, 1996; Dixon et al., 1997a; Wise et al., 1997). The majority of mutations identified lead to premature stop codons in the TCOF1 gene and possibly to truncated forms of its product, treacle. Based on these facts, it was suggested that TCS is caused by haploinsufficiency or by dominant-negative effects (Dixon, 1996; The Treacher Collins Syndrome Collaborative Group, 1996; Wise et al., 1997). Haploinsufficiency could be generated because truncated treacle loses its function and/or is rapidly digested, and/or its mRNA, containing premature termination codons, is degraded by the nonsense-mediated mRNA decay pathway (for review, see Frischmeyer and Dietz, 1999). Dominant-negative effects could be caused by truncated forms of treacle that interfere with the function of the full-length protein.
Treacle is a three-domain protein with a unique N and C terminus and a large central repeat domain (Dixon et al., 1997a; Wise et al., 1997). The repeat domain consists of 10 acidic serine clusters alternating with alanine-, lysine-, and proline-rich stretches. The only other protein containing such a characteristic repeat domain is the nucleolar and Cajal body (formerly coiled body; see Gall et al., 1999) phosphoprotein Nopp140 (Meier and Blobel, 1992, 1994). Nopp140 shuttles between the nucleolus, the cytoplasm, and the Cajal bodies and associates with small nucleolar ribonucleoprotein particles (snoRNPs) (Meier and Blobel, 1992; Isaac et al., 1998; Yang et al., 2000). Cajal bodies are distinct small nuclear organelles that are highly enriched in small nuclear ribonucleoprotein particles (for review, see Matera, 1999). Based on these findings we proposed Nopp140 to function as a chaperone of ribosome and snoRNP biogenesis.
Although treacle has been localized to the nucleolus by using green fluorescent protein (GFP)-tagged constructs in transient transfection studies (Marsh et al., 1998; Winokur and Shiang, 1998), the endogenous protein remains poorly characterized. The thorough characterization of nonribosomal nucleolar proteins, however, has become particularly important with the identification of novel nucleolar functions that range from tRNA biogenesis to control of tumor suppression (for review, see Olson et al., 2000).
MATERIALS AND METHODS
Cells and Antibodies
The fibroblast and Epstein-Barr virus-transformed lymphoblast cell lines were established from TCS patients and controls. All the cell lines were derived from patients with mutations that lead to premature stop codons except for the f4 fibroblasts, which were from a patient who was only clinically diagnosed to have TCS. The specific mutations were previously determined (Wise et al., 1997) and are as follows: f1 and l1, 2552delA and 2561delA; f2 and l2, 1408delAG; f3 and l3, 1408delAG (different patient than f2 and l2 but same mutation); l4, 497delATAC; l5, 2565delAG; l6, 422insA; l7, 2565delAG (different patient than l5 but same mutation); and l8, 2526delAG. The fibroblasts were grown in Eagle's minimum essential medium α with l-glutamine (Life Technologies, Gaithersburg, MD) supplemented with 15% fetal bovine serum (FBS) (Clontech, Palo Alto, CA) and had not been passed >11 times. The lymphoblasts were grown in RPMI-1640 (Life Technologies) containing 15% FBS, and the HeLa cells were maintained attached in DMEM with high glucose (Life Technologies) and 10% FBS. All media contained 100 units/ml penicillin and 0.1 mg/ml streptomycin.
Polyclonal rabbit antiserum against the N terminus of treacle, antitreacle(N), was described previously (Ab 014; Marsh et al., 1998). Antibodies against a synthetic peptide corresponding to amino acids 1231–1250 of human treacle, antitreacle(C), were raised in rabbits by Research Genetics, Inc. (Huntsville, AL). Antitreacle(C) immunoglobulins (IgGs) were affinity purified over a peptide column as described previously for anti-Nopp140 IgGs (Meier and Blobel, 1992). For this purpose, the synthetic peptide was coupled to N-hydroxysuccinimide-activated Sepharose (Amersham Pharmacia Biotech, Piscataway, NJ) as suggested by the manufacturer. Antibodies to the following antigens were from the sources indicated in parentheses, Nopp140 (RE10 IgGs; Meier and Blobel, 1992), nucleolin (7G2 ascites fluid; Pinol-Roma, 1999), fibrillarin (D77 monoclonal IgGs; Aris and Blobel, 1988), coilin (5P10 ascites fluid; Almeida et al., 1998), casein kinase 2 (CK2α polyclonal rabbit serum; Litchfield et al., 1994), HA hemagglutinin epitope (12CA5 ascites fluid; Wilson et al., 1984), and actin (ascites fluid C4; Boehringer Mannheim, Indianapolis, IN).
Indirect Immunofluorescence
Indirect immunofluorescence was exactly performed as previously described (Isaac et al., 1998) with the following exceptions. Presumably, to enhance accessibility to the antigen in fibroblasts, treacle(N) and treacle(C) antibodies required the presence of 0.5 M NaCl, in addition to phosphate-buffered saline (PBS) and 1% bovine serum albumin, during incubation. The following dilutions or concentrations of the antibodies were used: treacle(N) serum at 1:50 on fibroblasts, at 1:100 on HeLa cells, and at 1:250 on COS-1 cells; treacle(C) serum at 1:200 on HeLa cells; treacle(C) IgGs at 0.1 mg/ml on fibroblasts and at 5 μg/ml on HeLa cells; nucleolin ascites fluid at 1:2000 on fibroblasts; fibrillarin IgGs at 1 μg/ml on fibroblasts; coilin ascites fluid at 1:2000 on fibroblasts and at 1:10,000 on HeLa cells; and HA ascites fluid at 1:200 on COS-1 cells. For peptide competition, the treacle(C) antibodies were incubated concomitantly with 20 μg/ml of the synthetic competing peptide. Secondary antibodies were rhodamine-labeled goat anti-rabbit IgG and fluorescein isothiocyanate-labeled goat anti-mouse IgG antibodies (both from Boehringer Mannheim). Images were collected and processed, and pictures prepared for publication exactly as described (Meier, 1996; Isaac et al., 1998). In double immunofluorescence experiments, the rhodamine and fluorescein isothiocyanate images collected separately were assigned red and green colors, respectively, and superimposed by using Adobe Photoshop 5.0.2 software (Adobe Systems, San Jose, CA).
Protein Quantitation by Using Immunofluorescence
Fibroblasts were labeled with nucleolin antibodies in combination with either treacle(N) or treacle(C) antibodies as described above. Images were collected by using identical exposure times for each antigen and each cell line. Images were analyzed as follows with Metamorph 2.76 software (Universal Imaging, West Chester, PA). Nucleolin-stained areas were applied as a mask to define all nucleoli in a field of cells as areas for measurement. The nucleolar total fluorescence intensity (arbitrary units) in the corresponding treacle(N) or treacle(C) images as well as the corresponding pixel area was acquired. The average pixel intensity was then calculated by dividing the total fluorescence intensity by the pixel area.
Western Blots and Protein Quantitation
Tissue culture cells were scraped into ice-cold PBS, washed twice, lysed in hot sample buffer, and tip sonicated. Whole cell lysates corresponding to 1.8 × 105 fibroblast and 9.3 × 105 lymphoblast cells/lane were analyzed by SDS-PAGE, transferred to nitrocellulose, and stained with amido black. The nitrocellulose was cut into strips based on the migration of molecular weight standards (Bio-Rad Laboratories, Hercules, CA), and the appropriate strips incubated with primary antibodies at the dilutions or concentrations specified: antitreacle(N) serum at 1:1000, antitreacle(C) IgGs at 11 μg/ml (on HeLa cells at 1.1 μg/ml), anti-Nopp140 IgGs at 0.3 μg/ml, antinucleolin ascites fluid at 1:2000, antiactin ascites fluid at 1: 500, and anti-CK2α serum on HeLa cells at 1:5000. The primary antibodies were diluted in PBS containing 1% powdered milk with the exception of the incubation of antitreacle(C) IgGs on fibroblasts where in addition 0.1% Tween 20 (Bio-Rad Laboratories) was included. Immunodetection was performed as described (Meier, 1996) by using enhanced chemiluminescence (ECL) (Amersham Life Science, Arlington Heights, IL). The resulting films were scanned, the individual bands quantitated by using NIH (Scion) Image 1.62 software, and the background subtracted. The results from five separate Western blots were averaged for each antigen and expressed as ratios relative to the corresponding actin value averages.
Immunoprecipitation
Approximately 107 HeLa cells were lysed in a 0.5-ml volume and used for immunoprecipitation as described previously for buffalo rat liver cells (Li et al., 1997) with the following changes. The lysis buffer contained 50 mM Tris, pH 7.4, 200 mM NaCl, and 0.05% Triton X-100 in addition to the protease inhibitors but no SDS. Treacle(C) IgGs (20 μg) and 10 μg of competing peptide were used per 0.5 ml of precipitation reaction.
In Vitro Transcription/Translation and Phosphatase Treatment
Transient transfections and in vitro transcription/translation reactions were performed exactly as described (Isaac et al., 1998). The mouse Tcof1 cDNA constructs were described previously (Marsh et al., 1998). To dephosphorylate in vitro-translated treacle, 10 μl of in vitro transcription/translation mixture was treated with 10 units of calf intestine alkaline phosphatase (Boehringer Mannheim) for 30 min at 37°C after 10-fold dilution with phosphatase buffer (provided by the manufacturer). The phosphatase inhibitors were identical to those described previously with the addition of 50 mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid, and the samples were analyzed as published (Meier and Blobel, 1992).
RESULTS
Antitreacle Antibodies
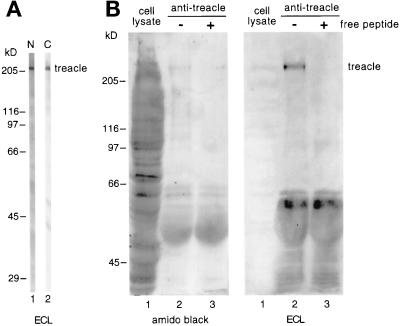
In this study, we used two different antibodies directed against the N- and C-terminal domains of human treacle flanking its Nopp140-related repeat domain. The first antiserum was raised against the N-terminal 55 amino acids of recombinant treacle (Marsh et al., 1998) and shall be referred to as antitreacle(N). The second serum was raised against a synthetic peptide encompassing amino acids 1231–1250 in the C terminus of treacle and was designated antitreacle(C). The latter serum was affinity purified over a peptide column as described in MATERIALS AND METHODS to obtain antitreacle(C) IgGs. The specificity of the antitreacle antibodies was tested on Western blots of HeLa whole cell lysates (Figure 1A). Both antibodies recognized a single major band of ∼220 kDa (Figure 1A). This slow mobility of treacle was in contrast to its predicted molecular weight of 144 kDa. To ascertain that the two antibodies indeed recognized the same antigen, we used the antitreacle(C) IgGs to immunoprecipitate treacle from HeLa whole cell lysates (Figure 1B, lanes 1) and probed the precipitate with antitreacle(N) antibodies (Figure 1B). Indeed, when the immunoprecipitate was analyzed by SDS-PAGE, transferred to nitrocellulose, and amido black stained, a faint band of ∼220 kDa could be distinguished (Figure 1B, left, lane 2). This band was recognized by the antitreacle(N) antibodies (Figure 1B, right, lane 2). The specificity of the immunoprecipitation was further confirmed by the ability of free peptide to compete for the precipitation (Figure 1B, lanes 3).
Figure 1.
Treacle migrates as a 220-kDa band on SDS-PAGE. (A) Western blots of HeLa whole cell lysates were cut into strips, probed with antitreacle(N) serum (lane 1) or antitreacle(C) IgGs (lane 2) and the immunoreactivity detected by ECL. (B) Treacle was immunoprecipitated from HeLa whole cell lysates (lanes 1) with antitreacle(C) IgGs in the absence (lanes 2) and presence of free competing peptide (lanes 3). The precipitates were analyzed by SDS-PAGE, transferred to nitrocellulose, amido black stained (left), and subsequently probed with antitreacle(N) serum as in A (right). Twenty times fewer equivalents of the whole cell lysate were loaded (lanes 1) than of the precipitates (lanes 2 and 3).
Treacle Is a Highly Phosphorylated Protein
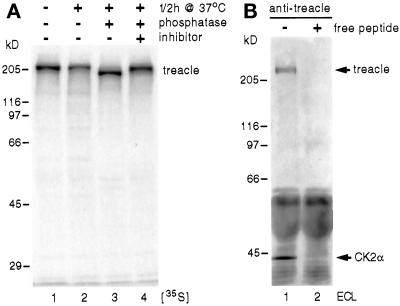
To further investigate the aberrant mobility of the putative treacle band on SDS-PAGE, we in vitro transcribed/translated treacle and analyzed it by SDS-PAGE and fluorography. For this purpose, we used the full-length cDNA of mouse treacle, which is 61.5% identical to human treacle (Dixon et al., 1997b; Paznekas et al., 1997). Despite being 109 amino acids shorter than its human ortholog, [35S]methionine- and [35S]cysteine-labeled mouse treacle migrated as a single band of ∼220 kDa, confirming the aberrant mobility of treacle on SDS-PAGE. This unusual behavior was very reminiscent of the treacle-related protein Nopp140, which migrates as a band of about twice its theoretical molecular weight mostly due to its high degree of phosphorylation (Meier and Blobel, 1992; Meier, 1996). To test whether treacle was phosphorylated like Nopp140, in vitro-translated treacle was incubated for 30 min at 37°C with alkaline phosphatase. Indeed, phosphatase treatment in the absence (Figure 2, lane 3) but not the presence (lane 4) of phosphatase inhibitors increased the mobility of treacle to a position corresponding to ∼180 kDa. This difference in migration between phosphorylated and dephosphorylated treacle amounted to 40 kDa and was identical to that of phosphatase-treated Nopp140 (Meier and Blobel, 1992), indicating a similar high degree of phosphorylation. In fact, treacle contains 82 potential CK2 phosphorylation sites, a number that is identical to that found in the repeat domain of Nopp140 (Meier and Blobel, 1992; Dixon et al., 1997a,b; Wise et al., 1997). The remaining difference between apparent and theoretical molecular weight of treacle after dephosphorylation is likely due to the high charge density of even the unphosphorylated protein. In a further parallel to Nopp140, the massive phosphorylation of treacle appeared to be all or none as indicated by the absence of intermediate forms of phosphorylation (Figures 1 and 2; Meier and Blobel, 1992).
Figure 2.
Treacle is a highly phosphorylated protein (A) and associates with CK2 (B). (A) Mouse treacle was in vitro transcribed/translated in reticulocyte lysate in the presence of [35S]methionine and [35S]cysteine and analyzed by SDS-PAGE and fluorography (lane 1). In vitro-translated treacle was incubated for 30 min at 37°C in the absence (lane 2) and presence of alkaline phosphatase (lanes 3 and 4), in the absence (lanes 2 and 3) and presence of phosphatase inhibitors (lane 4), and analyzed as described for lane 1. (B) Western blot of an immunoprecipitation of treacle in the absence (lane 1) and presence of competing peptide (lane 2) as described in Figure 1B. The nitrocellulose was probed with antibodies against the catalytic α subunit of CK2 and against treacle(N). The immunoreactivity was detected by ECL. Note the specific precipitation of CK2 and treacle (arrows) exclusively in the absence of competing peptide (lane 1), whereas the same amounts of IgGs are present in both lanes (dark bands below 60 kDa).
We showed previously that CK2 appears to be the kinase responsible for the unusual high degree of phosphorylation of Nopp140 and that CK2 interacts with Nopp140 (Meier, 1996; Li et al., 1997). To test whether CK2 also associated with treacle, we immunoprecipitated treacle from HeLa whole cell lysates with treacle(C) antibodies in the absence and presence of free competing peptide and probed the Western blot of the precipitates with antibodies against the catalytic α subunit of CK2 and against treacle(N) (Figure 2B). Indeed, CK2α antibodies detected a small but distinct band in the treacle precipitates in the absence (Figure 2B, lane 1) but not presence of competing peptide (lane 2). No Nopp140 could be detected in the treacle immunoprecipitates (our unpublished results). We conclude that treacle interacts with CK2 but not Nopp140. Whether this association is direct or mediated through other factors remains to be determined.
Localization of Treacle
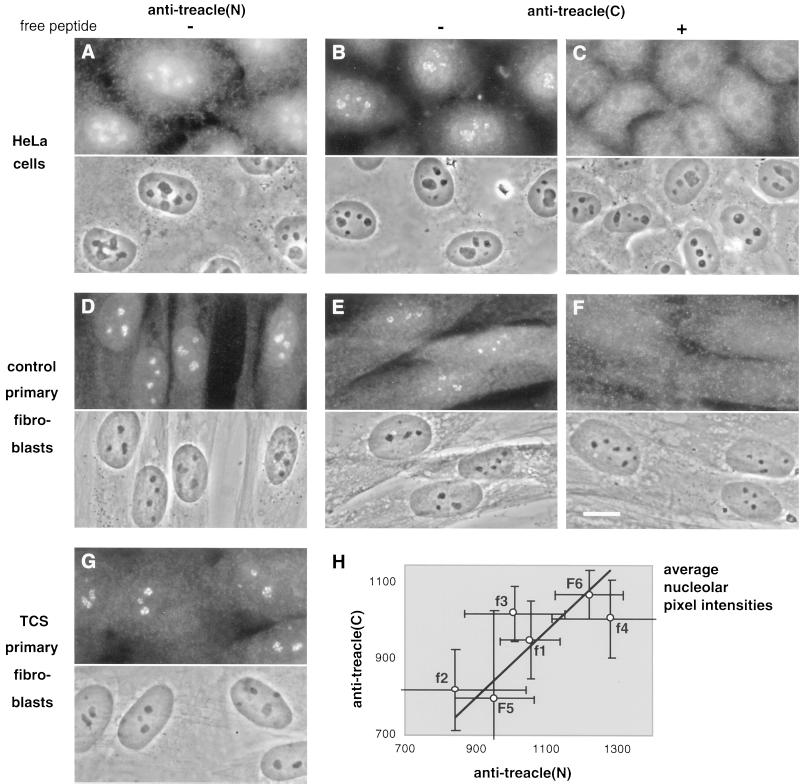
The treacle(N) and -(C) antibodies were used to determine the subcellular localization of the endogenous protein by indirect immunofluorescence. On HeLa cells, both antisera reacted most strongly and in a punctate manner with nucleoli (Figure 3, A and B). In addition, they diffusely labeled the cells throughout. The specificity of the nucleolar signal from the treacle(C) serum was tested by the addition of free competing peptide during the antiserum incubation. Indeed, the signal was removed exclusively from the nucleoli (Figure 3C). Interestingly, the immunoreactivity of the treacle(N) serum appeared more specific for nucleoli on primary human fibroblasts (Figure 3D) than on HeLa cells. In contrast, the nucleolar signal of the affinity-purified antitreacle(C) IgGs in the fibroblasts (Figure 3E) was not as strong as that in HeLa cells. The specificity of the antitreacle(C) IgG nucleolar reactivity however was confirmed by its removal with free peptide competition (Figure 3F). Taken together, therefore, endogenous treacle is a nucleolar protein. For unknown reasons, its C terminus appears better accessible in HeLa than in primary fibroblast cells, whereas the opposite is the case for its N terminus. The latter was confirmed with an independent antibody, an N-terminal peptide antiserum (our unpublished results). Finally, no difference in the localization of treacle or the cellular morphology could be observed on the light microscopic level between fibroblasts from healthy individuals (Figure 3D) and TCS patients (Figure 3G). This was underscored by a similar lack of difference in the localization of other nucleolar antigens tested, nucleolin, fibrillarin, and Nopp140 (our unpublished results). Finally, no increased labeling of the cytoplasm of TCS cells was detectable (Figure 3G). This indicates that no C-terminally truncated forms of treacle, which lack the ability to enter the nucleus (Figure 5c′; Marsh et al., 1998; Winokur and Shiang, 1998), are expressed in the cells of TCS patients.
Figure 3.
Treacle is a nucleolar protein. Indirect immunofluorescence with antitreacle(N) (A, D, and G) and antitreacle(C) serum (B and C) and antitreacle(C) IgGs (E and F) on fixed and permeabilized HeLa (A–C) or primary fibroblast cells (D–G). The specificity of the antitreacle(C) antibodies was confirmed by competition with free peptide (C and F). The fibroblasts were from a healthy individual (D–F) designated F5 and from a TCS patient (G) designated f1. The upper half of each panel shows the indirect immunofluorescence pattern and the lower half the corresponding phase contrast picture (A–G). Bar, 10 μm (F). (H) Plots the average nucleolar fluorescence pixel intensities (arbitrary units) of the antitreacle(N) serum (x-axis) and antitreacle(C) IgGs (y-axis) against each other in fibroblasts of two healthy individuals (F5 and F6) and of four TCS patients (f1–f4). The average nucleolar pixel intensities were determined as described in MATERIALS AND METHODS. The bars mark the SDs of the averages of 32 to 141 nucleoli analyzed. The line describes a linear regression between the six values. Note that only a small window of each axis is displayed, thus artificially magnifying the error bars. However, the size of each bar is significantly smaller than half of the pixel intensity for each point.
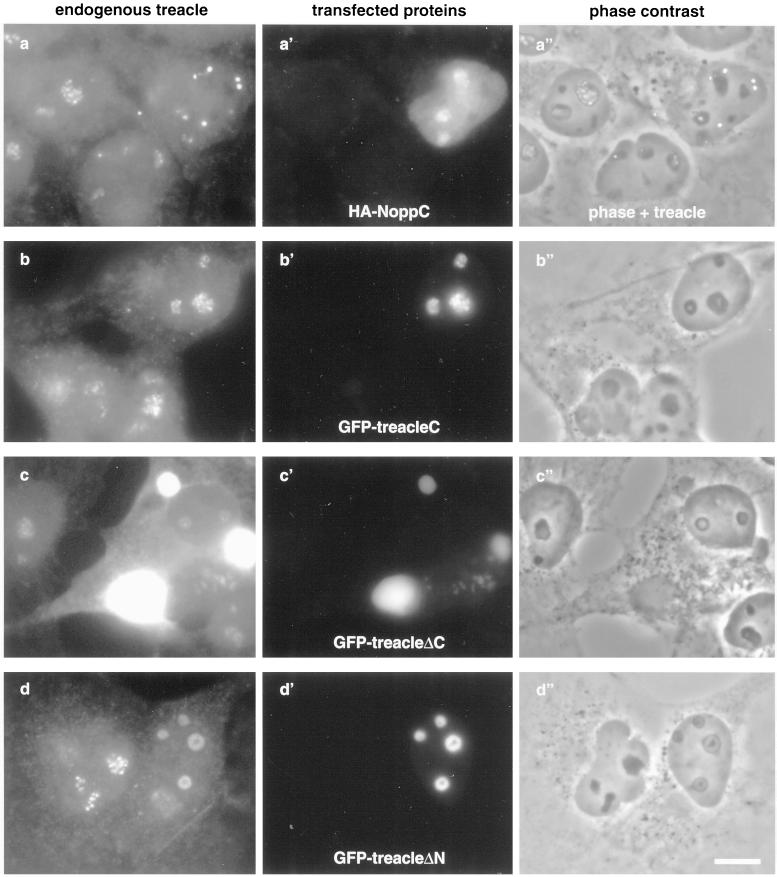
Figure 5.
Treacle is chased out of the nucleolus in NoppC-transfected cells (a) but unaffected by the C terminus or C-terminally truncated forms of exogenous treacle (b and c). N-terminally truncated treacle affects the structure of the nucleolus and the localization of endogenous treacle (d). COS-1 cells were transiently transfected with (a′) the HA-tagged C terminus of Nopp140 (HA-NoppC), (b′) the GFP-tagged C terminus of mouse treacle [GFP-treacle(C), amino acids 1158–1320], (c′) GFP-tagged treacle lacking the C terminus and the nuclear localization signals (GFP-treacleΔC, amino acids 1–1175), or (d′) GFP-tagged treacle without its N terminus (GFP-treacleΔN, amino acids 75–1320) and analyzed by indirect double immunofluorescence with antitreacle(N) serum (a–d), anti-HA antibodies (a′), and GFP fluorescence (b′–d′). (a′′–d′′) depicts the corresponding phase contrast images. Note that (a′′) is a transparent overlay of a and its phase contrast image to point out the extranucleolar localization of treacle in the transfected cell. Finally, the treacle(N) antibodies in c, unlike in a, b, and d, also recognize the transfected GFP-treacleΔC, which is highly overexpressed. Bar, 10 μm.
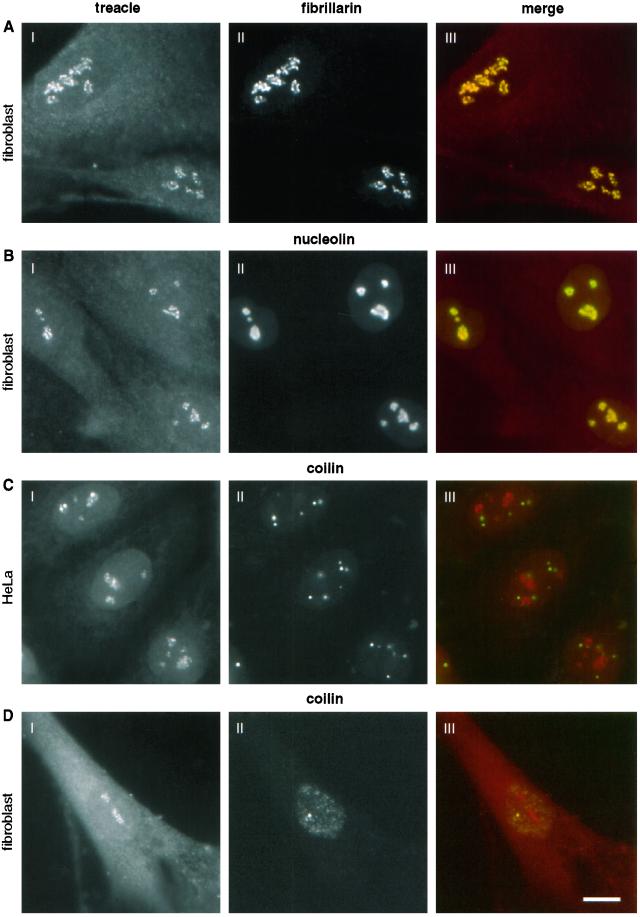
To further investigate the nucleolar localization of treacle, we performed indirect double immunofluorescence labeling experiments in fibroblasts. The localization of treacle (Figure 4A, I) and fibrillarin (Figure 4A, II) coincided exactly as indicated by the yellow color of the merged images (Figure 4A, III). Identical data were obtained when Nopp140 was colocalized with fibrillarin in these cells (our unpublished results). The nucleolar localization of nucleolin (Figure 4B, II), however, extended slightly beyond that of treacle when the two images were merged (Figure 4B, III, note yellow is surrounded by green). Because fibrillarin, like Nopp140, localizes specifically to the dense fibrillar component of the nucleolus, treacle is localized in that particular nucleolar compartment. Nucleolin, however, in addition to the dense fibrillar component, resides in the surrounding granular component of the nucleolus, which is devoid of treacle (Figure 4B, III). To test whether treacle, like fibrillarin and Nopp140, also localized to Cajal bodies, we compared its localization with that of the Cajal body marker protein coilin (Andrade et al., 1991; Raska et al., 1991). These experiments were performed in HeLa cells, which are particularly rich in Cajal bodies (Figure 4C). Surprisingly, treacle (Figure 4C, I) was completely absent from the Cajal bodies stained by coilin (Figure 4C, II), which is better evident when the two images are superimposed (Figure 4C, III). Because this result was unexpected, we tested whether treacle also was absent from Cajal bodies in human fibroblasts. Although only some of the fibroblasts contained Cajal bodies, treacle staining remained completely excluded from them (Figure 4D). Thus, treacle is the first known antigen of the dense fibrillar component of the nucleolus that fails to accumulate in the Cajal bodies.
Figure 4.
Treacle colocalizes with fibrillarin to the dense fibrillar component of the nucleolus but is absent from Cajal bodies. Double indirect immunofluorescence with treacle antibodies (I) and antibodies (II) to fibrillarin (A), nucleolin (B), and coilin (C and D). Fluorescence was detected on fibroblast (A, B, and D) or HeLa cells (C) and the treacle antibodies were treacle(N) serum for the fibroblasts and treacle(C) IgGs for the HeLa cells. III represents the merged images of I (red) and II (green) in artificial colors (precise overlap is shown in yellow). Bar, 10 μm.
We previously showed that the exogenous expression of the conserved C terminus of Nopp140, NoppC, exerted a dominant-negative effect on the endogenous protein and on all antigens of snoRNPs to which antibodies are available (Isaac et al., 1998; Yang et al., 2000). Interestingly, therefore, NoppC affected specifically the localization of all antigens of the dense fibrillar component but not those of the other nucleolar compartments. In addition to this effect, NoppC expression dispersed the Cajal bodies (Isaac et al., 1998). Because treacle was part of the dense fibrillar component but not the Cajal bodies we studied the effect of NoppC expression on its localization. Treacle was chased out of the nucleolus (Figure 5a) in NoppC-transfected cells (Figure 5a′) like all the other proteins of the dense fibrillar component. The extranucleolar location of treacle in the transfected cells was particulate and especially obvious when the phase contrast picture and the treacle immunofluorescence image were overlaid (Figure 5a′′). The particulate nature of the extranucleolar treacle is reminiscent of that of NAP57, fibrillarin, and endogenous Nopp140 under the same conditions (Isaac et al., 1998). This could reflect the expulsion of entire subcompartments of the dense fibrillar component from the nucleolus.
We further tested whether the C terminus or truncated forms of treacle had similar effects on endogenous treacle and other nucleolar proteins, thereby explaining potential dominant-negative effects in TCS. For this purpose, we expressed the GFP-tagged C terminus of mouse treacle or C-terminally truncated forms in COS cells. As shown previously, the C terminus alone accumulated in the nucleolus (Figure 5b′), whereas the C-terminally truncated forms, mimicking TCS mutations, remained completely excluded from the nucleus and accumulated in depots around the nuclear envelope in the cytoplasm (Figure 5c′; Marsh et al., 1998; our unpublished results). Additionally, the N-terminal first 37 and 75 amino acids of treacle fused to GFP accumulated in the nucleus but were not sufficient to target the central repeat domain or parts thereof to the nucleus (our unpublished results). However, the complementary C-terminal constructs (amino acids 37 and 75 to the C terminus) readily accumulated in the nucleolus (Figure 5d′) confirming the presence of nuclear localization and nucleolar retention signals in the C terminus of treacle (Marsh et al., 1998; Winokur and Shiang, 1998). The expression of all of these constructs left the localization of endogenous treacle, Nopp140, and nucleolin unaffected (Figure 5, b and c; our unpublished results) with the exception of the N-terminally truncated treacle (Figure 5d). The latter led to a reorganization of the nucleolus (Figure 5d′′) that affected all nucleolar antigens tested, including nucleolin, whose localization remained unaltered by all Nopp140 constructs (Isaac et al., 1998). Our observation that C-terminally truncated forms of treacle fail to interfere with the localization of the endogenous protein suggests that dominant-negative effects are unlikely to play a role in the pathogenesis of TCS. Indeed, no staining of C-terminally truncated treacle could be detected in the cytoplasm of cells from TCS patients nor was the localization of endogenous treacle or other nucleolar proteins affected (Figure 3G).
Cellular Amount of Treacle Is Indistinguishable in Individuals with and without TCS
In cells of TCS patients, one allele of the TCOF1 gene contains a mutation leading to a premature stop codon. Consequently, half of treacle in cells of TCS patients may be C-terminally truncated and degraded or missing due to nonsense-mediated mRNA decay (for review, see Frischmeyer and Dietz, 1999). To directly test this possibility, we quantitated the amount of treacle in primary fibroblasts from individuals with and without TCS by using our treacle(N) and -(C) antibodies. All cell lines were derived from TCS patients with verified nonsense mutations that lead to premature termination codons except for the f4 fibroblasts, which were from a patient who was only clinically diagnosed to have TCS (see MATERIALS AND METHODS; Wise et al., 1997). We used two approaches, first, quantitation of indirect immunofluorescence, and second, quantitation of the antibody reactivity on Western blots.
As described in MATERIALS AND METHODS, we used indirect immunofluorescence pictures of antitreacle(N) and -(C) antibodies as shown in Figure 3, D and E, respectively, and determined the average nucleolar fluorescence pixel intensity. Figure 3H presents the results of the statistical analysis from the primary fibroblasts of four TCS patients (f1–f4) and two control individuals (F5 and F6). Several observations can be made. First, the average nucleolar fluorescent pixel intensities from all cells and with both antibodies cluster closely together, i.e., within 25 and 30% of the maximal average pixel intensity for the treacle(C) and -(N) antibodies, respectively. This indicates that the amount of treacle varies less than twofold between cells from different individuals. Second, the variation within the cluster is completely independent of the phenotype of the individual from which the cells were derived. Thus, the two values of the control individuals nearly bracket those of the TCS patients at the minimum and maximum. Third and consequently, the variation of the amounts of treacle among the fibroblasts from the TCS patients was equal to that of the control individuals. Fourth, the values of the N- and C-terminal treacle antibodies roughly correlated with each other (Figure 3H, line). Thus, the fibroblasts with the lowest signal of one antibody also were among the lowest with the other, thereby validating our quantitation. In summary, these data indicate that the amount of full-length treacle in primary fibroblasts from TCS patients is indistinguishable from that of healthy control individuals. Finally, there is no twofold difference of the treacle(N) over the treacle(C) staining in the TCS cells compared with wild-type cells. Therefore, no C-terminally truncated forms of treacle are expressed in these cells, which is in agreement with the lack of cytoplasmic staining in TCS cells with the treacle(N) antibodies (Figure 3G).
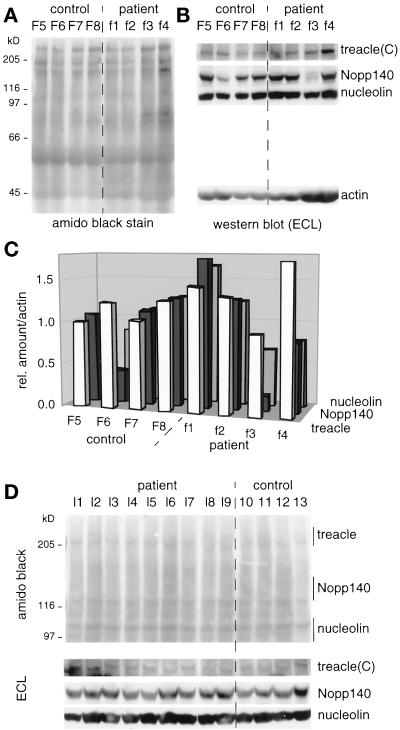
To corroborate this surprising finding we used an independent method of treacle quantitation in these cells, Western blotting (Figure 6). For this purpose, equal cell numbers of primary fibroblasts derived from four healthy individuals (F5–F8) and from four TCS patients (f1–f4) were lysed in sample buffer, analyzed by SDS-PAGE, transferred to nitrocellulose, and the proteins stained by amido black (Figure 6A). The immunoreactivity of treacle(C), Nopp140, nucleolin, and actin antibodies was tested on individual strips of nitrocellulose excised at their respective migration position and detected by ECL (Figure 6B). The relative amounts of these four antigens were determined by using NIH image software in five independent experiments, such as the one depicted in Figure 6, A and B and as detailed in MATERIALS AND METHODS. The average of the three nucleolar antigens, treacle, Nopp140, and nucleolin, was then expressed as their relative amount divided by the amount of actin (Figure 6C). The expression relative to actin was to control for differences in gel loading. In agreement with our indirect immunofluorescence results, the data show that the amount of full-length treacle [as detected by the antitreacle(C) IgGs] in fibroblasts from TCS patients is about equal to that in cells from healthy individuals. In particular, the overall amount of treacle varies less than twofold between all individuals, regardless of their phenotype. Furthermore, the relative amount of treacle correlates well in most cases with that of two other nucleolar antigens, Nopp140 and nucleolin. Surprisingly, the fibroblasts from one patient (f4) exhibited an unusual high relative amount of treacle (Figure 6, A–C). Because the gel loading of these fibroblasts appeared higher compared with the other cells, we repeated the measurements on gels where only one-fourth of the amount was loaded but the result was the same. Additionally, loading of different amounts of whole cell lysates showed that we were able to detect at least 16-fold differences in treacle content, thus validating our method (our unpublished results). Unfortunately, we were unable to get the antitreacle(N) antibodies to work consistently on Western blots of the primary fibroblasts. Therefore, it remains to be resolved whether any truncated forms of treacle are expressed in addition to the full-length one in cells of TCS patients although our quantitation of treacle immunofluorescence suggests not (Figure 3H). Finally, there was no significant difference in the cellular amount of treacle in the fibroblasts f1 compared with that in f2 and f3, although the truncation of treacle between these individuals differed by nearly 400 amino acids (see MATERIALS AND METHODS; Wise et al., 1997). Thus, there was no correlation between the degree of treacle truncation and the cellular amount of full-length treacle.
Figure 6.
The cellular amount of treacle varies less than twofold in cell lines derived from control individuals and TCS patients as determined by quantitative Western blotting. (A) Primary fibroblast whole cell lysates of equal cell numbers from control (F5–F8) and TCS individuals (f1–f4) were analyzed by SDS-PAGE, transferred to nitrocellulose, and amido black stained. (B) Nitrocellulose strips from A of the appropriate mobility range were probed with antitreacle(C), Nopp140, nucleolin, and actin antibodies and the immunoreactivity visualized by ECL. (C) Histogram of the relative amounts of treacle, Nopp140, and nucleolin compared with those of actin. The relative amounts were determined as described in MATERIALS AND METHODS and resulted from averaging five independent experiments, one of which is depicted in A and B. Note that the ratios of treacle/actin were artificially increased by a factor of 1.8 to allow a better comparison of their relative amounts to those of Nopp140 and nucleolin. (D) Same experiment as described in A and B but with lymphoblast cell lines derived from TCS patients (l1–l9) and control individuals (10–13). Top, amido black-stained nitrocellulose; bottom, immunoreactivity of treacle(C), Nopp140, and nucleolin antibodies visualized by ECL. Note, that the lymphoblasts l1–l3 were derived from the same individuals as the fibroblasts f1–f3, respectively.
To expand our quantitation studies to a different cell type, we investigated the amount of treacle in nine lymphoblast cell lines derived from TCS patients (Figure 6D, l1–l9) and four control lymphoblasts (10–13). Unfortunately, not only did the treacle(N) antibodies not recognize their antigen on Western blots of these cells but also even the treacle(C) IgGs reacted only very poorly with it. Nevertheless, the treacle(C) IgGs recognized a weak band of similar intensity in all lymphoblast cell lines, corroborating our data obtained from the primary fibroblasts (Figure 6D, bottom). The signal however was too weak to quantitate reliably. Nevertheless, it is evident that the amount of treacle in TCS lymphoblasts is not reduced compared with that in cells from healthy individuals. The amounts of Nopp140 and nucleolin appeared to be consistent with those of treacle (Figure 6D, bottom). Finally, amido black staining of the nitrocellulose confirmed that equal cell numbers and amounts of total protein were loaded per lymphoblast whole cell lysate (Figure 6D, top). We conclude therefore that our findings with regard to the treacle quantitation are not limited to fibroblasts but extend to lymphoblasts.
Aside from the described case of the f4 fibroblasts, the relative amounts of treacle, nucleolin, and Nopp140 appeared to be similar in cells of the different individuals except in the F6 and f3 fibroblasts (Figure 6, B and C). Surprisingly and reproducibly, the relative amount of Nopp140 in those cells was less than half of that in all the other cells. This finding was even more remarkable considering that the amount of Nopp140 in lymphoblasts derived from the same individual (l3) was comparable to those of other individuals (Figure 6D). Currently, it is not clear what causes this difference and it will require further investigation. However, the absence of such aberrant values for treacle further validates its quantitation on Western blots.
DISCUSSION
Our results demonstrate that treacle is a highly phosphorylated nucleolar protein that associates with CK2 similar to the previously identified nucleolar phosphoprotein Nopp140 (Meier and Blobel, 1990, 1992; Li et al., 1997). Thus, the similarities between the two proteins extend beyond their homologous central repeat domain. A closer look, however, also reveals important differences. In contrast to Nopp140 and all the other dense fibrillar component proteins of the nucleolus, therefore, treacle does not localize to the small nuclear ribonucleoprotein particle-enriched Cajal bodies. This differential localization could be explained by the interaction of treacle with proteins distinct from those associated with Nopp140. Indeed, treacle immunoprecipitates (Figure 1B) were devoid of Nopp140 and the snoRNP proteins, NAP57, and GAR1 (our unpublished results). Nopp140, however, associates with snoRNPs (Yang et al., 2000). Furthermore, the repeat domain is required for the localization of Nopp140 to the nucleolus and the Cajal bodies (Isaac et al., 1998) but it is not sufficient to target treacle to Cajal bodies. Additionally, partial constructs of treacle containing its repeat domain, unlike such Nopp140 constructs (Isaac et al., 1998), do not affect the localization of a specific subset of nucleolar proteins (Figure 5, b–d). Finally, the treacle-related repeat domain of Nopp140 is its evolutionarily least conserved part. In fact, it is the N and particularly the C termini of Nopp140, which are unrelated to treacle, that are conserved in all eukaryotes (Meier, 1996). Homologs of treacle, however, have currently been identified only in mammals (Dixon et al., 1997b; Paznekas et al., 1997). In summary, therefore, the function of the two proteins appears to be clearly distinct although overlapping. The overlap is based on the homologous repeat domain of treacle and Nopp140, their equal high degree of phosphorylation, and their colocalization in the dense fibrillar component of the nucleolus.
Most of the phosphorylation sites in the repeat domain of treacle are CK2 consensus sites and we show that CK2 indeed associates with treacle. Therefore, CK2 appears to be the kinase responsible for the unusually high degree of phosphorylation of treacle. Interestingly, such a CK2-like activity that phosphorylates the repeat domain of treacle, is present in all tissues and in the critical structures during embryonic development, from which the craniofacial features affected by TCS arise (Jones et al., 1999). It is thus not surprising that in all the experiments performed for this study treacle appeared in its fully phosphorylated state as judged by its mobility on SDS-PAGE. Treacle shares all these characteristics with Nopp140.
Our data indicate that the amount of full-length treacle in fibroblasts and lymphoblasts varies less than twofold and is independent of whether the cells originated from TCS patients or from healthy individuals. This result was surprising because the mutated allele of the TCOF1 gene in TCS patients should produce either truncated treacle or none, potentially causing dominant-negative effects or haploinsufficiency, respectively. Therefore, twofold expression of full-length treacle from the unaffected allele appears to compensate for the mutated one. Thus, mature cells are endowed with a mechanism to maintain wild-type levels of treacle from a single allele, indicating the importance of specific levels of this protein to the cell.
Truncated forms of treacle expressed from its mutated allele could interfere with the function and localization of the full-length treacle expressed from the healthy allele. In the case of Nopp140, truncated forms containing its repeat domain or parts thereof chase full-length Nopp140 out of the nucleolus and lead to arrest of RNA polymerase I transcription (Isaac et al., 1998; Chen et al., 1999; Isaac and Meier, unpublished data). In addition, partial constructs of Nopp140 containing its repeat domain induce novel structures, R-rings, in the nucleoplasm to which the endogenous full-length Nopp140 and other antigens are recruited (Isaac et al., 1998). Similarly, exogenous expression of C-terminally truncated treacle leads to its mislocalization and induction of novel structures (Figure 5c′; Marsh et al., 1998). Primary fibroblasts from TCS patients however, do not show any mislocalization of treacle or other nucleolar antigens (Figure 3G). Furthermore, the amount of treacle detected with N- and C-terminal antibodies was indistinguishable between TCS and control fibroblasts (Figure 3H). Thus, there is no evidence for the presence of truncated treacle in TCS fibroblasts. This is supported by the observation that TCS fibroblasts exhibited an efficient degradation of nonsense transcripts as determined by reverse transcription-polymerase chain reaction (to be published elsewhere). Even if truncated forms of treacle were expressed in these cells, our data indicate that they would leave the localization of the full-length treacle and other nucleolar proteins unaffected (Figure 5c). However, it cannot be excluded that the mislocalized truncated treacle might have deleterious effects in the cytoplasm. In summary, primary fibroblasts of TCS patients appear not to suffer from treacle haploinsufficiency or from dominant-negative effects caused by truncated treacle. How then do nonsense mutations in one allele of the TCOF1 gene lead to the characteristic phenotypes of the disease?
Based on the affected structures and on studies performed in rodents treated with cis- and trans-retinoic acid, TCS is caused by defects that specifically occur during the development of the first and second branchial arches (Poswillo, 1975; Wiley et al., 1983; Sulik et al., 1987). Indeed, during embryonic development, treacle is expressed at peak levels in the first and second branchial arches (Dixon et al., 1997b). Thus, TCS may be explained by haploinsufficiency or adverse effects of truncated treacle occurring specifically in those structures and exclusively during development. Either the required levels of treacle in these, unlike in adult cells, may be too high to be compensated for by a single allele, or the high levels allow truncated forms of treacle to become expressed. In fact, while this study was under review, Dixon et al. (2000) showed that deletion of one Tcof1 allele in mouse produced embryos with severe defects in craniofacial development, causing perinatal death. Because these findings favor haploinsufficiency as the cause for TCS, it is significant that patient cells express wild-type levels of treacle.
ACKNOWLEDGMENTS
We thank John Aris, Maria Carmo-Fonseca, Ed Krebs, Dongxia Li, and Serafin Piñol-Roma for antibodies; Shailesh Shenoi for help with the quantitative fluorescence analysis; and the Analytical Imaging Facility of AECOM for the use of their equipment. The research was in part funded by the National Institutes of Health P60-DE13078 (to E.W.J.) and GM-50725 (to U.T.M.), the Birth Defects Foundation 050591 (to M.J.D.), the Welcome Trust 058423 (to M.J.D.), and the Howard Hughes Medical Institute-Research Resources Program for Medical Schools (to U.T.M.).
Abbreviations used:
- N
amino
- C
carboxy
- CK2
casein kinase 2
- ECL
enhanced chemiluminescence
- GFP
green fluorescent protein
- HA
hemagglutinin
- snoRNP
small nucleolar ribonucleoprotein particle
- TCS
Treacher Collins syndrome
REFERENCES
- Almeida F, Saffrich R, Ansorge W, Carmo-Fonseca M. Microinjection of anti-coilin antibodies affects the structure of coiled bodies. J Cell Biol. 1998;142:899–912. doi: 10.1083/jcb.142.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade LEC, Chan EKL, Raska I, Peebles CL, Roos G, Tan EM. Human autoantibody to a novel protein of the nuclear coiled body: immunological characterization and cDNA cloning of p80 coilin. J Exp Med. 1991;173:1407–1419. doi: 10.1084/jem.173.6.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aris J, Blobel G. Identification and characterization of a yeast nucleolar protein that is similar to a rat liver nucleolar protein. J Cell Biol. 1988;107:17–31. doi: 10.1083/jcb.107.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HK, Pai CY, Huang JY, Yeh NH. Human Nopp140, which interacts with RNA polymerase I: implications for rRNA gene transcription and nucleolar structural organization. Mol Cell Biol. 1999;19:8536–8546. doi: 10.1128/mcb.19.12.8536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon J, Brakebusch C, Fässler R, Dixon MJ. Increased levels of apoptosis in the prefusion neural folds underlie the craniofacial disorder, Treacher Collins syndrome. Hum Mol Genet. 2000;9:1473–1480. doi: 10.1093/hmg/9.10.1473. [DOI] [PubMed] [Google Scholar]
- Dixon J, Edwards SJ, Anderson I, Brass A, Scambler PJ, Dixon MJ. Identification of the complete coding sequence and genomic organization of the Treacher Collins syndrome gene. Genome Res. 1997a;7:223–234. doi: 10.1101/gr.7.3.223. [DOI] [PubMed] [Google Scholar]
- Dixon J, Hovanes K, Shiang R, Dixon MJ. Sequence analysis, identification of evolutionary conserved motifs and expression analysis of murine tcof1 provide further evidence for a potential function for the gene and its human homologue, TCOF1. Hum Mol Genet. 1997b;6:727–737. doi: 10.1093/hmg/6.5.727. [DOI] [PubMed] [Google Scholar]
- Dixon MJ. Treacher Collins syndrome. Hum Mol Genet. 1996;5:1391–1396. doi: 10.1093/hmg/5.supplement_1.1391. [DOI] [PubMed] [Google Scholar]
- Fazen LE, Elmore J, Nadler HL. Mandibulo-facial dysostosis. (Treacher-Collins syndrome) Am J Dis Child. 1967;113:405–410. doi: 10.1001/archpedi.1967.02090190051001. [DOI] [PubMed] [Google Scholar]
- Frischmeyer PA, Dietz HC. Nonsense-mediated mRNA decay in health and disease. Hum Mol Genet. 1999;8:1893–1900. doi: 10.1093/hmg/8.10.1893. [DOI] [PubMed] [Google Scholar]
- Gall JG, Bellini M, Wu Z, Murphy C. Assembly of the nuclear transcription and processing machinery: cajal bodies (coiled bodies) and transcriptosomes. Mol Biol Cell. 1999;10:4385–4402. doi: 10.1091/mbc.10.12.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlin RJ, Cohen MM, Levin SL. Syndromes of the Head and Neck. 3rd ed. New York: Oxford: Oxford University Press; 1990. [Google Scholar]
- Isaac C, Yang Y, Meier UT. Nopp140 functions as a molecular link between the nucleolus and the coiled bodies. J Cell Biol. 1998;142:319–329. doi: 10.1083/jcb.142.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KL, Smith DW, Harvey MA, Hall BD, Quan L. Older paternal age and fresh gene mutation: data on additional disorders. J Pediatr. 1975;86:84–88. doi: 10.1016/s0022-3476(75)80709-8. [DOI] [PubMed] [Google Scholar]
- Jones NC, Farlie PG, Minichiello J, Newgreen DF. Detection of an appropriate kinase activity in branchial arches I and II that coincides with peak expression of the Treacher Collins syndrome gene product, treacle. Hum Mol Genet. 1999;8:2239–2245. doi: 10.1093/hmg/8.12.2239. [DOI] [PubMed] [Google Scholar]
- Li D, Meier UT, Dobrowolska G, Krebs EG. Specific interaction between casein kinase 2 and the nucleolar protein Nopp140. J Biol Chem. 1997;272:3773–3779. doi: 10.1074/jbc.272.6.3773. [DOI] [PubMed] [Google Scholar]
- Litchfield DW, Dobrowolska G, Krebs EG. Regulation of casein kinase II by growth factors: a reevaluation. Cell Mol Biol Res. 1994;40:373–381. [PubMed] [Google Scholar]
- Marsh KL, Dixon J, Dixon MJ. Mutations in the Treacher Collins syndrome gene lead to mislocalization of the nucleolar protein treacle. Hum Mol Genet. 1998;7:1795–1800. doi: 10.1093/hmg/7.11.1795. [DOI] [PubMed] [Google Scholar]
- Matera AG. Nuclear bodies: multifaceted subdomains of the interchromatin space. Trends Cell Biol. 1999;9:302–309. doi: 10.1016/s0962-8924(99)01606-2. [DOI] [PubMed] [Google Scholar]
- Meier UT. Comparison of the rat nucleolar protein Nopp140 to its yeast homolog SRP40: differential phosphorylation in vertebrates and yeast. J Biol Chem. 1996;271:19376–19384. [PubMed] [Google Scholar]
- Meier UT, Blobel G. A nuclear localization signal binding protein in the nucleolus. J Cell Biol. 1990;111:2235–2245. doi: 10.1083/jcb.111.6.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier UT, Blobel G. Nopp140 shuttles on tracks between nucleolus and cytoplasm. Cell. 1992;70:127–138. doi: 10.1016/0092-8674(92)90539-o. [DOI] [PubMed] [Google Scholar]
- Meier UT, Blobel G. NAP57, a mammalian nucleolar protein with a putative homolog in yeast and bacteria. J Cell Biol [correction appeared in 140: 447] 1994;127:1505–1514. doi: 10.1083/jcb.127.6.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson MO, Dundr M, Szebeni A. The nucleolus: an old factory with unexpected capabilities. Trends Cell Biol. 2000;10:189–196. doi: 10.1016/s0962-8924(00)01738-4. [DOI] [PubMed] [Google Scholar]
- Paznekas WA, Zhang N, Gridley T, Jabs EW. Mouse TCOF1 is expressed widely, has motifs conserved in nucleolar phosphoproteins, and maps to chromosome 18. Biochem Biophys Res Commun. 1997;238:1–6. doi: 10.1006/bbrc.1997.7229. [DOI] [PubMed] [Google Scholar]
- Phelps PD, Poswillo D, Lloyd GA. The ear deformities in mandibulofacial dysostosis (Treacher Collins syndrome) Clin Otolaryngol. 1981;6:15–28. doi: 10.1111/j.1365-2273.1981.tb01782.x. [DOI] [PubMed] [Google Scholar]
- Pinol-Roma S. Association of nonribosomal nucleolar proteins in ribonucleoprotein complexes during interphase and mitosis. Mol Biol Cell. 1999;10:77–90. doi: 10.1091/mbc.10.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poswillo D. The pathogenesis of the Treacher Collins syndrome (mandibulofacial dysostosis) Br J Oral Surg. 1975;13:1–26. doi: 10.1016/0007-117x(75)90019-0. [DOI] [PubMed] [Google Scholar]
- Raska I, Andrade LEC, Ochs RL, Chan EKL, Chang C-M, Roos G, Tan EM. Immunological and ultrastructural studies of the nuclear coiled body with autoimmune antibodies. Exp Cell Res. 1991;195:27–37. doi: 10.1016/0014-4827(91)90496-h. [DOI] [PubMed] [Google Scholar]
- Rovin S, Dachi SF, Borenstein DB, Cotter WB. Mandibulofacial dysostosis, a familial study of five generations. J Pediatr. 1964;65:215–221. doi: 10.1016/s0022-3476(64)80522-9. [DOI] [PubMed] [Google Scholar]
- Sulik KK, Johnston MC, Smiley SJ, Speight HS, Jarvis BE. Mandibulofacial dysostosis (Treacher Collins syndrome): a new proposal for its pathogenesis. Am J Med Genet. 1987;27:359–372. doi: 10.1002/ajmg.1320270214. [DOI] [PubMed] [Google Scholar]
- The Treacher Collins Syndrome Collaborative Group. Positional cloning of a gene involved in the pathogenesis of Treacher Collins syndrome. Nat Genet. 1996;12:130–136. doi: 10.1038/ng0296-130. [DOI] [PubMed] [Google Scholar]
- Wiley MJ, Cauwenbergs P, Taylor IM. Effects of retinoic acid on the development of the facial skeleton in hamsters: early changes involving cranial neural crest cells. Acta Anat. 1983;116:180–192. doi: 10.1159/000145741. [DOI] [PubMed] [Google Scholar]
- Wilson IA, Niman HL, Houghten RA, Cherenson AR, Connolly MR, Lerner RA. The structure of an antigenic determinant in a protein. Cell. 1984;37:767–778. doi: 10.1016/0092-8674(84)90412-4. [DOI] [PubMed] [Google Scholar]
- Winokur ST, Shiang R. The Treacher Collins syndrome (TCOF1) gene product, treacle, is targeted to the nucleolus by signals in its C-terminus. Hum Mol Genet. 1998;7:1947–1952. doi: 10.1093/hmg/7.12.1947. [DOI] [PubMed] [Google Scholar]
- Wise CA, Chiang LS, Paznekas WA, Sharma M, Musy MM, Ashley JA, Lovett M, Jabs EW. TCOF1 gene encodes a putative nucleolar phosphoprotein that exhibits mutations in Treacher Collins Syndrome throughout its coding region. Proc Natl Acad Sci USA. 1997;94:3110–3115. doi: 10.1073/pnas.94.7.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Isaac C, Wang C, Dragon F, Pogacic V, Meier UT. Conserved composition of mammalian box H/ACA and box C/D small nucleolar ribonucleoprotein particles and their interaction with the common factor Nopp140. Mol Biol Cell. 2000;11:567–577. doi: 10.1091/mbc.11.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]