Abstract
A widely dispersed interference group of retroviruses that includes the feline endogenous virus (RD114), baboon endogenous virus (BaEV), human endogenous virus type W (HERV-W), and type D primate retroviruses uses the human Na+-dependent neutral amino acid transporter type 2 (hASCT2; gene name, SLC1A5) as a common cell surface receptor. Although hamster cells are fully resistant to these viruses and murine cells are susceptible only to BaEV and HERV-W pseudotype viruses, these rodent cells both become highly susceptible to all of the viruses after treatment with tunicamycin, an inhibitor of protein N-linked glycosylation. A partial explanation for these results was recently provided by findings that the orthologous murine transporter mASCT2 is inactive as a viral receptor, that a related (ca. 55% identity) murine paralog (mASCT1; gene name, SLC1A4) mediates infections specifically of BaEV and HERV-W, and that N-deglycosylation of mASCT1 activates it as a receptor for all viruses of this interference group. Because the only two N-linked oligosaccharides in mASCT1 occur in the carboxyl-terminal region of extracellular loop 2 (ECL2), it was inferred that this region contributes in an inhibitory manner to infections by RD114 and type D primate viruses. To directly and more thoroughly investigate the receptor active sites, we constructed and analyzed a series of hASCT2/mASCT2 chimeras and site-directed mutants. Our results suggest that a hypervariable sequence of 21 amino acids in the carboxyl-terminal portion of ECL2 plays a critical role in determining the receptor properties of ASCT2 proteins for all viruses in this interference group. In addition, we analyzed the tunicamycin-dependent viral susceptibility of hamster cells. In contrast to mASCT1, which contains two N-linked oligosaccharides that partially restrict viral infections, hamster ASCT1 contains an additional N-linked oligosaccharide clustered close to the others in the carboxyl-terminal region of ECL2. Removal of this N-linked oligosaccharide by mutagenesis enabled hamster ASCT1 to function as a receptor for all viruses of this interference group. These results strongly suggest that combinations of amino acid sequence changes and N-linked oligosaccharides in a critical carboxyl-terminal region of ECL2 control retroviral utilization of both the ASCT1 and ASCT2 receptors.
Recent investigations have identified a human Na+-dependent transporter for polar neutral amino acids (hASCT2; gene name, SLC1A5) as the common receptor for a large widely dispersed interference group of retroviruses that includes the feline endogenous virus (RD114), baboon endogenous virus (BaEV), human endogenous virus type W (HERV-W), and type D primate or simian retroviruses (SRVs) that cause infectious immunodeficiencies (6, 23, 28, 33). Additional evidence suggests that the horizontally transmitted spleen necrosis viruses and reticuloendotheliosis viruses originated by a rare zoonosis of a bird by a retrovirus that was closely related to BaEV, followed by adaptive radiation into gallinaceous and anseriform birds (4, 16, 18, 19). These avian retroviruses may also occur in the same interference group as the above viruses (19). Although HERV-W is not present as a complete provirus in the human genome, there is at least one full-length HERV-W envelope glycoprotein gene that is expressed naturally in placenta and in several inflammatory diseases (5, 6, 9, 14, 17, 27). The HERV-W envelope glycoprotein is highly fusogenic and can pseudotype human immunodeficiency virus type 1 (HIV-1) virions, implying that it might contribute to the spread of HIV-1 into CD4-negative privileged tissues such as the placenta or brain (1, 23).
ASCT2 is a member of the glutamate transporter superfamily, which also contains a closely related (ca. 54% identity) transporter ASCT1 (gene name, SLC1A4). ASCT1 and ASCT2 transport an overlapping but nonidentical set of neutral amino acids, with an important difference being the transport of glutamine only by ASCT2 (3, 8, 15, 35). The transporters in this superfamily all contain an associated Cl− channel that is gated open by a transportable amino acid (13, 30). However, the flux of Cl− is uncoupled from the amino acid flux (8, 30, 38). The topology of the glutamate transporters has also been investigated (7, 29). The model of Brocke et al. (7) is consistent with our evidence for the ASCT proteins and is used in this work. Our evidence derived from finding that the carboxyl termini are in the cytosol and that the regions identified as extracellular loop 2 (ECL2) contain N-linked oligosaccharides, which is consistent with an extracellular orientation (25).
Although all of the mammalian retroviruses in this interference family infect human cells and use hASCT2 as a common receptor, the viruses have distinct host ranges. For example, only BaEV and HERV-W pseudotyped viruses are able to infect murine cells, whereas Chinese hamster ovary (CHO) cells are fully resistant to all of these viruses. In contrast, murine cells become highly susceptible to all of the viruses after exposure to tunicamycin, an inhibitor of protein N-linked glycosylation (20, 25). Based on these results, it was initially surprising to find that the murine ASCT2 ortholog (mASCT2) is inactive as a receptor for all of these retroviruses and that removal of its N-linked oligosaccharides by mutagenesis did not enable it to mediate infections of RD114 or primate type D retroviruses (25). However, a partial explanation for these viral host range properties was derived from the findings that the mouse protein mASCT1 is a specific receptor for BaEV and HERV-W pseudotyped viruses and that N-deglycosylation of mASCT1 by mutagenesis activates it as a receptor for the other mammalian retroviruses of this family (23, 25). Thus, the abilities of these viruses to infect murine cells appear to be determined by mASCT1 rather than by mASCT2.
The active site(s) for viral interaction with the ASCT1 and ASCT2 receptors has not previously been investigated in detail. Indeed, the only evidence reported on this matter derived from the observation that N-deglycosylation of mASCT1 converts it into an active receptor for type D primate retroviruses and RD114 (25). Because the only two N-linked oligosaccharides in mASCT1 occur in the carboxyl-terminal region of ECL2, it was inferred that this region is likely to be important for reception of RD114 and type D primate retroviruses but perhaps unimportant for BaEV and HERV-W (23, 25). These two N-linked oligosaccharides function cooperatively to inhibit mASCT1 utilization by RD114 and type D primate retroviruses, with elimination of either glycosylation site being sufficient to alleviate the inhibition (25).
We now report additional studies that pertain to this issue which involved analyses of chimeras and site-directed substitution mutations of the active hASCT2 receptor and the inactive mASCT2 ortholog. Our results suggest that a hypervariable sequence of 21 amino acids in the carboxyl-terminal region of ECL2 has a major influence on reception of all viruses in this interference family. Although N-linked oligosaccharides occur in these ECL2 regions of human and mouse ASCT2, their effects on viral reception appear to be less important than the amino acid sequence differences. In addition to these studies of ASCT2, we also analyzed the basis for the tunicamycin-dependent susceptibility of CHO cells to all of these viruses. In contrast to mASCT1, which has only two N-linked oligosaccharides, hamster ASCT1 contains an additional nearby N-linked oligosaccharide in the same critical ECL2 region. Removal of this N-linked oligosaccharide by mutagenesis converted hamster ASCT1 into an active receptor for all of the viruses in this family. Thus, the remaining two N-linked oligosaccharides block infections by RD114 and type D primate retroviruses in the context of mASCT1 but not in the context of hamster ASCT1. Considered together, our results strongly suggest that the carboxyl-terminal regions of ECL2 in both ASCT1 and ASCT2 play critical roles in controlling retroviral reception. This control is determined by a combination of amino acid sequence changes and N-linked oligosaccharides, with the consequences of individual changes being dependent on the overall sequence context in which they occur. These results have important implications for understanding virus-receptor interactions and host factors that prevent zoonoses.
MATERIALS AND METHODS
Cell lines, plasmids, and viruses.
Human embryonal kidney HEK293T cells (ATCC CRL-1573) were grown in Dulbecco's modified Eagle's high-glucose medium supplemented with 10% fetal bovine serum (FBS). TE671 human rhabdomyosarcoma cells (ATCC CRL8805) were grown in Dulbecco's modified Eagle's medium supplemented with 10% FBS. CHO cells (ATCC CCL-61) and derived clones were grown in alpha-modified minimal essential medium supplemented with 10% FBS. Clones of CHO cells stably expressing hamster ASCT1, hamster ASCT1 N194T, N194T-N201T, N194T-N206T, and N201T-N206T were made by transfection using myc-tagged receptor-expressing pcDNA3.1 vectors. Transfected cells were selected with G418, and individual Geneticin-resistant colonies were isolated. Highly expressing cell clones were chosen, as determined by Western immunoblotting, using a monoclonal antibody specific for the myc tag. We employed the human, mouse, and hamster common names, ASCT1 and ASCT2, for the receptor proteins. The standard nomenclature for their genes is SLC1A4 and SLC1A5, respectively (OMIM database, The National Center for Biotechnology Information, National Institutes of Health; http://www.ncbi.nlm.nih.gov/entrez).
The phCMV expression vectors encoding HERV-W, HERV-Wcyt16, BaEV-Rless, and RD-Rless envelopes were described elsewhere (23). The pCMVdR8.74 vector, which encodes the structural proteins of HIV-1 except for the envelope, and pRRLsin18.cPPT.CMV.eGFP.WPRE, which encodes an enhanced green fluorescent protein (GFP)-encoding reporter gene, were kindly provided by Luigi Naldini (University of Torino Medical School, Turin, Italy) (36).
LacZ(RD114) was produced by TELCeB6/RDF-7 helper free packaging cells (10). LacZ(BaEV) was rescued by infection of mink Mv-1-Lu cells harboring a lacZ vector (34) with a replication-competent BaEV stock. TELCeB6 cells infected with a replication-competent SRV-2 stock were used to produce LacZ(SRV-2) (33).
HIV/HERV-W pseudotype viruses were made by cotransfecting human HEK293T cells with the phCMV-HERV-W vector expressing the HERV-W envelope, pCMVdR8.74 plus pRRLsin18.cPPT.CMV.eGFP.WPRE, using PolyFect reagent (Qiagen, Valencia, Calif.) (23).
Hamster ASCT1 (CHO.ASCT1) cDNA cloning.
Hamster ASCT1 (CHO.ASCT1) receptor cDNA was isolated by reverse transcription-PCR amplification with total RNA. Total RNA was prepared from CHO cells with an RNeasy Midi kit (Qiagen). The 1.599-kb CHO.ASCT1 cDNA was amplified by using primers complementary to the 5′ and 3′ ends of the mASCT1 coding region (upstream primer, 5′-AAAAGCTTATGGAGAAGAGCGGCGAGACC-3′, containing a HindIII restriction site [underlined sequence]; downstream primer, 5′-AACTCGAGTCACAGCACTGACTCC-TTGGA-3′, containing a XhoI restriction site [underlined sequence]). The PCR products were subsequently cloned into the pCDNA3.1V5His-TOPO mammalian expression vector (Invitrogen, Carlsbad, Calif.) and sequenced by the Microbiology and Molecular Immunology Core Facility on the PE/ABD 377 sequencer using dye terminator cycle sequencing chemistry (Applied Biosystems, Foster City, Calif.).
Construction of chimeric receptors and site-directed mutagenesis.
Human-mouse ASCT2 chimera constructs were made by using the overlapping PCR technique. The corresponding cDNAs were amplified by using primers complementary to the conserved coding regions for putative intracellular loop 2 (h/m- and m/hASCT2), putative transmembrane 3 and 4 (hECL2/mASCT2 and mECL2/hASCT2), and putative ECL2 (mhECL2- and hmECL2/hASCT2). The PCR products were subsequently cloned into the pCDNA3.1V5His-TOPO mammalian expression vector (Invitrogen) and sequenced as described above. Hamster ASCT1 receptor residues were mutated by PCR mutagenesis using two complementary mutagenic primers containing the targeted point mutation and QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, Calif.). Plasmid DNA from three independent clones was sequenced to confirm the mutations. The DNA sequence was determined as described above. The mutants were designated by parental receptor name followed by the mutated amino acid followed by the residue number and the new amino acid.
Infection and cell-cell fusion assays.
CHO cells expressing hASCT2, mASCT2, human-mouse ASCT2 chimera, hamster ASCT1 (CHO.ASCT1), or CHO.ASCT1 N-deglycosylated mutants were generated by transient transfections of the corresponding cDNAs by using SuperFect transfection reagent (Qiagen). Transient transfectants were challenged with either LacZ(RD114), LacZ(BaEV), or LacZ(SRV-2) pseudotype virus, with overnight incubations with virus beginning 24 h after transfection. Infected cells were stained by treating cells with 0.25% glutaraldehyde and assayed for β-galactosidase activity with 5-bromo-4-chloro-3-indoyl-β-d-galactopyranoside as a substrate (24). The blue CFU were counted, and the titer of infection was expressed as the number of CFU per milliliter of virus supernatant. For HIV/HERV-W pseudotype virus infection assays, clones of CHO cells stably expressing hASCT2, hASCT1, hamster ASCT1, hamster ASCT1 N194T, N194T-N201T, N194T-N206T, and N201T-N206T were seeded in 48-well plates at a density of 0.5 × 104 cells per well and incubated overnight at 37°C. A 250-μl aliquot of diluted virus sample containing 8 μg of Polybrene per ml was added to the cells and centrifuged for spinoculation at 1,200 × g for 2 h at 25°C. After removal of the supernatants, the cells were incubated in regular medium for 48 to 72 h at 37°C. For cell-cell fusion assays, the cells were seeded in 12-well plates at a density of 105 cells per well. Twenty-four hours later, the cells were transfected with phCMV-RD114-Rless, phCMV-BaEV-Rless, or phCMV-HERV-W by using PolyFect reagent (Qiagen). At 24 to 36 h posttransfection, cells were fixed and stained with May-Grunwald and Giemsa solutions (Merck) according to the manufacturer's recommendations.
l-[3H]alanine transport.
The l-[3H]alanine transport assay was performed in HEK293T cells transiently expressing ASCT receptors. The initial rate of l-[3H]alanine uptake was analyzed at 48 h after the transfections were begun, as previously described (37).
Immunoblot analyses.
For studies of cell surface expression of CHO.ASCT1 and its N-deglycosylated mutants, human HEK293T cells were transiently transfected with the corresponding myc-tagged expression vectors by using SuperFect transfection reagent (Qiagen). At 48 h posttransfection HEK293T cells were surface biotinylated by the addition of 2 mM sulfo-NHS-LC-biotin (Pierce, Rockford, Ill.) to 2 × 107 cells for 1 h at 4°C. The reaction was quenched with 20 mM glycine for 15 min. Washed cells were scraped off the culture dishes in cold phosphate-buffered saline and centrifuged at 200 × g at 4°C for 5 min. The cell pellets were then resuspended in lysis buffer (50 mM Tris-HCl [pH 8.8], 150 mM NaCl, 0.1% sodium dodecyl sulfate, 1% NP-40, 0.5% sodium deoxycholate, and protease inhibitor cocktail [Complete; Boehringer Mannheim]) and incubated on ice for 30 min. The cell debris and nuclei were removed by centrifugation at 15,000 × g at 4°C for 10 min. The biotinylated molecules were adsorbed onto streptavidin-agarose beads (GIBCO BRL) at 4°C. Beads were washed three times with lysis buffer and resuspended in 20 μl of lysis buffer. Ten microliters of beads was treated with N-glycosidase F (2 h; 37°C). The treated and untreated beads were boiled with an equal volume of 2× Laemmli sample buffer (21) and subsequently analyzed by electrophoresis in 12% polyacrylamide gels in the presence of 0.1% sodium dodecyl sulfate. The proteins were then transferred to nitrocellulose membranes, which were then treated with 5% milk powder in phosphate-buffered saline. The nitrocellulose blots were probed with anti-Myc tag monoclonal antibody 9E10 (Sigma) and developed by using a horseradish peroxidase-conjugated goat anti-mouse antibody (Southern Biotechnology Associates, Inc.) and an enhanced chemiluminescence kit (NEN Life Research Products, Boston, Mass.).
Nucleotide sequence accession number.
The GenBank accession number for the Chinese hamster ASCT1 cDNA is AF537346.
RESULTS
A divergent sequence of 21 amino acids in the carboxyl-terminal region of ECL2 controls viral receptor activity of human and mouse ASCT2.
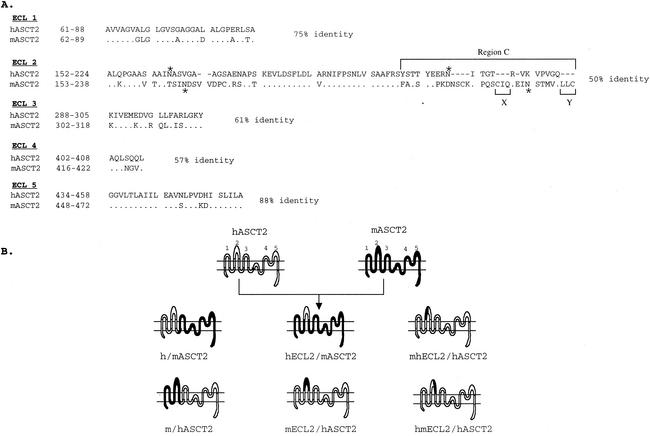
Figure 1 shows the putative topology of the ASCT proteins based on investigations of the closely related glutamate transporters (7) and on our compatible evidence for N-linked glycosylation of ECL2 in ASCT1 and ASCT2 (25; also, see below). The model includes two reentrant loops between ECL 3-4 and ECL 4-5 which apparently move in the membrane and are involved in amino acid transport (7). Although the human and mouse ASCT2 orthologs are 81% identical, a notable clustering of divergences occurs in two regions of ECL2 that are separated by a sequence that is highly conserved not only in ASCT1 and -2 but also in other members of the glutamate transporter superfamily (Fig. 1A) (30).
FIG. 1.
Construction of hASCT2/mASCT2 chimeras. (A) Amino acid sequence comparison of the putative ECL regions of hASCT2 and mASCT2. Numbers at the left of the sequences correspond to the positions of the first and last amino acids shown. Dots indicate amino acid identity. Deletions in sequences are indicated by dashes. N-glycosylation sites are indicated by asterisks. Numbers at the right of the sequences indicate the percent amino acid identity. (B) The topologies and the nomenclatures of the wild-type and chimeric ASCT2 proteins. The representation on the top shows the putative topology of the hASCT2 and mASCT2 cell surface receptors; the putative ECLs are numbered 1 through 5.
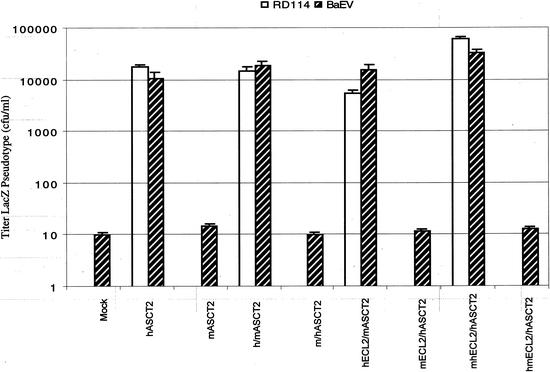
We analyzed the viral receptor properties of human and mouse ASCT2 by constructing and analyzing the reciprocal chimeras diagrammed in Fig. 1B. Initial studies indicated that h/mASCT2 was active as a receptor for LacZ(RD114) and LacZ(BaEV) pseudotyped viruses, whereas the reciprocal m/hASCT2 chimera was inactive (Fig. 2), and we subsequently extended this analysis by studying the other chimeras diagrammed in Fig. 1B. The results indicated that all chimeras that contained a stretch of 21 amino acids from the carboxyl-terminal region of ECL2 in hASCT2 were active receptors, whereas all chimeras lacking this sequence were inactive. This sequence is identified in Fig. 1A as the highly divergent region C.
FIG. 2.
Mediation of infections by hASCT2, mASCT2, and chimeric receptors. Infectivity assays were done with CHO cells that had been transiently transfected with expression vectors for the receptors. Infections with LacZ(RD114) and LacZ(BaEV) pseudotype viruses were initiated 24 h after the transfections were begun. The titers are averages of three independent experiments ± standard errors. RD114 titers were zero for some of the receptors, and in these cases the bars are not shown.
HEK293T cells transiently transfected with expression vectors for these wild-type and chimeric ASCT2 proteins all had increased rates of uptake of l-[3H]alanine, suggesting that they were active transporters that were expressed on cell surfaces (Table 1, analysis A). The results were significant to the 95% confidence level in all cases except for the m/hASCT2 chimera, for which the result was somewhat less significant (see footnotes of Table 1). However, our conclusion that hASCT2 region C is necessary for viral receptor function does not depend on the data obtained with the m/hASCT2 chimera (Fig. 1 and 2).
TABLE 1.
l-[3H]alanine uptake by HEK293T cells expressing wild-type, mutant, and chimeric ASCT2 and ASCT1 proteins
| Exogenous receptor assayeda | l-[3H]alanine uptake (nmol/min/mg of protein) |
|---|---|
| Analysis Ab | |
| pcDNA3.1 | 29.7 ± 1.5 |
| hASCT2 | 45.6 ± 2.4 |
| mASCT2 | 61.2 ± 1.6 |
| h/mASCT2 | 43.7 ± 1.9 |
| m/hASCT2 | 33.6 ± 1.9 |
| hECL2/mASCT2 | 41.9 ± 2.0 |
| mECL2/hASCT2 | 39.5 ± 2.2 |
| mhECL2/hASCT2 | 35.3 ± 2.5 |
| hmECL2/hASCT2 | 42.3 ± 1.5 |
| Analysis Bc | |
| pcDNA3.1 | 8.4 ± 1.6 |
| CHO.ASCT1 | 19.0 ± 4.7 |
| CHO.ASCT1.N194T | 15.7 ± 3.0 |
| CHO.ASCT1.N201T | 19.4 ± 3.8 |
| CHO.ASCT1.N206T | 16.2 ± 3.0 |
| CHO.ASCT1.N194T-N201T | 14.0 ± 3.0 |
| CHO.ASCT1.N194T-N206T | 17.2 ± 4.3 |
| CHO.ASCT1.N201T-N206T | 17.7 ± 4.1 |
| CHO.ASCT1.N194T-N201T-N206T | 14.4 ± 2.7 |
The rates of l-[3H]alanine transport were measured in HEK293T cells transiently transfected with either the empty vector (pcDNA3.1) or the receptor-expression vectors. Uptake of l-[3H]alanine was measured for 1 min at 37°C. The data were obtained in three separate assays, with each assay being done on four replica cultures. Thus, the data are each composite values derived from 12 cultures ± standard error. Different commercial batches of l-[3H]alanine were used for assays A and B, which was responsible for the differences in the relative incorporation estimates.
l-[3H]alanine uptake mediated by wild-type hASCT2, mASCT2, and their chimeras. Based on the paired-comparison t test, the rates of uptake in the cultures transfected with ASCT2 expression vectors were significantly greater than the rates in the control cultures to a confidence level of >95%, except for the m/hASCT2 chimera, which was significant to a confidence level of only approximately 90%.
l-[3H]alanine uptake mediated by wild-type CHO.ASCT1 and its N-deglycosylated mutants. Based on the paired-comparison t test, the rates of uptake in the cultures transfected with CHO.ASCT1 expression vectors were significantly greater than the rates in the control cultures to a confidence level of >95%, except for the CHO.ASCT1.N201T and CHO.ASCT1N194T-N201T results, which were significant to a confidence level of approximately 90%.
As shown in Fig. 1A, the C regions of hASCT2 and mASCT2 were almost completely different, which made their alignment uncertain and complicated our efforts to compare them by substitution mutagenesis. Nevertheless, we made the small deletions in mASCT2 identified in Fig. 1A as X and Y, and we found that the mutants were inactive as viral receptors (results not shown). In addition, we previously reported that removal of the N-linked oligosaccharides from ECL2 of mASCT2 did not activate receptor activity for BaEV, RD114, or type D primate retroviruses, although the N-deglycosylated mASCT2 was partially active as a receptor for HERV-W pseudotyped viruses (23, 25).
Tunicamycin-dependent susceptibility of hamster cells to infection by these retroviruses.
The above assays for viral receptor activity (Fig. 2) were done using CHO cells because they are highly resistant to all of the viruses in this interference group. However, as shown in Table 2, CHO cells also become susceptible to infections after they are treated with tunicamycin. Because the tunicamycin-dependent susceptibility of murine cells to RD114 and type D primate retroviruses was previously shown to depend on ASCT1 rather than ASCT2, we isolated RNA from CHO cells and used reverse transcription-PCR to isolate Chinese hamster ASCT1 cDNA. Sequence analysis of the hamster ASCT1 cDNA (accession number AF537346) implied that the encoded protein is 93% identical to mASCT1. Figure 3 compares the ECL2 sequences of the mASCT1 and hamster ASCT1 proteins. Interestingly, the hamster sequence contains three closely clustered consensus sites for N-linked glycosylation, with two (at N201 and N206) being identical to those in mASCT1 and an additional one at N194.
TABLE 2.
Tunicamycin treatment of hamster cells enables their infection by RD114 and simian type D retroviruses
| Cells | Titer of LacZ pseudotype (CFU/ml)a
|
|||||
|---|---|---|---|---|---|---|
| RD114
|
SRV-2
|
BaEV
|
||||
| (−)Tunic | (+)Tunic | (−)Tunic | (+)Tunic | (−)Tunic | (+)Tunic | |
| CHO | 0 | 1.0 × 105 | 0 | 2.2 × 104 | 4.1 | 5.2 × 104 |
CHO cells were tested for their susceptibility to LacZ(RD114), LacZ(SRV-2), and LacZ(BaEV) pseudotype viruses before treatment with tunicamycin [(−)Tunic] and after treatment of the cells with 250 ng of tunicamycin/ml [(+)Tunic] for 24 h. The viruses used in these assays had titers of approximately 105 CFU/ml when examined in human TE671 cells.
FIG. 3.
Amino acid sequence comparison of the putative ECL2 of mASCT1 and hamster ASCT1 (CHO.ASCT1) indicates 87% sequence identity. Numbers at the left of the sequences correspond to the positions of the first and last amino acids shown. Dots indicate amino acid identity. N-linked glycosylation sites are indicated by asterisks.
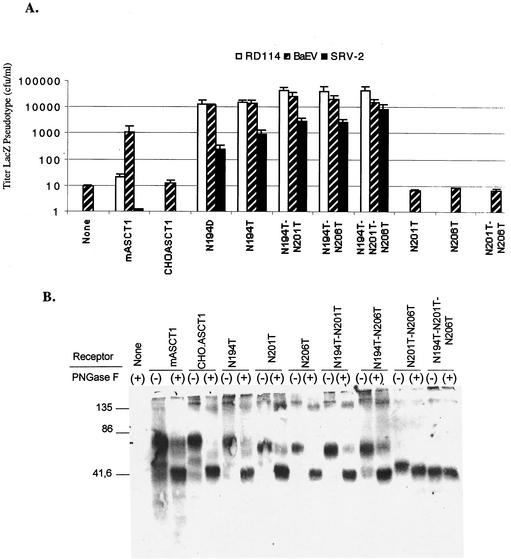
To determine whether N-linked glycosylation of hamster ASCT1 at position N194 might be involved in the tunicamycin-dependent susceptibility of CHO cells to infections by RD114, BaEV, and SRV-2 type D retroviruses, we added a Myc epitope tag at the carboxyl terminus of this protein and we constructed the single mutants N194T, N201T, and N206T, the double mutants N194T-N201T, N194T-N206T, and N201T-N206T, and the triple mutant N194T-N201T-N206T by site-directed mutagenesis of this myc-tagged hamster ASCT1 cDNA clone. We transiently expressed these tagged hamster ASCT1 proteins and a control tagged mASCT1 protein in CHO cells, and we then analyzed the cells for susceptibility to infection by RD114, BaEV, and SRV-2 viruses. As shown in Fig. 4A, all of the hamster ASCT1 mutants that contain N194T mediated virus infections, whereas none of the other mutants were active. Thus, the N194 residue has a dominant inhibitory effect on infections. However, as further confirmed below, the double mutants and triple mutants that contain the N194T substitution are several times more active in viral reception than the single N194T mutant. Consistent with the results in Fig. 4A, we previously showed that wild-type mASCT1, which contains N201 and N206 (Fig. 3), is active in mediating BaEV and HERV-W infections but is at least 50- to 100-fold less active in mediating infections by RD114 and type D simian retroviruses (23, 25). Moreover, removal of the N-linked oligosaccharides at N201 and/or N206 of mASCT1 by mutagenesis eliminated this inhibition of RD114 and type D primate virus infectivities (25). Consequently, we were surprised to find that the single mutant hamster ASCT1 (N194T) is a strong receptor for all of these viruses (Fig. 4A). To determine whether the D at position 194 of mASCT1 might selectively inhibit infections of RD114 and SRV-2, we constructed the N194D mutant of hamster ASCT1. As shown in Fig. 4A, the hamster ASCT1(N194D) mutant was similar to the N194T mutant in mediating these viral infections. This result implies that the viral reception differences between mASCT1 and hamster ASCT1(N194D) must be caused by other amino acid sequence differences between these proteins. Furthermore, the viral inhibitory effects of N201 and N206 clearly depend on the amino acid sequence context in which they occur.
FIG. 4.
Surface expression and viral receptor properties of wild-type and mutant hamster ASCT1 proteins. (A) Titers of infection of LacZ pseudotype viruses in CHO cells that were transiently expressing CHO.ASCT1 and its N-glycosylation mutants. Infections with LacZ pseudotype viruses were initiated 24 h after the transfections were begun. The titers of infection are averages of three independent experiments ± standard errors. With several of the receptors, the titers of the RD114 or SRV-2 viruses were zero, in which cases the bars are not shown. (B) Expression of wild-type and mutant hamster ASCT1 proteins on the surface of HEK293T cells and analysis of the N-linked oligosaccharides. HEK293T cells transiently expressing Myc-tagged CHO.ASCT1 and its N-deglycosylation mutants were surface biotinylated as described in Materials and Methods. The biotinylated proteins were purified by affinity chromatography. The samples that were untreated (−) or treated (+) with PNGaseF were analyzed by Western immunoblotting with anti-Myc tag monoclonal antibody 9E10 (Sigma).
Because the hamster and mouse ASCT1 proteins contained Myc epitope tags, we were able to analyze their cell surface expression by using a membrane-impermeant biotinylation reagent (22, 23, 32). After affinity purification of the biotinylated proteins using streptavidin-agarose beads, sample aliquots were either treated or untreated with protein N-glycanase F (PNGaseF) before electrophoresis and Western immunoblotting with the anti-Myc monoclonal antibody 9E10 (Fig. 4B). The results clearly showed that the ASCT1 proteins were all expressed to similar extents on the cell surfaces. Treatment of wild-type mouse or hamster ASCT1 with PNGaseF increased its electrophoretic mobility, consistent with the expected Mr of 55,000 for the unglycosylated protein. In addition, the single hamster ASCT1 mutants N194T, N201T, and N206T and the double mutants N194T-N201T, N194T-N206T, and N201T-N206T all had slightly faster electrophoretic mobilities than the undigested wild-type hamster ASCT1 protein, and their mobilities were further increased to the apparent Mr of 55,000 by PNGaseF digestions. In contrast, the triple hamster ASCT1 mutant N194T-N201T-N206T had the same size as the fully deglycosylated wild-type protein and was unaffected by PNGaseF. These results indicate that hamster ASCT1 is N-glycosylated at all three positions, N194, N201, and N206. However, close inspection of the double mutant results in Fig. 4B suggests that the N-linked oligosaccharide at position N194 may have a relatively small size compared to those at N201 and N206.
N-linked glycosylation of hamster ASCT1 also blocks the HERV-W envelope.
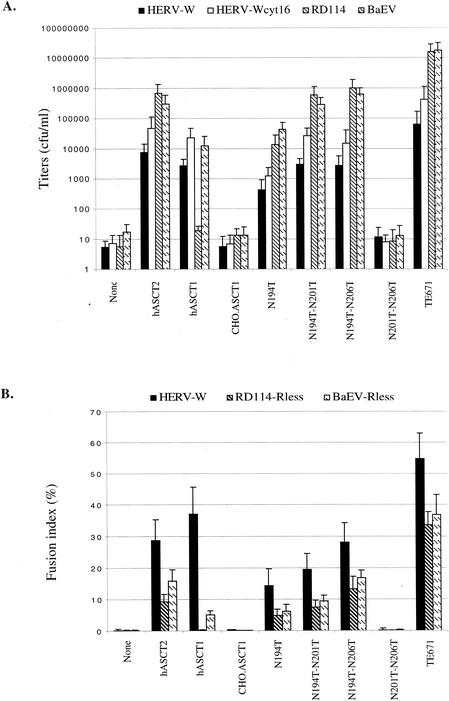
After the previous studies were substantially completed, we confirmed that the HERV-W envelope glycoprotein can pseudotype HIV-1 virion cores, and we also found that these virions could use fully glycosylated mASCT1 in a manner similar to BaEV (23). To verify and to extend the above results, we made CHO cell derivatives that constitutively express vectors that encode hamster ASCT1 or its single N194T or double N194T-N201T, N194T-N206T, or N201T-N206T N-deglycosylated mutants. HIV-GFP virions pseudotyped with either HERV-W or HERV-Wcyt16 envelopes were then used to infect these cells as described in Materials and Methods. The HERV-Wcyt16 envelope has a shortened cytosolic tail containing only 16 amino acids, and it forms pseudotyped virions more efficiently than the full-length HERV-W envelope (23). As controls for these assays, we also used MuLV-LacZ(RD114) and LacZ(BaEV) pseudotyped virions, CHO cells that constitutively express hASCT2, hASCT1, and human TE671 cells. As shown by the infectivity data in Fig. 5A, CHO cells that expressed hamster ASCT1 with the N194T mutation were highly susceptible to viruses pseudotyped with HERV-W envelope glycoproteins, whereas the control CHO cells and those expressing wild-type CHO hamster ASCT1 or the N201T-N206T double mutant were resistant. Thus, glycosylation of hamster ASCT1 at N194 has a dominant inhibitory effect on infections by all of these viruses that cannot be alleviated by eliminating the oligosaccharides at N201 and N206. However, the latter N-linked oligosaccharides clearly contribute to the inhibition, since the N194T-N201T and N194T-N206T double mutants were reproducibly 4 to 20 times more active in mediating infections by all of these viruses than the N194T single mutant (Fig. 4A and 5A). In addition, these results show that human ASCT1 functions similarly to mASCT1 in selectively mediating infections of BaEV and HERV-W but not of RD114. Studies of l-[3H]alanine uptake suggested that the wild-type and mutant ASCT1 proteins were all expressed on cell surfaces (Table 1), thus confirming the biotinylation results shown in Fig. 4B.
FIG. 5.
N-linked glycosylation of hamster ASCT1 (CHO.ASCT1) blocks infectivity and membrane fusion mediated by the HERV-W envelope glycoprotein. (A) Mediation of HIV/HERV-W pseudotype virus infections by CHO.ASCT1 and its N-deglycosylated mutants. Infectivity assays were done with CHO cell clones that constitutively express the proteins. The titers are averages of three independent infection experiments ± standard errors. (B) Histogram showing the mean fusion index of each virus-receptor combination in the cell-cell fusion assay (error bars are standard deviations; n = 3). The fusion index represents the percent fusion events in a cell population and is defined as [(N − S)/T] × 100, where N is the number of nuclei in syncytia, S is the number of syncytia, and T is the total number of nuclei counted (2).
The HERV-W envelope glycoprotein is highly fusogenic and may contribute to the formation of syncytiatrophoblasts in the placenta (6, 26). Consequently, we transiently transfected the HERV-W Env expression vector into the CHO cell clones described above that stably express hamster CHO.ASCT1 or its N-deglycosylated mutant derivatives, and we then analyzed the cultures for syncytia. As expected from the above analyses, normal CHO cells and their derivative that expresses wild-type hamster ASCT1 do not form syncytia after expression of HERV-W Env (Fig. 5B), whereas the cells that express hamster ASCT1(N194T) form abundant syncytia. Interestingly, the CHO derivatives that express the double mutants N194T-N201T or N194T-N206T form substantially more syncytia than the N194T single mutant. Furthermore, these CHO cell derivatives expressed similar amounts of the wild-type and mutant ASCT1 proteins, as indicated by immunostaining with the Myc antibody and by cell surface biotinylation as described above. These results strongly support our conclusion that N-linked glycosylation of hamster ASCT1 at N194 has a strong inhibitory effect on HERV-W-mediated infection and membrane fusion processes and that the oligosaccharides at N201 and N206 also contribute to these inhibitions.
DISCUSSION
General conclusions.
These results provide strong evidence that a sequence termed region C in the carboxyl-terminal portion of ECL2 (Fig. 1 and 3) plays a major role in controlling the retroviral receptor functions of the ASCT1 and ASCT2 proteins of different mammalian species and that this control is determined for all the viruses in this interference family in a cooperative manner by a combination of amino acid sequence changes and variably situated inhibitory N-linked oligosaccharides. The hypervariability of the region C sequences is consistent with the hypothesis that they have been a critical battlefield in host-virus coevolution (23, 31). In the case of mASCT2, the resistance is principally dependent on the divergent amino acid sequence of region C compared with the susceptibility human ortholog hASCT2. However, it seems likely that the N-linked oligosaccharides also contribute to this resistance, since an N-deglycosylated mutant derivative of mASCT2 is active in mediating infectivity and membrane fusion by the HERV-W envelope (23). Similarly, as discussed below, our results strongly imply that a combination of amino acid sequence changes and N-linked oligosaccharides in region C of ASCT1 control its ability to mediate infections of different retroviruses in this interference family. However, in the case of ASCT1 the N-linked oligosaccharides appear to have a predominant influence.
The C-regions may be negative control barriers rather than viral attachment sites.
Although our results strongly suggest that the C regions of ASCT1 and ASCT2 are critically important for their retroviral receptor functions, we emphasize that our data cannot exclude the possibility that other sites in these proteins might also contribute to infections. This uncertainty derives from a limitation that is inherent in comparative studies of receptor orthologs from susceptible and resistant species. In particular, it has been generally assumed in previous studies that the sequence differences between such orthologs can be used to identify the active sites needed for viral infection. On the contrary, as we have suggested elsewhere (31), there are excellent reasons for believing that many retroviruses have survived in part because they have become adapted to recognize invariant sites that are important for the normal function of the receptor and that are consequently difficult for the host to modify without loss of fitness. For this reason, we propose that the hypervariable sequences as exemplified by region C might be principally negative control areas that have been elaborated as defense bulwarks to inhibit viral access to nearby invariant viral interaction sites. According to this interpretation, substitution of a divergent sequence from a functional receptor into a resistant ortholog might enable infection merely because the substituted sequence is less inhibitory than the sequence that is replaced. In essence, the comparative approach overlooks the possible influences of sequences that are conserved in the proteins being compared.
Several lines of evidence support the hypothesis that the C regions of ASCT1 and ASCT2 are primarily negative control regions as defined above. First, although hASCT2 and N-deglycosylated derivatives of mASCT1 and hamster ASCT1 are active receptors for all retroviruses in this interference group, there is very little sequence similarity in the C regions of these proteins. Moreover, the deglycosylated derivative of mASCT2 is also an active receptor for HERV-W pseudotyped viruses (23). Consequently, it seems very unlikely that the divergent portions of the C regions could be specific recognition sites for these viruses. Second, the C regions occur adjacent to a central region of approximately 25 amino acids within ECL2 that is very highly conserved, not only in the ASCT1 and ASCT2 proteins but also in the other members of the glutamate transporter family (23, 30, 31). Similarly, as described in more detail elsewhere, hypervariable control regions in other gammaretrovirus receptors also occur adjacent to highly conserved sequences (31). Third, the region C sequences appear to have been under strong selection pressure to diversify, as suggested by the high ratio of nonsynonymous to synonymous nucleotide sequence changes in the corresponding coding region of the ASCT2 gene (23, 31). This strongly supports the hypothesis that the penetration of this diversity into the host C regions was not caused simply by genetic drift but that it occurred because the sequence diversification was advantageous. It is unreasonable to assume that the negative selection pressure on region C occurred only in the resistant species, because susceptible species are most threatened by the viruses. In contrast, previous chimera and mutagenesis studies of receptors have assumed that the divergent sequence in the susceptible species makes a positive contribution to infection and that the corresponding sequence in the resistant species has a neutral effect. Fourth, our evidence establishes that the N-linked oligosaccharides in the C regions have inhibitory effects on viral infections (Fig. 4 and 5 and references 23 and 25), consistent with the idea that they function to block access of viruses to an essential recognition site in the receptor. Accordingly, we interpret our chimera and mutagenesis studies of ASCT2 (i.e., Fig. 2) as implying that region C of hASCT2 may be a less formidable barrier to the viruses than the corresponding region of mASCT2.
Cooperative roles of N-linked glycosylation and amino acid sequence changes in controlling receptor functions of ASCT1 proteins.
A striking result of this investigation is that an N-linked oligosaccharide in region C of hamster ASCT1 at position N194 plays a critical dominant inhibitory role in preventing infection of CHO cells by RD114, BaEV, type D primate retroviruses, and HERV-W pseudotyped viruses, whereas the two additional oligosaccharides at N201 and N206 have weaker inhibitory effects. Thus, as shown in Fig. 4A and 5A, the hamster ASCT1 N-deglycosylation mutants N194T, N194D, N194T-N201T, N194T-N206T, and N194T-N201T-N206T were active receptors for all of these viruses, whereas the wild-type hamster ASCT1 protein and the N201T, N206T, and N201T-N206T mutants were inactive. Nevertheless, the N201 and N206 oligosaccharides clearly had adjunct inhibitory effects on infectivity, because the N194T-N201T and N194T-N206T double mutants and the N194T-N201T-N206T triple mutant were reproducibly approximately 5- to 20-times-more-active viral receptors than the single N194T mutant (Fig. 4A and 5A). Similar enhancement effects were seen in syncytium assays, implying that the N201 and N206 oligosaccharides also influence the membrane fusion process that is mediated by the HERV-W envelope glycoprotein (Fig. 5B). It is somewhat surprising that the oligosaccharide at position N194 in hamster ASCT1 has a predominant inhibitory effect compared to those at positions N201 and N206, because the N194 oligosaccharide appears to be relatively small, as seen by its effect on the protein's apparent Mr as determined by electrophoresis (Fig. 4B).
In agreement with our laboratory's previous results (23, 25), the evidence in this report confirms that mASCT1 and human ASCT1 are at least 50 to 100 times more active in mediation of BaEV and HERV-W infections than in mediation of RD114 or type D primate retrovirus infections (Fig. 4 and 5). In addition, we previously showed that the N201T, N206T, and N201T-N206T mutants of mASCT1 were all efficient receptors for the entire interference group of retroviruses, including RD114 and the type D primate viruses (23, 25). Thus, in the context of mASCT1 the N201 and N206 oligosaccharides cooperate to strongly and selectively inhibit infections of RD114 and the type D simian retroviruses. In view of these results with mASCT1 and hASCT1, we were surprised to find that the N194T and N194D mutants of hamster ASCT1, which also contain N-linked oligosaccharides at N201 and N206, are highly and uniformly active in mediating infections by all viruses in this interference group, including RD114 and the primate type D retrovirus SRV-2 (Fig. 3, 4, and 5). Consequently, there must be an amino acid sequence difference between mASCT1 and hamster ASCT1 that modulates the influence of the N201 and N206 oligosaccharides. These oligosaccharides are clearly more inhibitory in the context of mASCT1 than in the context of hamster ASCT1. However, even in the context of mASCT1 these N201 and N206 oligosaccharides are not completely inhibitory, because they cannot prevent infections by BaEV and HERV-W pseudotyped viruses and because neither of these oligosaccharides alone is sufficient to block infections by any virus (23, 25). Similar results were previously reported by Eiden and coworkers in studies of the CAT1 receptor for ecotropic murine leukemia viruses that occurs in Mus dunni fibroblasts (11, 12). Specifically, they found that the inability of the M. dunni CAT1 receptor to mediate infections by the Moloney strain of ecotropic murine leukemia virus could be alleviated either by eliminating an N-linked oligosaccharide by mutagenesis, by treating the cells with tunicamycin, or by an ILeu-to-Val substitution 15 amino acids away from the site of the N-linked oligosaccharide (11, 12). Considered together, these results suggest that inhibitory effects of N-linked glycosylation on retroviral receptor functions can depend on the nearby amino acid sequences of the receptor.
Acknowledgments
This research was supported by NIH grants CA25810 and CA67358.
We are grateful to Susan Kozak, Kristine Rose, Patrick Rose, and Emily Platt for helpful advice.
REFERENCES
- 1.An, D. S., Y. Xie, and I. S. Chen. 2001. Envelope gene of the human endogenous retrovirus HERV-W encodes a functional retrovirus envelope. J. Virol. 75:3488-3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen, K. B. 1994. A domain of murine retrovirus surface protein gp70 mediates cell fusion, as shown in a novel SC-1 cell fusion system. J. Virol. 68:3175-3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arriza, J. L., M. P. Kavanaugh, W. A. Fairman, Y. N. Wu, G. H. Murdoch, R. A. North, and S. G. Amara. 1993. Cloning and expression of a human neutral amino acid transporter with structural similarity to the glutamate transporter gene family. J. Biol. Chem. 268:15329-15332. [PubMed] [Google Scholar]
- 4.Barbacid, M., E. Hunter, and S. A. Aaronson. 1979. Avian reticuloendotheliosis viruses: evolutionary linkage with mammalian type C retroviruses. J. Virol. 30:508-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blond, J.-L., F. Besème, L. Duret, O. Bouton, F. Bedin, H. Perron, B. Mandrand, and F. Mallet. 1999. Molecular characterization and placental expression of HERV-W, a new human endogenous retrovirus family. J. Virol. 73:1175-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blond, J. L., D. Lavillette, V. Cheynet, O. Bouton, G. Oriol, S. Chapel-Fernandes, B. Mandrand, F. Mallet, and F. L. Cosset. 2000. An envelope glycoprotein of the human endogenous retrovirus HERV-W is expressed in the human placenta and fuses cells expressing the type D mammalian retrovirus receptor. J. Virol. 74:3321-3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brocke, L., A. Bendahan, M. Grunewald, and B. I. Kanner. 2002. Proximity of two oppositely oriented reentrant loops in the glutamate transporter GLT-1 identified by paired cysteine mutagenesis. J. Biol. Chem. 277:3985-3992. [DOI] [PubMed] [Google Scholar]
- 8.Broer, A., C. Wagner, F. Lang, and S. Broer. 2000. Neutral amino acid transporter ASCT2 displays substrate-induced Na+ exchange and a substrate-gated anion conductance. Biochem. J. 346:705-710. [PMC free article] [PubMed] [Google Scholar]
- 9.Conrad, B., R. N. Weissmahr, J. Boni, R. Arcari, J. Schupbach, and B. Mach. 1997. A human endogenous retroviral superantigen as candidate autoimmune gene in type I diabetes. Cell 90:303-313. [DOI] [PubMed] [Google Scholar]
- 10.Cosset, F. L., Y. Takeuchi, J. L. Battini, R. A. Weiss, and M. K. Collins. 1995. High-titer packaging cells producing recombinant retroviruses resistant to human serum. J. Virol. 69:7430-7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eiden, M. V., K. Farrell, J. Warsowe, L. C. Mahan, and C. A. Wilson. 1993. Characterization of a naturally occurring ecotropic receptor that does not facilitate entry of all ecotropic murine retroviruses. J. Virol. 67:4056-4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eiden, M. V., K. Farrell, and C. A. Wilson. 1994. Glycosylation-dependent inactivation of the ecotropic murine leukemia virus receptor. J. Virol. 68:626-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanai, Y. 1997. Family of neutral and acidic amino acid transporters: molecular biology, physiology and medical implications. Curr. Opin. Cell Biol. 9:565-572. [DOI] [PubMed] [Google Scholar]
- 14.Karlsson, H., S. Bachmann, J. Schroder, J. McArthur, E. F. Torrey, and R. H. Yolken. 2001. Retroviral RNA identified in the cerebrospinal fluids and brains of individuals with schizophrenia. Proc. Natl. Acad. Sci. USA 98:4634-4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kekuda, R., P. D. Prasad, Y. J. Fei, V. Torres-Zamorano, S. Sinha, T. L. Yang-Feng, F. H. Leibach, and V. Ganapathy. 1996. Cloning of the sodium-dependent, broad-scope, neutral amino acid transporter Bo from a human placental choriocarcinoma cell line. J. Biol. Chem. 271:18657-18661. [DOI] [PubMed] [Google Scholar]
- 16.Kewalramani, V. N., A. T. Panganiban, and M. Emerman. 1992. Spleen necrosis virus, an avian immunosuppressive retrovirus, shares a receptor with the type D simian retroviruses. J. Virol. 66:3026-3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kolson, D. L., and F. Gonzalez-Scarano. 2001. Endogenous retroviruses and multiple sclerosis. Ann. Neurol. 50:429-430. [DOI] [PubMed] [Google Scholar]
- 18.Koo, H. M., A. M. Brown, Y. Ron, and J. P. Dougherty. 1991. Spleen necrosis virus, an avian retrovirus, can infect primate cells. J. Virol. 65:4769-4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koo, H. M., J. Gu, A. Varela-Echavarria, Y. Ron, and J. P. Dougherty. 1992. Reticuloendotheliosis type C and primate type D oncoretroviruses are members of the same receptor interference group. J. Virol. 66:3448-3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koo, H. M., S. Parthasarathi, Y. Ron, and J. P. Dougherty. 1994. Pseudotyped REV/SRV retroviruses reveal restrictions to infection and host range within members of the same receptor interference group. Virology 205:345-351. [DOI] [PubMed] [Google Scholar]
- 21.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 22.Lapham, C. K., M. B. Zaitseva, S. Lee, T. Romanstseva, and H. Golding. 1999. Fusion of monocytes and macrophages with HIV-1 correlates with biochemical properties of CXCR4 and CCR5. Nat. Med. 5:303-308. [DOI] [PubMed] [Google Scholar]
- 23.Lavillette, D., M. Marin, A. Ruggieri, F. Mallet, F. L. Cosset, and D. Kabat. 2002. The envelope glycoprotein of human endogenous retrovirus type W uses a divergent family of amino acid transporters/cell surface receptors. J. Virol. 76:6442-6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacGregor, G. R., A. E. Mogg, J. F. Burke, and C. T. Caskey. 1987. Histochemical staining of clonal mammalian cell lines expressing E. coli beta galactosidase indicates heterogeneous expression of the bacterial gene. Somat. Cell Mol. Genet. 13:253-265. [DOI] [PubMed] [Google Scholar]
- 25.Marin, M., C. S. Tailor, A. Nouri, and D. Kabat. 2000. Sodium-dependent neutral amino acid transporter type 1 is an auxiliary receptor for baboon endogenous retrovirus. J. Virol. 74:8085-8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mi, S., X. Lee, X. Li, G. M. Veldman, H. Finnerty, L. Racie, E. LaVallie, X. Y. Tang, P. Edouard, S. Howes, J. C. Keith, Jr., and J. M. McCoy. 2000. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature 403:785-789. [DOI] [PubMed] [Google Scholar]
- 27.Perron, H., J. A. Garson, F. Bedin, F. Beseme, G. Paranhos-Baccala, F. Komurian-Pradel, F. Mallet, P. W. Tuke, C. Voisset, J. L. Blond, B. Lalande, J. M. Seigneurin, B. Mandrand, et al. 1997. Molecular identification of a novel retrovirus repeatedly isolated from patients with multiple sclerosis. Proc. Natl. Acad. Sci. USA 94:7583-7588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rasko, J. E., J. L. Battini, R. J. Gottschalk, I. Mazo, and A. D. Miller. 1999. The RD114/simian type D retrovirus receptor is a neutral amino acid transporter. Proc. Natl. Acad. Sci. USA 96:2129-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seal, R. P., B. H. Leighton, and S. G. Amara. 2000. A model for the topology of excitatory amino acid transporters determined by the extracellular accessibility of substituted cysteines. Neuron 25:695-706. [DOI] [PubMed] [Google Scholar]
- 30.Slotboom, D. J., W. N. Konings, and J. S. Lolkema. 1999. Structural features of the glutamate transporter family. Microbiol. Mol. Biol. Rev. 63:293-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tailor, C. S., D. Lavillette, M. Marin, and D. Kabat. Cell surface receptors for gammaretroviruses. Curr. Top. Microbiol. Immunol., in press. [DOI] [PubMed]
- 32.Tailor, C. S., M. Marin, A. Nouri, M. P. Kavanaugh, and D. Kabat. 2001. Truncated forms of the dual function human ASCT2 neutral amino acid transporter/retroviral receptor are translationally initiated at multiple alternative CUG and GUG codons. J. Biol. Chem. 276:27221-27230. [DOI] [PubMed] [Google Scholar]
- 33.Tailor, C. S., A. Nouri, Y. Zhao, Y. Takeuchi, and D. Kabat. 1999. A sodium-dependent neutral-amino-acid transporter mediates infections of feline and baboon endogenous retroviruses and simian type D retroviruses. J. Virol. 73:4470-4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takeuchi, Y., F. L. Cosset, P. J. Lachmann, H. Okada, R. A. Weiss, and M. K. Collins. 1994. Type C retrovirus inactivation by human complement is determined by both the viral genome and the producer cell. J. Virol. 68:8001-8007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Utsunomiya-Tate, N., H. Endou, and Y. Kanai. 1996. Cloning and functional characterization of a system ASC-like Na+-dependent neutral amino acid transporter. J. Biol. Chem. 271:14883-14890. [DOI] [PubMed] [Google Scholar]
- 36.Vigna, E., and L. Naldini. 2000. Lentiviral vectors: excellent tools for experimental gene transfer and promising candidates for gene therapy. J. Gene Med. 2:308-316. [DOI] [PubMed] [Google Scholar]
- 37.Wang, H., E. Dechant, M. Kavanaugh, R. A. North, and D. Kabat. 1992. Effects of ecotropic murine retroviruses on the dual-function cell surface receptor/basic amino acid transporter. J. Biol. Chem. 267:23617-23624. [PubMed] [Google Scholar]
- 38.Zerangue, N., and M. P. Kavanaugh. 1996. ASCT-1 is a neutral amino acid exchanger with chloride channel activity. J. Biol. Chem. 271:27991-27994. [DOI] [PubMed] [Google Scholar]