Abstract
Potent drugs such as cyclosporine have provided effective probes of signal transduction pathways and, as well, of human immunodeficiency virus type 1 (HIV-1) replication mechanisms. Recently, it was reported that As2O3, a drug used to treat acute promyelocytic leukemia (PML), stimulates HIV-1 replication. We found that As2O3 accelerates the kinetics of a spreading HIV-1 infection in human T cells and increases the number of cells bearing HIV-1 provirus after a single round of infection. The stimulatory effect occurred after membrane fusion and resulted in increased steady-state levels of newly synthesized viral cDNA. Stimulation was independent of HIV-1 env and most viral accessory genes, and As2O3 had no detectable effects on viral expression postintegration or virion assembly. Murine leukemia virus (MLV) transduction was enhanced by As2O3 to the same extent as HIV-1 transduction, but As2O3 had no additional effect on Fv1 restriction. In contrast, As2O3 largely overcame the specific block to N-tropic MLV reverse transcription posed by human Ref1. As2O3 disrupts PML bodies, nuclear structures named for a major component, the PML protein. We observed no changes in PML bodies in response to HIV-1 infection. Experiments with PML-null target cells indicated that PML has no effect on HIV-1 infectivity and is dispensable for the stimulatory effect of As2O3. As2O3 caused cell death in uninfected cells at the same concentrations which stimulate HIV-1 replication. Among four additional apoptosis-inducing agents, a boost in HIV-1 infectivity was observed only with carbonyl cyanide m-chlorophenylhydrazone, a compound which, like As2O3, disrupts the mitochondrial transmembrane potential. In summary, As2O3 stimulates retroviral reverse transcription, perhaps via effects on mitochondria, and provides a useful tool for characterizing Ref1.
Cellular factors are required at virtually every phase of retrovirus replication. This has been amply demonstrated by analysis of specific conditions that fail to maintain productive infection. Mouse cells, for example, are nonpermissive for human immunodeficiency virus type 1 (HIV-1) replication and have provided a particularly fruitful experimental system. HIV-1 will not enter mouse cells because the viral env-encoded gp120 does not bind to mouse homologues of the human cell surface receptors, CD4 and CCR5 (6, 35). If the entry block is bypassed, a provirus can be established, but HIV-1 transcription is defective because mouse cyclin T1 does not interact with viral Tat (62). If the transcriptional block is bypassed, viral proteins fail to assemble new virions, due to an apparent deficiency in mouse cells of a factor that is present in human cells (10, 39, 40). Other experimental approaches have demonstrated that, via direct interaction with Tsg101, HIV-1 usurps the vesicular transport system in order to complete the virion budding process (23).
Similarly, cellular factors have been identified that specifically inhibit retroviral replication. The n and b alleles of Fv1 encode retroviral Gag-like elements, each of which specifically decreases the efficiency of integration of murine retroviruses bearing either of two capsid (CA) susceptibility determinants (9, 11, 33). New host factors regulating retroviral replication may ultimately be identified by analysis of additional nonpermissive conditions. Interestingly, cells from mammals other than mice restrict reverse transcription of N-tropic murine leukemia virus (MLV), but not B-tropic MLV, even though Fv1 is absent from these species (57); the putative factor mediating restriction in these cells is now called Ref1 (57, 58). Additional factors are being sought based on the observation that HIV-1 is unable to efficiently complete reverse transcription after infection of nonhuman primate cells (21, 26, 27, 41, 54).
Host factors have also been identified which must be present during the assembly process to alter particle infectivity in the subsequent round of infection. Cyclophilin A enhances the infectivity of particles in this manner (14). In contrast, if expressed at the time of virus production, CEM15 inhibits postentry steps of HIV-1 replication (53); the significance of CEM15 is emphasized by the fact that all primate lentiviruses encode a factor, Vif, which counteracts its antiviral activity. A putative regulatory factor has also been suggested by the observation that the nuclear transport efficiency of MLV preintegration complexes is determined by the particular cell line used for virion production (52).
The understanding of cellular factors that regulate retroviral replication has also been advanced by study of the action of drugs that alter infectivity. Cyclosporine, a competitive inhibitor of the HIV-1 Gag-cyclophilin A interaction, helped establish the significance of cyclophilin A for HIV-1 replication (12, 22). The finding that proteasome inhibitors decrease virion assembly (48, 50) helped establish the importance of ubiquitin for virion release. Proteasome inhibitors also increase the infectivity of incoming viruses, suggesting that ubiquitin-dependent degradation plays an antiviral role during virus entry (51). The antiviral activity of particular chemokines has been exploited to clarify the importance of particular chemokine receptors for virion entry (19).
Recently, arsenic trioxide (As2O3) was reported to enhance HIV-1 infectivity (60). As2O3 is used to treat acute promyelocytic leukemia (APL), which bears an oncogenic PML-retinoic acid receptor alpha fusion protein resulting from chromosomal translocation (17, 34, 43, 66). PML protein is normally required for the formation of PML bodies, multiprotein nuclear structures of unknown function that are often disrupted by infection with DNA viruses (2, 3, 15, 31). The fact that As2O3 promotes the degradation of PML protein and alters the morphology of PML bodies (17, 34, 43, 66) suggested that the drug might promote HIV-1 infection by antagonizing an antiviral activity associated with PML. Consistent with this hypothesis, HIV-1 infection has been reported to alter PML protein localization (60), though others failed to confirm this (7).
We became interested in the reported effects of As2O3 on HIV-1 infectivity when we discovered in the two-hybrid system that HIV-1-encoded proteins interact with known components of PML bodies. Detailed analysis of the effect of As2O3 on HIV-1 and MLV replication demonstrated that As2O3 causes increased steady-state levels of newly synthesized retroviral cDNA. The drug has no additional effect on Fv1 restriction but significantly counteracts the reverse transcription block due to Ref1. Our data fail to demonstrate that the effects of As2O3 on HIV-1 are related to effects of the drug on PML protein. Rather, the effect of the drug on HIV-1 seems more likely to be related to other effects of the drug, such as its mitochondrial-uncoupling activity.
MATERIALS AND METHODS
Plasmid DNAs.
pNL4-3 (1) contains a complete infectious HIV-1 provirus, HIV-1NL4-3. pNL-GFP, a gift from Dana Gabuzda, is pNL4-3 with an env-inactivating mutation and enhanced green fluorescent protein (EGFP) (Clontech) expressed in place of Nef (24). FUGW, a gift from David Baltimore and Carlos Lois, is an HIV-1-derived vector expressing EGFP from the human ubiquitin promoter (38). TRIP-GFP, a gift from Pierre Charneau, is an HIV-1-derived vector expressing EGFP from the cytomegalovirus immediate-early (CMVie) promoter (64). ΔR8.2, ΔR8.9, and pMD-G, gifts from Didier Trono, encode HIV-1 structural proteins and the vesicular stomatitis virus (VSV) G protein and have been extensively described (67, 68). pCIG3 N and B, constructs expressing MLV N- and B-tropic structural proteins, have been described (11). pCNCG is an MLV-derived vector expressing EGFP from the CMVie promoter (44). pHXB2Env, a construct expressing HXB2 gp160 (46), was provided by Nathaniel Landau.
Cells and tissue culture.
Wild-type and PML−/− 129/Sv mouse embryonic fibroblasts (MEFs) were produced as previously described (61). 293T, HeLa, TE671, NIH 3T3, and BALB/c 3T3 cells and MEFs were maintained in Dulbecco's modified Eagle's medium supplemented with antibiotics and either 10 (293T, HeLa, BALB/c 3T3, and TE671 cells) or 20% (MEFs) fetal calf serum or 10% calf serum (NIH 3T3 cells). Jurkat T cells were maintained in RPMI supplemented with antibiotics and 10% fetal calf serum.
Virus production and determination of titers of viral stocks.
To produce HIV-1 virions, 293T cells were transfected as previously described (13) with 10 μg of pNL4-3 or pNL-GFP. In some cases, as indicated, cells were cotransfected with 5 μg of pMD-G. To produce HIV-1 vectors, 293T cells were cotransfected with (i) 10 μg of pFUGW or TRIP-GFP, (ii) 10 μg of ΔR8.9 or ΔR8.2, or (iii) 5 μg of pMDG or 10 μg of pHXBEnv. HIV-1 virion- or vector-containing supernatant was collected 2 days posttransfection, clarified by low-speed centrifugation, filtered (0.45-μm-pore-size Pall Acrodisc), and stored at −80°C. Virion quantities were normalized prior to infection using the NEN HIV-1 Capsid (CAp24) enzyme-linked immunosorbent assay kit. To determine titers, 2 × 105 Jurkat T cells were incubated in serial dilutions of virus stocks (0.5-ml final volume). Two days postinfection, the percentage of cells expressing either GFP or p24 was determined by flow cytometry, as described below. The titers of VSV G-pseudotyped virions in HeLa cells were similar to those determined using Jurkat T cells.
For MLV-derived vectors, 293T cells were transfected using the Fugene 6 procedure (Roche) with 1.5 μg of pCNCG, 1 μg of pCIG3 N or B, and 1 μg of pMDG. Titers for MLV vectors were determined on Mus dunni tail fibroblasts (MDTF) as described previously (49, 57).
Infections.
For viruses bearing HIV-1 Env, single-cycle infections in Jurkat T cells and in MEFs were performed at a multiplicity of infection (MOI) of 0.01; for viruses bearing VSV G, the MOI was 0.05 to 0.2. For Southern analysis of viral cDNA synthesis, an MOI of 2.0 was used. For spreading infections, MOIs of 0.005 or 0.0005 were used, and HIV-1 proliferation was monitored by measuring the accumulation of reverse transcriptase (RT) activity in cell supernatants as described previously (18).
Drugs.
0.1 M As2O3 (Sigma) was prepared in 1 N NaOH, diluted to 10−3 M in PBS, and adjusted to pH 7.0 using HCl. As2O3 was diluted further with PBS, and the solutions were kept at 4°C for up to 3 months without loss of activity. Dextran sulfate (5 mg/ml; Sigma) was prepared in H2O and stored frozen. Dextran sulfate was used at 100 to 200 μg/ml, a concentration that completely prevented HIV-1 Env-mediated entry with <5% decrease in target cell viability (data not shown). Zidovudine (Sigma) was added to the cells 2 h prior to infection (100 μM final concentration). Lamivudine (3TC) was extracted from an Epivir pill (GlaxoWellcome) with 3 ml of dimethyl sulfoxide and kept at −80°C. A 1:200 dilution of this stock solution was used to inhibit HIV-1 replication. The solution caused no toxicity to host cells, and the concentration was 250-fold greater than the 50% inhibitory concentration for HIV-1 in a multinuclear activated galactosidase indicator (MAGI) assay (data not shown). cis-Platinum(II)diammine dichloride (cisplatin), camptothecin, and carbonyl cyanide m-chlorophenylhydrazone (m-Cl-CCP) were from Sigma and were prepared in dimethyl sulfoxide. The anti-Fas antibody (clone CH-11; Medical Biological Laboratories) was diluted in phosphate-buffered saline (PBS).
Assay for cell viability.
After cells were exposed to drugs for 2 days, cell viability was measured using an XTT assay (63). Adherent cells were washed in PBS and put in 1 mg of XTT (Sigma)/ml-0.01 mM phenazine methosulfate (Sigma) in phenol red-free medium. Cells in suspension were maintained in phenol red-free RPMI for at least 1 week before 2× XTT-phenazine methosulfate was added. XTT assay mixtures were incubated for 15 min at 37°C; 200 μl of supernatant was transferred to a 96-well plate, which was read at 450 nm with a reference wavelength of 690 nm.
Flow cytometry.
For analysis of GFP expression, cells were fixed for 30 min in 2% formaldehyde- PBS, washed twice with PBS, and resuspended in PBS at 106 cells/ml. For analysis of p24 expression, 106 cells were fixed and washed as described above and permeabilized in 300 μl of 1× PermWash solution (Pharmingen) for 30 min at room temperature. The cells were pelleted and incubated in 50 μl of 1× PermWash containing a 1:100 dilution of RD1-labeled anti-HIV-1 CA (p24) monoclonal antibody (KC57-RD1; Coulter), for 1 h at 4°C in the dark. The cells were then washed three times in 1× PermWash and resuspended in 0.5 ml of PBS. For the coanalysis of CAp24 expression and annexin V binding, 106 cells were washed once in ice-cold PBS, resuspended in 100 μl of annexin V binding buffer (Pharmingen) containing 5 μl of annexin V-fluorescein isothiocyanate (Pharmingen). After 15 min of incubation at room temperature, the cells were washed twice in the annexin V binding buffer and then fixed and processed for CAp24 staining as detailed above. Flow cytometric analysis was done on a FACScalibur using CellQuest software (Becton Dickinson). Intact cells were identified based on light scatter profiles, and only those cells were included in the analysis. GFP and fluorescein isothiocyanate fluorescences were recorded on the FL1 channel; RD1 fluorescence was recorded on the FL2 channel. For double-fluorescence recordings, compensation was done using the relevant controls. Ten thousand cells per sample were processed (except where indicated), and cells positive for either marker were gated and counted as a percentage of total intact cells. False-positive results were insignificant, as shown by controls corresponding to uninfected cells. As2O3 did not cause false-positive fluorescent cells at the concentrations used.
Immunofluorescence microscopy.
HeLa cells (104) plated on Permanox eight-chamber slides (LabTek) were infected with VSV G-pseudotyped HIV-1 virus or vector at an MOI of 40. The cells were washed with PBS, fixed for 30 min in freshly prepared 2% paraformaldehyde, washed three times in PBS, and then permeabilized in 1× PermWash solution. Hybridizations and subsequent washing were all performed in 1× PermWash containing 10% normal goat serum (Vector). PML and Daxx were detected using the antibodies PG-M3 and M-112 (Santa Cruz), respectively, at a 1:200 dilution. Bound primary antibodies were revealed using Alexa-594-conjugated goat anti-rabbit or anti-mouse immunoglobulin G antibodies (Molecular Probes) at a 1:500 dilution. DNA was stained with DAPI (4′,6′-diamidine-2-phenylindole) (1 μM; Molecular Probes) added along with the secondary antibodies. The slides were mounted in Vectashield (Vector), and photographs were immediately taken using a Nikon E800 microscope equipped with a 100× objective and coupled to a DAGE 330 camera.
Monitoring HIV-1 cDNA synthesis.
Jurkat T cells (107) were infected in 5 ml of medium with VSV G-pseudotyped HIV-1NL4-3 at an MOI of 2. The cells were washed with PBS at 24 h, and total cellular DNA was extracted using the Nucleobond Midikit (Clontech). Four micrograms of DNA was digested with a mixture of XhoI, MscI, and DpnI (DpnI restricts only DNA synthesized in Escherichia coli and thus serves to reduce contamination with transfected plasmid DNA). The products were resolved on a 0.7% agarose gel, transferred to a nylon membrane (Amersham Hybond N+), and probed with 32P-labeled DNA probes (Amersham Rediprime kit). Viral DNA was detected with a probe spanning the 5′ MscI site in HIV-1NL4-3 (nucleotides 1818 to 3498 in the provirus). As a control for even loading, the same membrane was rehybridized with a probe specific for mitochondrial DNA that was generated using the following oligonucleotides: 5′-CCACTCCACCTTACTACCAGCA-3′ and 5′-GTAATGCTAGGGTGAGTGGTAGG-3′.
Monitoring MLV cDNA synthesis.
Human TE671 cells (2 × 105) were infected with 7.5 × 103 infectious units (titers were determined on nonrestrictive Mus dunni tail fibroblasts [MDTF]) of MLV-based GFP-transducing vectors. Total DNA was extracted at 6 h (Dneasy; Qiagen), and Taqman PCR was performed on 100 ng of DNA as previously described (59) using a primer-probe combination specific to GFP (29).
RESULTS
As2O3 stimulates HIV-1 replication in human T cells.
Previously, it was shown that As2O3 increases the efficiency of HIV-1 transduction (60). We became interested in the effects of As2O3 on HIV-1 infectivity when we discovered that HIV-1 p6 interacts with SUMO-1, Ubc9, and Daxx (data not shown). These three proteins all localize to PML bodies, nuclear structures that are disrupted by As2O3 (17, 34, 43, 66).
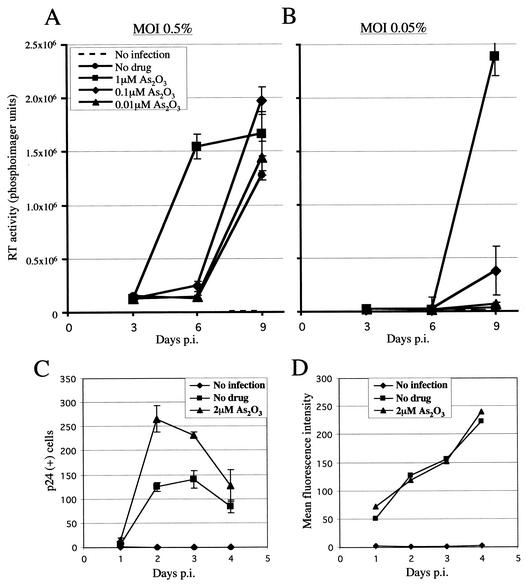
In vivo, among other cell types, HIV-1 infects CD4+ T cells. The effect of As2O3 on wild-type HIV-1NL4-3 propagation in Jurkat T cells was therefore examined. Jurkat T cells were infected with HIV-1NL4-3 in the presence of As2O3 concentrations from 10−5 to 10−8 M. A concentration of 10−5 M As2O3 resulted in virtually total cell death, and therefore, viral replication could not be assessed. In the presence of 10−6 M As2O3, viral replication initiated at an MOI of 0.5% reached its plateau by about day 6, at which time RT activity was still undetectable in the absence of the drug (Fig. 1A). After infection at an MOI of 0.05%, 10−6 M As2O3 was associated with a >10-fold increase in RT activity on day 9 compared to no drug (Fig. 1B). A concentration of 10−7 M As2O3 had a small but detectable positive effect on HIV-1 propagation at both MOIs.
FIG. 1.
As2O3 stimulates HIV-1 replication in T cells. (A and B) Jurkat T cells were exposed to the indicated concentrations of As2O3 and infected the next day with HIV-1NL4-3 at an MOI of 0.5 (A) or 0.05% (B). Cultures were replenished with fresh media and drug on days 3 and 6 postinfection (p.i.). RT activity was measured in supernatants collected on days 3, 6, and 9. The data represent the average (± standard deviation) of three independent infections. (C and D) Jurkat T cells, incubated with or without As2O3, were infected overnight with HIV-1NL4-3 and then treated with dextran sulfate to prevent the spread of virus to new target cells. One-third of the culture was collected daily and replaced with fresh medium supplemented with dextran sulfate, with or without As2O3. (C) Number of CA (p24)-expressing cells per 25,000 cells analyzed by flow cytometry. (D) Level of CA expression as represented by mean fluorescence for the fraction of cells expressing CA. The results represent the average of three independent infections.
As2O3 increases the number of cells bearing HIV-1 provirus after a single round of infection.
To determine if As2O3 enhances HIV-1 replication in a single cycle, Jurkat T cells were infected overnight with HIV-1NL4-3, and the spread of the virus to new target cells was blocked by addition of dextran sulfate, an inhibitor of virus entry. The frequency of p24-expressing cells (Fig. 1C), as well as the magnitude of p24 expression (Fig. 1D), was monitored every 24 h by flow cytometry.
In the absence of drug, the percentage of p24-expressing cells in the culture peaked on day 3 and then decreased by ∼2-fold per day for the next 4 days (Fig. 1C and data not shown). Presumably, the loss of p24-expressing cells over time was due to toxicity or cell cycle arrest mediated by viral products or to silencing of viral expression. When 2 μM As2O3 was added at the time of infection, the frequency of cells expressing p24 increased by ∼2-fold (Fig. 2A, day 2), indicating that a step early in the infection process was enhanced by As2O3. No boost to infectivity was observed if As2O3 was washed out 2 h postinfection, and the effect of the drug was still evident, though at reduced magnitude, if it was added as much as 24 h postinfection (data not shown). Similarly, pretreating cells with As2O3 prior to infection did not provide additional enhancement of replication (data not shown).
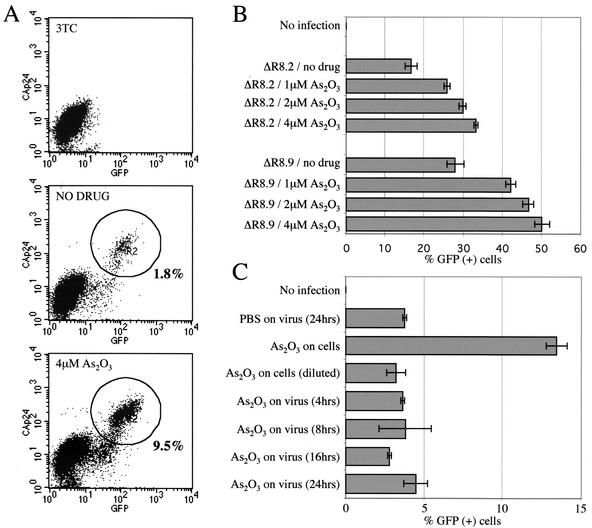
FIG. 2.
Stimulation of HIV-1 infection by As2O3 is independent of env and most viral accessory genes. (A) Jurkat T cells were infected with VSV G-pseudotyped HIV-1NL-GFP, an env-deficient virus encoding GFP in place of Nef, in the presence or absence of 4 μM As2O3. Expression of GFP, an early Rev-independent product, and of CA, a late Rev-dependent product, were analyzed by flow cytometry on day 1 postinfection. The circles indicate gating windows for CA-GFP-double-positive cells. As a control to ensure that signals were due to de novo infection, the RT inhibitor 3TC was added 3 h before infection. (B) Effects of As2O3 on the infectivities of HIV-1-derived vectors. VSV G-pseudotyped particles were produced with the FUGW HIV-1 GFP vector and either of two helper proviruses, ΔR8.2 or ΔR8.9, the latter of which bears inactivating mutations in vif, vpr, vpu, and nef. The particles were equalized by p24 enzyme-linked immunosorbent assay and used to infect Jurkat T cells in the presence of the indicated concentrations of As2O3. The cells were fixed 2 days later and analyzed by flow cytometry for GFP expression. The error bars indicate standard deviations. (C) As2O3 acts postentry to increase HIV-1 replication. VSV-G pseudotyped HIV-1NL-GFP was incubated for 24 h at 37°C with or without As2O3 added for the times indicated (2 μM final concentration). The virus was diluted 40-fold and added to Jurkat T cells. Alternatively, target cells were incubated in 2 μM As2O3 or 0.05 μM As2O3 (40-fold dilution) at the time of infection. Two days postinfection, the percentage of GFP-expressing cells was determined by flow cytometry.
The rates of decrease in p24-expressing cells after day 3 were similar for As2O3-treated and untreated cells, suggesting that As2O3 was not indirectly increasing the efficiency of HIV-1 infection by enhancing the survival of infected cells (Fig. 1C). Among p24-positive cells, mean fluorescence intensity was not increased by As2O3 (Fig. 1D), indicating that transcription and translation of unspliced HIV-1 RNA was not enhanced. Consistent with this finding, quantitation of virus particle release from 293T cells transfected with HIV-1NL4-3 showed that As2O3 did not increase particle production (data not shown). Therefore, As2O3 appears to enhance a viral replication step prior to, and not following, integration.
The effect of As2O3 is independent of HIV-1 env and most accessory genes.
To determine which viral factors are required for stimulation of HIV-1 replication by As2O3, Jurkat T cells were infected with VSV G-pseudotyped HIV-1NL-GFP virions in the presence of increasing As2O3 concentrations. HIV-1NL-GFP bears inactivating mutations in nef and env and a GFP gene insertion in place of nef (24). VSV G pseudotyping modifies the mechanism of HIV-1 entry (4, 16) and permits assessment of the importance of HIV-1 envelope-mediated entry for the As2O3 effect. Since Nef is an early Rev-independent HIV-1 product, GFP expression from the HIV-1NL-GFP provirus is representative of early HIV-1 gene expression. p24 expression, in contrast, serves as a reporter for Rev-dependent gene expression.
Expression of CAp24 and GFP after infection was monitored using two-color flow cytometry. One day postinfection, three populations were evident by flow cytometry: uninfected cells, cells expressing GFP but not CAp24, and cells expressing both GFP and CAp24 (Fig. 2A). As2O3 strongly enhanced infection by the virus, indicating that the effect of the drug on HIV-1 replication was independent of HIV-1 env. Moreover, As2O3 increased the number of GFP- and CAp24-expressing cells to the same extent but had no effect on the intensity of fluorescence of either of the markers; these findings demonstrate that As2O3 does not alter the ratio of early to late gene expression and provide further evidence that the drug acts at a pretranscriptional level.
The effect of As2O3 on the titer of HIV-1 vectors in Jurkat T cells was tested next. GFP was transduced with particles produced using either a ΔR8.2 (which bears intact open reading frames for all HIV-1 accessory genes) or ΔR8.9 (which has inactivating mutations in vif, vpr, vpu, and nef) helper construct. As2O3 increased the percentage of cells expressing GFP independently of the accessory genes deleted in ΔR8.9, showing that they are not relevant to the effect of the drug (Fig. 2B). Identical results were obtained using particles bearing HIV-1 Env instead of VSV G (data not shown). The magnitude of increase in the titers of the vectors due to As2O3 was consistently smaller than that observed with complete HIV-1NL4-3 or with HIV-1NL-GFP. This difference did not result from the use of VSV G, as similar results were obtained with a vector bearing HIV-1 Env. Identical results were also obtained when GFP was expressed using the human ubiquitin promoter (Fig. 2B) or the CMVie promoter (data not shown).
As2O3 stimulates virus replication after virion membrane fusion.
The finding that As2O3 stimulates an early step of infection raised the possibility that the drug acts directly on virions prior to entry. To test this, VSV G-pseudotyped HIV-1NL-GFP virions were incubated in 2 μM As2O3 for up to 24 h prior to infection of Jurkat T cells. No increase in infectivity was observed (Fig. 2C). In contrast, incubation of target cells in 2 μM As2O3 immediately after infection resulted in a roughly threefold increase in the frequency of GFP-expressing cells. This result demonstrated that As2O3 does not act on cell-free virions.
As2O3 stimulates viral cDNA synthesis.
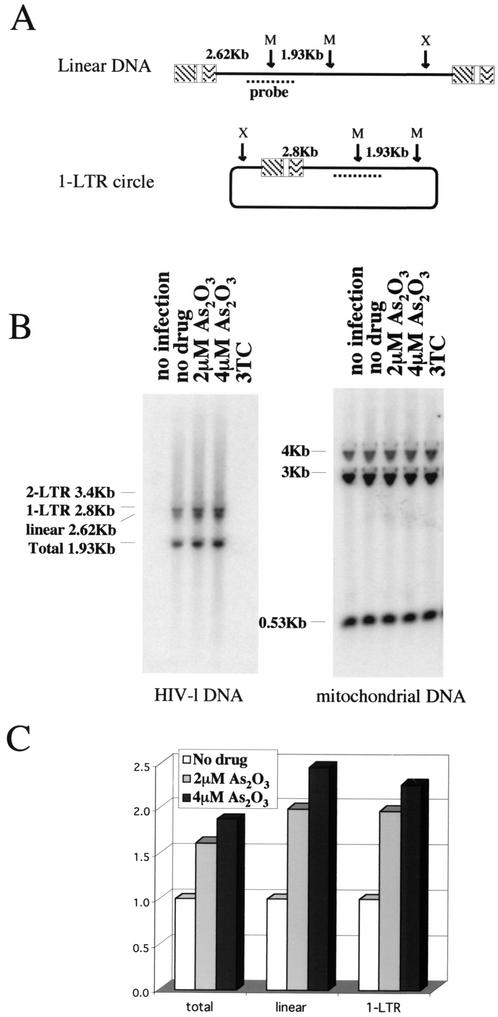
To characterize further which early step of HIV-1 replication was enhanced by As2O3, Jurkat T cells were infected with VSV-G pseudotyped HIV-1NL4-3 at an MOI of 2. Total cellular DNA was subjected to Southern blot analysis designed to reveal HIV-1 linear, 1-long terminal repeat (LTR), and 2-LTR DNA species, as well as total HIV-1 DNA, which includes newly generated provirus (Fig. 3A). DNA was harvested at 24 h based on previous optimization for monitoring the various DNA species by this method (64), and the profile obtained (Fig. 3B) closely resembled that which was previously reported (64). The 2-LTR form was barely visible and was not included in the analysis. As2O3 treatment increased steady-state levels of all HIV-1 DNA species ∼2-fold (Fig. 3C). Since circular viral DNA forms result from processing by nuclear enzymes, it can be concluded that As2O3 did not modify the kinetics of nuclear transport of the viral DNA.
FIG. 3.
As2O3 increases steady-state levels of viral cDNA. VSV G-pseudotyped HIV-1NL4-3 was used to infect Jurkat T cells in the presence of As2O3 as indicated. As a control, the HIV-1 RT inhibitor 3TC was added 2 h prior to infection. Twenty-four hours postinfection, total cellular DNA was processed for Southern blotting. (A) Schematic view of linear and 1-LTR DNA species showing the positions of restriction sites and probe. X, XhoI site, M, Msc1 site. (B) The membrane was probed with the HIV-1-specific DNA probe (left) and then stripped and reprobed for human mitochondrial DNA. (C) The amounts of the different viral DNA species that accumulated in the presence of As2O3 were quantitated by phosphorimager and normalized for mitochondrial DNA and are presented relative to the amount of DNA in the absence of As2O3 (no drug).
As2O3 stimulates MLV transduction but does not rescue Fv1 restriction.
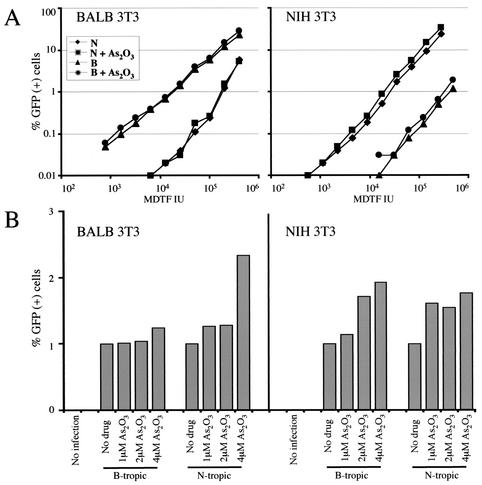
Since As2O3 enhances an early step of HIV-1 replication, it was determined if the drug similarly enhances MLV replication and if it overcomes Fv1-mediated restriction. As described in the introduction, the Fv1 locus of particular mouse strains encodes Gag-like factors which decrease the efficiency with which MLV strains bearing particular CA residues establish proviruses (9, 11, 33, 57). A matched pair of MLV vectors, one restricted by the Fv1 b allele and the other restricted by the n allele, were used to transduce GFP into murine NIH 3T3 cells (B restrictive) and murine BALB/c 3T3 cells (N restrictive). As2O3 exhibited toxicities in these two murine cell lines similar to that in human cells (50% toxic dose [TD50], 4 μM), suggesting that cellular uptake of the drug was comparable. The titers of the two MLV vectors were normalized by determining the titeris on M.dunni cells, which exhibit no Fv1 restriction. In the absence of As2O3, the two cell lines exhibited the expected phenotypes with respect to Fv1 restriction: the infectivity of the restricted vector was 1.2 to 2 log10 units less than that of the unrestricted vector (Fig. 4A). As2O3 (2 μM) did not rescue the observed Fv1-mediated restriction, regardless of the MOI (Fig. 4A). The extent of As2O3-mediated stimulation of GFP transduction was then tested over a range of drug concentrations, using quantities of virus that resulted in an MOI of ∼0.01 in the absence of drug. As2O3 treatment resulted in increased transduction efficiencies in both cell lines (up to 2.2-fold), with the exception of BALB/c 3T3 infection by the B-tropic vector (Fig. 4B). The magnitude of stimulation by As2O3 was comparable to that observed with HIV-1 vectors (Fig. 2B), suggesting that As2O3 enhances the same postentry replication step for both HIV-1 and MLV.
FIG. 4.
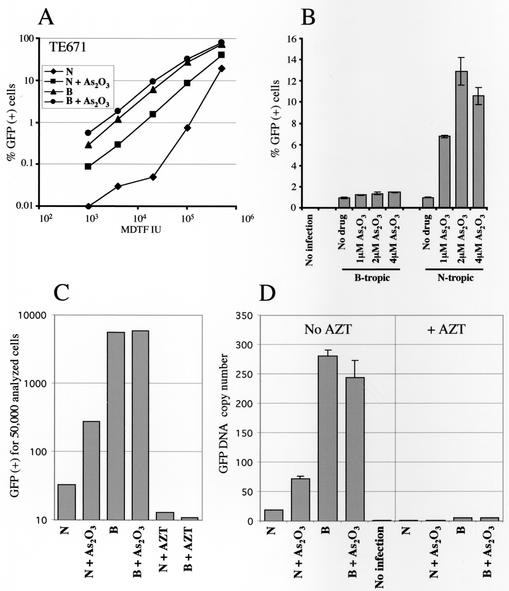
As2O3 increases MLV infectivity but does not counteract the specific block due to Fv1. (A) BALB/c (BALB) 3T3 cells or NIH 3T3 cells were infected with either N- or B-tropic MLV-derived GFP vectors in the presence or absence of 2 μM As2O3. The amount of virus added (titers were determined [in infectious units] on nonrestrictive MDTF [MDTF IU]) is indicated on the abscissa. Two days later, the percentage of GFP-expressing cells was determined by flow cytometry (50,000 cells were analyzed). (B) Cells were infected with quantities of N- or B-tropic MLV vectors that render roughly 1% of the cells GFP+ in the absence of As2O3. The percentage of GFP-expressing cells after infection in the presence of the indicated As2O3 concentrations was then determined as for panel A.
As2O3 rescues Ref1-mediated restriction to MLV.
Replication of N-tropic MLV, and not B-tropic MLV, is inhibited by an antiviral activity called Ref1 present in human cells (57, 58). To determine if As2O3 overcomes the block due to Ref1, the N-restrictive human rhabdomyosarcoma cell line TE671 was used as a target. As2O3 toxicity in TE671 cells was similar to that in the other cells tested (TD50, roughly 4 μM). As expected, transduction by the N-tropic vector was up to 2 log10 units less efficient in these cells than that by the B-tropic vector (Fig. 5A). As2O3 enhanced the transduction of GFP by N-tropic MLV in TE671 cells (increase, ∼1 log10 unit) but had only a small effect on B-tropic MLV (Fig. 5A). Keeping the MOI fixed and varying the drug concentration, a maximum increase in N-tropic MLV transduction efficiency of ∼13-fold was observed using 2 μM As2O3 (Fig. 5B). The effect of the drug on transduction by the B-tropic vector was similar to that observed with HIV-1 vectors. Therefore, As2O3 specifically rescued Ref1-mediated restriction to MLV in TE671 cells.
FIG. 5.
As2O3 rescues the block to N-tropic MLV replication in human cells. TE671 cells were infected with N- or B-tropic MLV-derived vectors (titers were determined as for Fig. 4). (A) Infection at various MOIs in the presence or absence of 2 μM As2O3. (B) Cells were infected with quantities of N- or B-tropic MLV vectors that render roughly 1% of the cells GFP+ in the absence of As2O3. The percentage of GFP-expressing cells after infection in the presence of the indicated As2O3 concentrations was then determined as for Fig. 4A. The error bars indicate standard deviations. (C and D). As2O3-mediated enhancement of human cell infection by N-tropic MLV correlates with increased steady-state levels of viral cDNA. TE671 cells were infected with 7.5 × 103 infectious units of N- or B-tropic MLV vectors (titers were determined on M.dunni cells) in the presence or absence of 2 μM As2O3. To demonstrate that signals resulted from de novo infection, where indicated, zidovudine (AZT) was added to the cells 2 h prior to infection. (C) Two days postinfection, the number of GFP-expressing cells was determined by flow cytometry. (D) In parallel, total DNA was extracted 6 h postinfection. Vector-specific GFP cDNA was quantitated using Taqman PCR. The results are expressed as DNA copy numbers, as determined using a standard curve derived from amplification of plasmid DNA.
As2O3 stimulates MLV DNA synthesis in TE671 cells.
Unlike Fv1, which causes a block prior to integration, Ref1 activity results in decreased steady-state levels of viral cDNA shortly after infection (57). The effect of As2O3 on N-tropic MLV cDNA synthesis following acute infection of TE671 cells was therefore examined. TE671 cells were infected, with or without 2 μM As2O3, using identical numbers of infectious units of N- and B-tropic MLV vectors, as determined on nonrestrictive M.dunni cells. A fraction of the culture was used to analyze vector-mediated GFP transduction (Fig. 5C). The remainder was lysed 6 h postinfection, and steady-state viral cDNA was quantitated using real-time Taqman PCR (Fig. 5D). In the absence of drug, GFP transduction by N-tropic MLV was again less than that of B-tropic MLV by >2 log10 units (Fig. 5C). Vector-specific cDNA produced in the absence of drug was 16 times lower for the N-tropic MLV than for the B-tropic MLV (Fig. 5D). As2O3 stimulated N-tropic transduction by ∼8-fold, with minimal effect on B-tropic virus (Fig. 5C). Similarly, the drug had no effect on the synthesis of B-tropic MLV cDNA but increased the synthesis of N-tropic MLV cDNA fourfold (Fig. 5D).
PML protein does not regulate HIV-1 infectivity.
As2O3 promotes degradation of PML protein and alters the morphology of nuclear PML bodies (34, 43). It has been reported that HIV-1 infection of HeLa cells disrupts PML bodies, that PML body components colocalize with incoming viral DNA, and that PML protein overexpression counteracts the positive effect of As2O3 on viral replication (60). These observations suggest that PML protein exhibits anti-HIV-1 activity and that As2O3 promotes viral infectivity by counteracting PML protein. Going against this model, another group failed to detect alteration of PML bodies after HIV-1 infection, or colocalization between PML protein and unintegrated HIV-1 DNA, using either HeLa cells or HOS osteosarcoma cells (7).
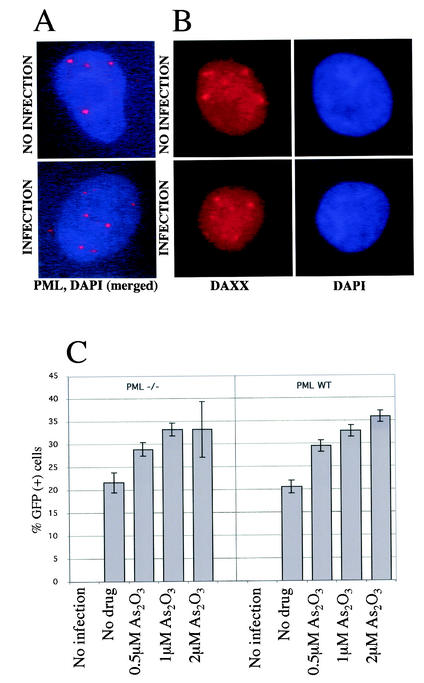
We assessed the integrity of PML bodies following HIV-1 infection of HeLa cells at a high MOI, conditions similar to those under which HIV-1 was reported to induce PML protein relocalization (60). As2O3 increased HIV-1 infectivity to the same extent in HeLa cells as it did in Jurkat T cells (data not shown). HeLa cells were infected at an MOI of 40 with a GFP-expressing HIV-1 vector similar to the one used in the experiment shown in Fig. 2B. Virtually all cells were efficiently infected, as shown by intense intracellular p24 staining 2 h following exposure and by GFP expression in most cells at 30 h postinfection (data not shown). By immunofluorescence, it could be seen that PML protein localized to discrete nuclear structures (PML bodies). This pattern was not altered 2.5 h following infection (Fig. 6A) or at any time tested, from 30 min to 30 h (data not shown). Occasionally, PML protein was detected outside the nucleus, but this sporadic finding was independent of HIV-1 infection.
FIG. 6.
As2O3-mediated enhancement of HIV-1 infectivity is retained in cells lacking PML. (A) HeLa cells were either uninfected or infected for 2.5 h with the HIV-1-derived vector TRIP-GFP at an MOI of 40. Immunofluorescence microscopy was performed using the DNA marker DAPI and an antibody against PML, and the two images were merged. (B) The experiment was repeated using DAPI and an antibody against Daxx, another PML body component. (C) MEFs derived from PML−/− or wild-type (WT) mice were infected with an HIV-1 vector (TRIP-CMV-GFP) in the presence of the indicated concentrations of As2O3. Two days postinfection, the cells were analyzed by flow cytometry for GFP expression. The bars represent the average (± standard deviation) of three independent infections.
PML protein recruits Daxx to PML bodies. Under conditions where PML protein is disrupted, Daxx no longer localizes to PML bodies (30, 65). One would therefore predict that relocalization of PML protein to the cytoplasm would result in a redistribution of Daxx. In HeLa cells, Daxx concentrated in nuclear structures that were confirmed to be PML bodies by reactivity with antibodies against other PML body components (Fig. 6B and data not shown). Daxx localization was not modified by HIV-1 infection, confirming that PML bodies were not disrupted. The same results were obtained with antibodies specific for SUMO-1, another PML body component (data not shown). Finally, we saw no evidence of PML body disruption when cells were infected with VSV G-pseudotyped HIV-1 at the same MOI (data not shown).
To further test the importance of PML protein as an anti-HIV-1 replication factor, or as a cellular target of As2O3 relevant for HIV-1 replication, wild-type and PML−/− MEFs were infected with a GFP-transducing HIV-1 vector in the presence of increasing As2O3 concentrations. PML protein and PML bodies are undetectable in the PML−/− MEFs used here (61, 65). In the absence of the drug, the efficiencies of infection using wild-type or PML−/− target cells were identical (Fig. 6C), suggesting that PML protein does not regulate early steps of HIV-1 replication. In addition, As2O3 treatment caused increases in GFP transduction in the two cell types that were of equal magnitude, suggesting that the effect of As2O3 on HIV-1 replication is PML protein independent (Fig. 6C).
As2O3 stimulates HIV-1 replication at toxic concentrations.
In addition to disrupting PML bodies, As2O3 generates proapoptotic signals, including a decrease in the potential across the internal mitochondrial membrane and release into the cytoplasm of factors such as diablo and cytochrome c (56). The uncoupling effect is elicited using purified mitochondria, suggesting that mitochondria are direct targets of As2O3 (36). Late apoptotic events, such as DNA fragmentation, are also associated with As2O3 action and probably derive from the initial action of the drug on mitochondria (17, 56).
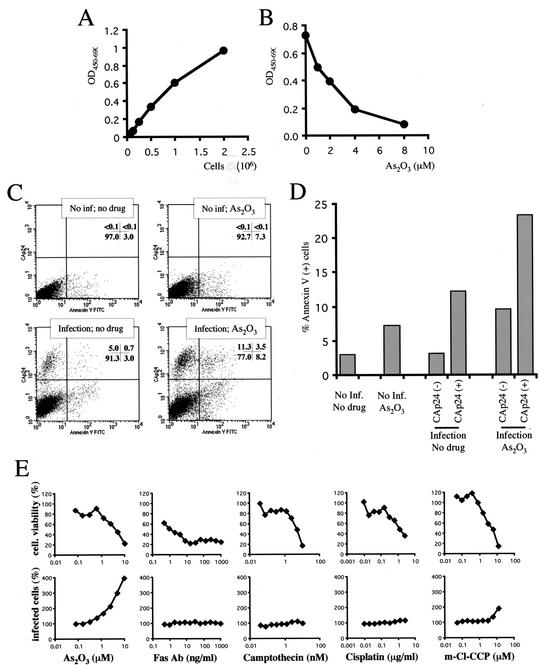
Since As2O3 inhibits tumor cell proliferation generally (32, 37, 47), the effect of As2O3 on the viability of Jurkat T cells, at doses relevant to the effect on HIV-1 replication, was addressed. Jurkat T cells were treated with As2O3, and the relative number of live cells remaining was estimated by XTT assay. The values obtained in this assay reflect the number of viable cells in the culture, as demonstrated using a standard dilution (Fig. 7A). As2O3 reduced Jurkat cell viability in a concentration-dependent fashion, with a TD50 of ∼2 × 10−6 M (Fig. 7B). Optimal stimulation of HIV-1 replication in Jurkat T cells was observed at concentrations higher than 1 μM As2O3, concentrations which result in significant decreases in Jurkat cell viability (Fig. 7B).
FIG. 7.
As2O3 induces cell death in T cells at concentrations which promote HIV-1 infection. (A) Linearity of an XTT-based viability assay as assessed on a serial dilution of Jurkat cells. OD450-690, optical density at 450 nm with a reference wavelength of 690 nm. (B) Jurkat T cells were cultured for 2 days in the presence of the indicated concentrations of As2O3. Viability was then assessed using the XTT assay. (C) VSV G-pseudotyped HIV-1NL4-3 was used to infect Jurkat T cells in the presence or absence of 2 μM As2O3, as indicated. Three days later, the cells were double stained for surface annexin V binding (x axis) and internal CA (p24) expression (y axis). The percentage of cells in each quadrant is indicated. inf, infection. (D) The percentage of annexin V binding cells was calculated for each uninfected and infected population (as judged by CA expression) in each graph of panel C. (E) Jurkat T cells were infected with HIV-1NL-GFP and simultaneously treated with various apoptosis-inducing drugs at the indicated concentrations. Two days later, the numbers of live cells were determined by the XTT assay (top) and the frequency of GFP-expressing intact cells was monitored by fluorescence-activated cell sorter (bottom). In both cases, the numbers shown are percentages of the values obtained in the absence of drug.
Toxicities caused by HIV-1 infection and by As2O3 are additive.
We designed an experiment to test whether As2O3 induced significant cell death and whether this could explain the effect of the drug on HIV-1 replication. HIV-1 infection might protect cells against the drug's toxic effects. Alternatively, As2O3 could protect cells against toxic effects caused by HIV-1 infection. We thus assessed cell death after treatment with As2O3, infection with HIV-1, or both together. Jurkat T cells were infected with VSV G-pseudotyped HIV-1NL4-3 in the presence or absence of As2O3. Viral spread to uninfected cells, as well as syncytium formation, were blocked with dextran sulfate. Induction of cell death was then assayed by an annexin V binding assay. Simultaneously, HIV-1-infected cells were identified by internal CA (p24) expression (Fig. 7C).
As expected, As2O3 enhanced HIV-1 infection of Jurkat T cells, as reflected by an almost threefold increase in the total number of p24-expressing cells (compare the top quadrants of the two bottom graphs in Fig. 7C). The p24-annexin V double-staining procedure permitted determination of the percentage of cells undergoing cell death among both HIV-1-infected and uninfected populations (Fig. 7D). As2O3 caused an increase in annexin V binding of two- to threefold. Among HIV-1-infected cells, the frequency of annexin V binding cells was increased fourfold in the absence of As2O3. Cells from the same well that did not express p24 displayed basal levels of annexin V binding, indicating that HIV-1 infection directly induced cell death, in accord with what has been published by others using annexin V and terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling assays (25). Infection in the presence of As2O3 resulted in increased annexin V binding for both infected and uninfected cells compared to infection in the absence of drug. This shows that cell deaths induced by the drug and by infection were cumulative and that HIV-1 infection did not protect cells from As2O3-induced cell death, nor did As2O3 protect cells against HIV-1-induced toxicity.
Enhancement of infectivity is not caused by apoptosis.
To investigate further a possible connection between apoptosis and efficiency of HIV-1 infection, we tested the effects of various apoptotis-inducing agents on the infection of Jurkat T cells by VSV G-pseudotyped HIV-1NL-GFP. Camptothecin triggers both cell cycle arrest (at S phase) and apoptosis by degradation of topoisomerase I (42). Cisplatin, a DNA-damaging agent, arrests cells at the S and G2 phases and kills them through apoptotic pathways (45). m-Cl-CCP was studied because, like As2O3, it is known to strongly induce mitochondrial depolarization (5, 56). Conversely, apoptosis induced by activation of the Fas (CD95) pathway appears to be independent of mitochondria, as reflected by the fact that it is not counteracted by overexpression of Bcl-2 (28).
Cells were infected with HIV-1NL-GFP in the presence of a range of concentrations of As2O3, anti-Fas antibody, camptothecin, cisplatin, or m-Cl-CCP. Cellular viability and HIV-1 infection were monitored (Fig. 7E). Only As2O3 strongly enhanced infection, showing that enhancement of HIV-1 infection was drug specific. Interestingly, the mitochondrial-uncoupling agent, m-Cl-CCP, had a small but significant effect at the highest concentration tested; assessment of higher concentrations was precluded by drug toxicity.
DISCUSSION
As2O3 enhances retroviral reverse transcription.
Our results demonstrate that As2O3 stimulates retroviral replication, either in a spreading-infection assay in T cells or in single-cycle infection of various cell lines. There were no detectable effects of As2O3 on late phases of HIV-1 replication, as indicated by the quantity of virions released by 293T cells or Jurkat T cells or by the infectivity of the particles released from these cells (data not shown). Since As2O3 alters the structure of PML bodies and the HIV-1 transactivator Tat has recently been found to interact with PML through its cellular partner, cyclin T1 (A. Marcello and M. Giacca, personal communication), the potential effects of As2O3 on HIV-1 expression were carefully analyzed. The level of expression (mean fluorescence intensity) of either early or late HIV-1 products was not altered by As2O3 (Fig. 2), indicating that the drug did not modify transcription or posttranscriptional processing and translation.
As2O3 increased the percentage of cells infected by either replication-competent viruses or replication-deficient vectors, indicating that an early phase of replication was enhanced by the drug. The drug had similar effects on viruses bearing either HIV-1 Env or VSV G, molecules which target different entry pathways, and had no effect on cell-free virions, indicating that it acted on a postentry step. Indeed, the drug increased the steady-state levels of HIV-1 cDNA that accumulated acutely after infection (Fig. 3). Despite the use of an effective RT inhibitor to clearly demonstrate that the signal in our assay was due to de novo cDNA synthesis, steady-state levels of viral cDNA reflect the rates of both synthesis and degradation. As2O3 might simply increase the efficiency of cDNA synthesis itself, or perhaps As2O3 inhibits cellular nucleases that degrade the viral cDNA, especially considering that unintegrated retroviral cDNA is unstable in infected cells (20, 55). Alternatively, As2O3 might increase the efficiency of a step prior to reverse transcription, such as virion uncoating.
Viral determinants of responsiveness to As2O3.
The effect of As2O3 on HIV-1 transduction was independent of Env or of the viral accessory genes vif, vpr, vpu, and nef (Fig. 2). This is consistent with the fact that As2O3 increased the infectivity of MLV, a virus which bears none of these genes. The magnitudes of stimulation were similar with vectors derived from HIV-1 and MLV—two very dissimilar retroviruses—suggesting that the same stimulatory effect would be observed with all retroviruses.
The magnitude of the As2O3 effect on transduction by complete HIV-1 was consistently larger than the effect on HIV-1-derived vectors, even when all viral protein products were identical. This raises the possibility that genome size or structure influences the magnitude of the effect of the drug, and it is consistent with the effect of As2O3 on viral cDNA levels. If As2O3 stimulates viral cDNA synthesis, the effect of the drug might be less noticeable if a given genome is reverse transcribed more efficiently. Alternatively, a more efficiently synthesized genome would be less subject to the degradation machinery, which preferentially targets incompletely synthesized genomes (20).
As2O3 counteracts Ref1 antiviral activity.
As2O3 significantly rescued Ref1 restriction to N-tropic MLV replication in human TE671 cells but not the Fv1 restriction to the same virus in murine 3T3 cells (Fig. 4 and 5). This result is consistent with the fact that these two restriction mechanisms are distinct; indeed, Fv1 has been cloned and has no homologue in human cells (9). The Fv1 block occurs for the most part after viral cDNA synthesis, while with Ref1, the products of reverse transcription are decreased (57). Accordingly, we found that rescue by As2O3 of N-tropic MLV infectivity in TE671 cells was accompanied by an increase in the levels of viral cDNA (Fig. 5). Thus, rescue of Ref1 restriction by As2O3 altered the same step in the virus life cycle, reverse transcription, that was stimulated by As2O3 for retroviruses more generally (Fig. 5).
The literature contains evidence for cellular factors other than Fv1 and Ref1 that restrict early postentry steps in the retrovirus life cycle. For instance, cells from most Old World monkeys restrict the efficiency of entry of HIV-1, while cells from most New World monkeys restrict entry of simian immunodeficiency virus strain MAC (27). We tested the effects of As2O3 on three simian cell lines, OMK, FRhK4, and CV-1, each of which restricts HIV-1 postentry steps (8, 21, 27). As2O3, at concentrations ranging from 1 to 20 μM, had no effect on the efficiency of HIV-1 infection in any of these cell lines (data not shown). However, no toxic effects of the drug were evident in these cells, even at concentrations as high as 10 μM. This suggests either that cellular uptake of the drug was inefficient in these cells or that the putative cellular target of As2O3 is not present.
PML protein does not regulate retroviral infection.
Incubation of cell-free virions in As2O3 did not increase subsequent infectivity (Fig. 2), suggesting that As2O3 does not act directly on retroviral components. Though the drug might interact directly with viral components after virions fuse with target cells, it seems more likely that stimulation of retroviral replication by As2O3 is mediated via cellular factors. It was recently hypothesized that PML protein exhibits antiviral activity which is inhibited by As2O3 (60). Though PML protein overexpression blocked the effect of As2O3, PML protein antiviral activity, in the absence of As2O3, was not demonstrated. Moreover, it was suggested that PML protein overexpression blocked the As2O3 effect by preventing As2O3-induced PML protein degradation or trapping it in the nucleus, but others report that As2O3 induces degradation of PML protein to undetectable levels, even when PML protein is overexpressed (34, 66). Here, we found that the effects of As2O3 on HIV-1 replication were identical in PML-null and wild-type PML cells (Fig. 6), demonstrating that PML protein is not necessary for the action of the drug. Perhaps more importantly, the levels of infection of wild-type and PML-null cells were identical, indicating that, with or without As2O3, PML protein does not influence early steps of HIV-1 replication. Consistent with this idea, in our hands, infection with HIV-1 or vectors did not alter the morphology or localization of PML bodies (Fig. 6).
What is the mechanism by which As2O3 stimulates retroviral replication?
As2O3 stimulates HIV-1 replication in Jurkat T cells (as well as in HeLa and myelomonocytic U937 cells [data not shown]) at concentrations which disrupt cellular viability (Fig. 7). In agreement with others (37), we found that micromolar As2O3 induced cell death, as seen by annexin V binding (Fig. 7). Any connection between viral stimulatory effects and cellular toxicity might be trivial. For example, infected cells might become resistant to the toxic effects of As2O3 or As2O3 might render cells insensitive to the toxic effects of HIV-1. These possibilities seem unlikely, since As2O3 induced similar levels of cell death in productively infected Jurkat T cells and in uninfected cells in the same culture (Fig. 7) and HIV-1-infected cells disappeared from the cell population at the same rate (or even slightly faster) in the presence of As2O3 (Fig. 1).
In addition, we find that other apoptosis-inducing drugs have little or no effect on the early steps of HIV-1 replication, indicating that apoptosis as a general mechanism is not relevant to the observed effect of As2O3 on HIV-1. Consistent with this idea, the general caspase inhibitor Z-VAD-FMK did not suppress the effect of As2O3 on HIV-1 (data not shown), indicating that late apoptotic events were not required. It is possible, though, that an earlier apoptotic event induced by As2O3, such as the suppression of the transmembrane potential of mitochondria (36), is relevant to the activation of HIV-1. In support of this hypothesis is the fact that another mitochondrial-uncoupling agent, m-Cl-CCP, also stimulated HIV-1 infection (though to a lesser extent than As2O3).
Acknowledgments
We thank David Baltimore, Pierre Charneau, Dana Gabuzda, Nathaniel Landau, Carlos Lois, Ali Naini, and Veronique Zennou for reagents. We also thank Ed Laufer (Columbia University) for sharing his microscope facility.
This work was supported by a scholarship from the Elizabeth Glaser Pediatric AIDS Foundation (to L.B.), by Research Career Development Fellowship no. 064257 from the Wellcome Trust (to G.T.), and by the Columbia-Rockefeller Center for AIDS Research (P30 AI42848).
REFERENCES
- 1.Adachi, A., H. E. Gendelman, S. Koenig, T. Folks, R. Willey, A. Rabson, and M. A. Martin. 1986. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J. Virol. 59:284-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adamson, A. L., and S. Kenney. 2001. Epstein-Barr virus immediate-early protein BZLF1 is SUMO-1 modified and disrupts promyelocytic leukemia bodies. J. Virol. 75:2388-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahn, J. H., E. J. Brignole III, and G. S. Hayward. 1998. Disruption of PML subnuclear domains by the acidic IE1 protein of human cytomegalovirus is mediated through interaction with PML and may modulate a RING finger-dependent cryptic transactivator function of PML. Mol. Cell. Biol. 18:4899-4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aiken, C. 1997. Pseudotyping human immunodeficiency virus type 1 (HIV-1) by the glycoprotein of vesicular stomatitis virus targets HIV-1 entry to an endocytic pathway and suppresses both the requirement for Nef and the sensitivity to cyclosporin A. J. Virol. 71:5871-5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstrong, J. S., K. K. Steinauer, J. French, P. L. Killoran, J. Walleczek, J. Kochanski, and S. J. Knox. 2001. Bcl-2 inhibits apoptosis induced by mitochondrial uncoupling but does not prevent mitochondrial transmembrane depolarization. Exp. Cell Res. 262:170-179. [DOI] [PubMed] [Google Scholar]
- 6.Atchison, R. E., J. Gosling, F. S. Monteclaro, C. Franci, L. Digilio, I. F. Charo, and M. A. Goldsmith. 1996. Multiple extracellular elements of CCR5 and HIV-1 entry: dissociation from response to chemokines. Science 274:1924-1926. [DOI] [PubMed] [Google Scholar]
- 7.Bell, P., L. J. Montaner, and G. G. Maul. 2001. Accumulation and intranuclear distribution of unintegrated human immunodeficiency virus type 1 DNA. J. Virol. 75:7683-7691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Besnier, C., Y. Takeuchi, and G. Towers. 2002. Restriction of lentivirus in monkeys. Proc. Natl. Acad. Sci. USA 99:11920-11925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Best, S., P. Le Tissier, G. Towers, and J. P. Stoye. 1996. Positional cloning of the mouse retrovirus restriction gene Fv1. Nature 382:826-829. [DOI] [PubMed] [Google Scholar]
- 10.Bieniasz, P. D., and B. R. Cullen. 2000. Multiple blocks to human immunodeficiency virus type 1 replication in rodent cells. J. Virol. 74:9868-9877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bock, M., K. N. Bishop, G. Towers, and J. P. Stoye. 2000. Use of a transient assay for studying the genetic determinants of Fv1 restriction. J. Virol. 74:7422-7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braaten, D., C. Aberham, E. K. Franke, L. Yin, W. Phares, and J. Luban. 1996. Cyclosporine A-resistant human immunodeficiency virus type 1 mutants demonstrate that Gag encodes the functional target of cyclophilin A. J. Virol. 70:5170-5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braaten, D., E. K. Franke, and J. Luban. 1996. Cyclophilin A is required for an early step in the life cycle of human immunodeficiency virus type 1 before the initiation of reverse transcription. J. Virol. 70:3551-3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braaten, D., and J. Luban. 2001. Cyclophilin A regulates HIV-1 infectivity, as demonstrated by gene targeting in human T cells. EMBO J. 20:1300-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burkham, J., D. M. Coen, and S. K. Weller. 1998. ND10 protein PML is recruited to herpes simplex virus type 1 prereplicative sites and replication compartments in the presence of viral DNA polymerase. J. Virol. 72:10100-10107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chazal, N., G. Singer, C. Aiken, M. L. Hammarskjold, and D. Rekosh. 2001. Human immunodeficiency virus type 1 particles pseudotyped with envelope proteins that fuse at low pH no longer require Nef for optimal infectivity. J. Virol. 75:4014-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen, G. Q., J. Zhu, X. G. Shi, J. H. Ni, H. J. Zhong, G. Y. Si, X. L. Jin, W. Tang, X. S. Li, S. M. Xong, Z. X. Shen, G. L. Sun, J. Ma, P. Zhang, T. D. Zhang, C. Gazin, T. Naoe, S. J. Chen, Z. Y. Wang, and Z. Chen. 1996. In vitro studies on cellular and molecular mechanisms of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia: As2O3 induces NB4 cell apoptosis with downregulation of Bcl-2 expression and modulation of PML-RAR alpha/PML proteins. Blood 88:1052-1061. [PubMed] [Google Scholar]
- 18.Cimarelli, A., and J. Luban. 2000. Human immunodeficiency virus type 1 virion density is not determined by nucleocapsid basic residues. J. Virol. 74:6734-6740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cocchi, F., A. L. DeVico, A. Garzino-Demo, S. K. Arya, R. C. Gallo, and P. Lusso. 1995. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science 270:1811-1815. [DOI] [PubMed] [Google Scholar]
- 20.Collin, M., and S. Gordon. 1994. The kinetics of human immunodeficiency virus reverse transcription are slower in primary human macrophages than in a lymphoid cell line. Virology 200:114-120. [DOI] [PubMed] [Google Scholar]
- 21.Cowan, S., T. Hatziioannou, T. Cunningham, M. A. Muesing, H. G. Gottlinger, and P. D. Bieniasz. 2002. Cellular inhibitors with Fv1-like activity restrict human and simian immunodeficiency virus tropism. Proc. Natl. Acad. Sci. USA 99:11914-11919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franke, E. K., and J. Luban. 1996. Inhibition of HIV-1 replication by cyclosporine A or related compounds correlates with the ability to disrupt the Gag-cyclophilin A interaction. Virology 222:279-282. [DOI] [PubMed] [Google Scholar]
- 23.Garrus, J. E., U. K. von Schwedler, O. W. Pornillos, S. G. Morham, K. H. Zavitz, H. E. Wang, D. A. Wettstein, K. M. Stray, M. Cote, R. L. Rich, D. G. Myszka, and W. I. Sundquist. 2001. Tsg101 and the vacuolar protein sorting pathway are essential for HIV-1 budding. Cell 107:55-65. [DOI] [PubMed] [Google Scholar]
- 24.He, J., Y. Chen, M. Farzan, H. Choe, A. Ohagen, S. Gartner, J. Busciglio, X. Yang, W. Hofmann, W. Newman, C. R. Mackay, J. Sodroski, and D. Gabuzda. 1997. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature 385:645-649. [DOI] [PubMed] [Google Scholar]
- 25.Herbein, G., C. Van Lint, J. L. Lovett, and E. Verdin. 1998. Distinct mechanisms trigger apoptosis in human immunodeficiency virus type 1-infected and in uninfected bystander T lymphocytes. J. Virol. 72:660-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Himathongkham, S., and P. A. Luciw. 1996. Restriction of HIV-1 (subtype B) replication at the entry step in rhesus macaque cells. Virology 219:485-488. [DOI] [PubMed] [Google Scholar]
- 27.Hofmann, W., D. Schubert, J. LaBonte, L. Munson, S. Gibson, J. Scammell, P. Ferrigno, and J. Sodroski. 1999. Species-specific, postentry barriers to primate immunodeficiency virus infection. J. Virol. 73:10020-10028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang, D. C., S. Cory, and A. Strasser. 1997. Bcl-2, Bcl-XL and adenovirus protein E1B19kD are functionally equivalent in their ability to inhibit cell death. Oncogene 14:405-414. [DOI] [PubMed] [Google Scholar]
- 29.Ikeda, Y., M. K. Collins, P. A. Radcliffe, K. A. Mitrophanous, and Y. Takeuchi. 2002. Gene transduction efficiency in cells of different species by HIV and EIAV vectors. Gene Ther. 9:932-938. [DOI] [PubMed] [Google Scholar]
- 30.Ishov, A. M., A. G. Sotnikov, D. Negorev, O. V. Vladimirova, N. Neff, T. Kamitani, E. T. Yeh, J. F. Strauss III, and G. G. Maul. 1999. PML is critical for ND10 formation and recruits the PML-interacting protein daxx to this nuclear structure when modified by SUMO-1. J. Cell Biol 147:221-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishov, A. M., R. M. Stenberg, and G. G. Maul. 1997. Human cytomegalovirus immediate early interaction with host nuclear structures: definition of an immediate transcript environment. J. Cell Biol. 138:5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang, X. H., B. C. Wong, S. T. Yuen, S. H. Jiang, C. H. Cho, K. C. Lai, M. C. Lin, H. F. Kung, S. K. Lam, and B. Chun-Yu Wong. 2001. Arsenic trioxide induces apoptosis in human gastric cancer cells through up-regulation of p53 and activation of caspase-3. Int. J. Cancer 91:173-179. [DOI] [PubMed] [Google Scholar]
- 33.Kozak, C. A., and A. Chakraborti. 1996. Single amino acid changes in the murine leukemia virus capsid protein gene define the target of Fv1 resistance. Virology 225:300-305. [DOI] [PubMed] [Google Scholar]
- 34.Lallemand-Breitenbach, V., J. Zhu, F. Puvion, M. Koken, N. Honore, A. Doubeikovsky, E. Duprez, P. P. Pandolfi, E. Puvion, P. Freemont, and H. de The. 2001. The role of promyelocytic leukemia (PML) sumolation in nuclear body formation, 11S proteasome recruitment, and As2O3-induced PML or PML/retinoic acid receptor alpha degradation. J. Exp. Med. 193:1361-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Landau, N. R., M. Warton, and D. R. Littman. 1988. The envelope glycoprotein of the human immunodeficiency virus binds to the immunoglobulin-like domain of CD4. Nature 334:159-162. [DOI] [PubMed] [Google Scholar]
- 36.Larochette, N., D. Decaudin, E. Jacotot, C. Brenner, I. Marzo, S. A. Susin, N. Zamzami, Z. Xie, J. Reed, and G. Kroemer. 1999. Arsenite induces apoptosis via a direct effect on the mitochondrial permeability transition pore. Exp. Cell Res. 249:413-421. [DOI] [PubMed] [Google Scholar]
- 37.Lehmann, S., S. Bengtzen, A. Paul, B. Christensson, and C. Paul. 2001. Effects of arsenic trioxide (As2O3) on leukemic cells from patients with non-M3 acute myelogenous leukemia: studies of cytotoxicity, apoptosis and the pattern of resistance. Eur. J. Haematol. 66:357-364. [DOI] [PubMed] [Google Scholar]
- 38.Lois, C., E. J. Hong, S. Pease, E. J. Brown, and D. Baltimore. 2002. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science 295:868-872. [DOI] [PubMed] [Google Scholar]
- 39.Mariani, R., B. A. Rasala, G. Rutter, K. Wiegers, S. M. Brandt, H. G. Krausslich, and N. R. Landau. 2001. Mouse-human heterokaryons support efficient human immunodeficiency virus type 1 assembly. J. Virol. 75:3141-3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mariani, R., G. Rutter, M. E. Harris, T. J. Hope, H. G. Krausslich, and N. R. Landau. 2000. A block to human immunodeficiency virus type 1 assembly in murine cells. J. Virol. 74:3859-3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McClure, M. O., Q. J. Sattentau, P. C. Beverley, J. P. Hearn, A. K. Fitzgerald, A. J. Zuckerman, and R. A. Weiss. 1987. HIV infection of primate lymphocytes and conservation of the CD4 receptor. Nature 330:487-489. [DOI] [PubMed] [Google Scholar]
- 42.Morris, E. J., and H. M. Geller. 1996. Induction of neuronal apoptosis by camptothecin, an inhibitor of DNA topoisomerase-I: evidence for cell cycle-independent toxicity. J. Cell Biol. 134:757-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muller, S., W. H. Miller, Jr., and A. Dejean. 1998. Trivalent antimonials induce degradation of the PML-RAR oncoprotein and reorganization of the promyelocytic leukemia nuclear bodies in acute promyelocytic leukemia NB4 cells. Blood 92:4308-4316. [PubMed] [Google Scholar]
- 44.Neil, S., F. Martin, Y. Ikeda, and M. Collins. 2001. Postentry restriction to human immunodeficiency virus-based vector transduction in human monocytes. J. Virol. 75:5448-5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ormerod, M. G., C. O'Neill, D. Robertson, L. R. Kelland, and K. R. Harrap. 1996. cis-Diamminedichloroplatinum(II)-induced cell death through apoptosis in sensitive and resistant human ovarian carcinoma cell lines. Cancer Chemother. Pharmacol. 37:463-471. [DOI] [PubMed] [Google Scholar]
- 46.Page, K. A., N. R. Landau, and D. R. Littman. 1990. Construction and use of a human immunodeficiency virus vector for analysis of virus infectivity. J. Virol. 64:5270-5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park, J. W., Y. J. Choi, M. A. Jang, S. H. Baek, J. H. Lim, T. Passaniti, and T. K. Kwon. 2001. Arsenic trioxide induces G2/M growth arrest and apoptosis after caspase-3 activation and bcl-2 phosphorylation in promonocytic U937 cells. Biochem. Biophys. Res. Commun. 286:726-734. [DOI] [PubMed] [Google Scholar]
- 48.Patnaik, A., V. Chau, and J. W. Wills. 2000. Ubiquitin is part of the retrovirus budding machinery. Proc. Natl. Acad. Sci. USA 97:13069-13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qi, C. F., F. Bonhomme, A. Buckler-White, C. Buckler, A. Orth, M. R. Lander, S. K. Chattopadhyay, and H. C. Morse III. 1998. Molecular phylogeny of Fv1. Mamm. Genome 9:1049-1055. [DOI] [PubMed] [Google Scholar]
- 50.Schubert, U., D. E. Ott, E. N. Chertova, R. Welker, U. Tessmer, M. F. Princiotta, J. R. Bennink, H. G. Krausslich, and J. W. Yewdell. 2000. Proteasome inhibition interferes with gag polyprotein processing, release, and maturation of HIV-1 and HIV-2. Proc. Natl. Acad. Sci. USA 97:13057-13062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwartz, O., V. Marechal, B. Friguet, F. Arenzana-Seisdedos, and J. M. Heard. 1998. Antiviral activity of the proteasome on incoming human immunodeficiency virus type 1. J. Virol. 72:3845-3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Serhan, F., N. Jourdan, S. Saleun, P. Moullier, and G. Duisit. 2002. Characterization of producer cell-dependent restriction of murine leukemia virus replication. J. Virol. 76:6609-6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sheehy, A. M., N. C. Gaddis, J. D. Choi, and M. H. Malim. 2002. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature 418:646-650. [DOI] [PubMed] [Google Scholar]
- 54.Shibata, R., H. Sakai, M. Kawamura, K. Tokunaga, and A. Adachi. 1995. Early replication block of human immunodeficiency virus type 1 in monkey cells. J. Gen. Virol. 76:2723-2730. [DOI] [PubMed] [Google Scholar]
- 55.Simon, J. H., and M. H. Malim. 1996. The human immunodeficiency virus type 1 Vif protein modulates the postpenetration stability of viral nucleoprotein complexes. J. Virol. 70:5297-5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sordet, O., C. Rebe, I. Leroy, J. M. Bruey, C. Garrido, C. Miguet, G. Lizard, S. Plenchette, L. Corcos, and E. Solary. 2001. Mitochondria-targeting drugs arsenic trioxide and lonidamine bypass the resistance of TPA-differentiated leukemic cells to apoptosis. Blood 97:3931-3940. [DOI] [PubMed] [Google Scholar]
- 57.Towers, G., M. Bock, S. Martin, Y. Takeuchi, J. P. Stoye, and O. Danos. 2000. A conserved mechanism of retrovirus restriction in mammals. Proc. Natl. Acad. Sci. USA 97:12295-12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Towers, G., M. Collins, and Y. Takeuchi. 2002. Abrogation of Ref1 retrovirus restriction in human cells. J. Virol. 76:2548-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Towers, G. J., D. Stockholm, V. Labrousse-Najburg, F. Carlier, O. Danos, and J. C. Pages. 1999. One step screening of retroviral producer clones by real time quantitative PCR. J. Gene Med. 1:352-359. [DOI] [PubMed] [Google Scholar]
- 60.Turelli, P., V. Doucas, E. Craig, B. Mangeat, N. Klages, R. Evans, G. Kalpana, and D. Trono. 2001. Cytoplasmic recruitment of INI1 and PML on incoming HIV preintegration complexes: interference with early steps of viral replication. Mol. Cell 7:1245-1254. [DOI] [PubMed] [Google Scholar]
- 61.Wang, Z. G., L. Delva, M. Gaboli, R. Rivi, M. Giorgio, C. Cordon-Cardo, F. Grosveld, and P. P. Pandolfi. 1998. Role of PML in cell growth and the retinoic acid pathway. Science 279:1547-1551. [DOI] [PubMed] [Google Scholar]
- 62.Wei, P., M. E. Garber, S. M. Fang, W. H. Fischer, and K. A. Jones. 1998. A novel CDK9-associated C-type cyclin interacts directly with HIV-1 Tat and mediates its high-affinity, loop-specific binding to TAR RNA. Cell 92:451-462. [DOI] [PubMed] [Google Scholar]
- 63.Weislow, O. S., R. Kiser, D. L. Fine, J. Bader, R. H. Shoemaker, and M. R. Boyd. 1989. New soluble-formazan assay for HIV-1 cytopathic effects: application to high-flux screening of synthetic and natural products for AIDS-antiviral activity. J. Natl. Cancer Inst. 81:577-586. [DOI] [PubMed] [Google Scholar]
- 64.Zennou, V., C. Petit, D. Guetard, U. Nerhbass, L. Montagnier, and P. Charneau. 2000. HIV-1 genome nuclear import is mediated by a central DNA flap. Cell 101:173-185. [DOI] [PubMed] [Google Scholar]
- 65.Zhong, S., S. Muller, S. Ronchetti, P. S. Freemont, A. Dejean, and P. P. Pandolfi. 2000. Role of SUMO-1-modified PML in nuclear body formation. Blood 95:2748-2752. [PubMed] [Google Scholar]
- 66.Zhu, J., M. H. Koken, F. Quignon, M. K. Chelbi-Alix, L. Degos, Z. Y. Wang, Z. Chen, and H. de The. 1997. The arsenic-induced PML targeting onto nuclear bodies: implications for the treatment of acute promyelocytic leukemia. Proc. Natl. Acad. Sci. USA 94:3978-3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zufferey, R., T. Dull, R. J. Mandel, A. Bukovsky, D. Quiroz, L. Naldini, and D. Trono. 1998. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J. Virol. 72:9873-9880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zufferey, R., D. Nagy, R. J. Mandel, L. Naldini, and D. Trono. 1997. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat. Biotechnol. 15:871-875. [DOI] [PubMed] [Google Scholar]