Abstract
Human parvovirus B19 infects predominantly erythroid precursor cells, leading to inhibition of erythropoiesis. This erythroid cell damage is mediated by the viral nonstructural protein 1 (NS1) through an apoptotic mechanism. We previously demonstrated that B19 virus infection induces G2 arrest in erythroid UT7/Epo-S1 cells; however, the role of NS1 in regulating cell cycle arrest is unknown. In this report, by using paclitaxel, a mitotic inhibitor, we show that B19 virus infection induces not only G2 arrest but also G1 arrest. Interestingly, UV-irradiated B19 virus, which has inactivated the expression of NS1, still harbors the ability to induce G2 arrest but not G1 arrest. Furthermore, treatment with caffeine, a G2 checkpoint inhibitor, abrogated the B19 virus-induced G2 arrest despite expression of NS1. These results suggest that the B19 virus-induced G2 arrest is not mediated by NS1 expression. We also found that NS1-transfected UT7/Epo-S1 and 293T cells induced cell cycle arrest at the G1 phase. These results indicate that NS1 expression plays a critical role in G1 arrest induced by B19 virus. Furthermore, NS1 expression significantly increased p21/WAF1 expression, a cyclin-dependent kinase inhibitor that induces G1 arrest. Thus, G1 arrest mediated by NS1 may be a prerequisite for the apoptotic damage of erythroid progenitor cells upon B19 virus infection.
Human parvovirus B19 is known to cause several diseases, such as aplastic crisis in patients with chronic hemolytic anemia, erythema infectiosum, persistent infections manifesting as chronic anemia in immunocompromised patients, nonimmune hydrops fetalis leading to intrauterine fetal death, and arthralgia/arthritis (6). The mechanistic basis for these B19 virus-associated diseases is poorly understood; however, most symptoms appear to be related to the unique tissue tropism of B19 virus. Cells permissive for B19 virus replication are proliferating erythroid precursors in human bone marrow and fetal liver tissues, and B19 virus infection to the target cells directly induces cell death (6, 16).
B19 virus is a single-stranded DNA virus that lacks an envelope and has a genome length of 5.4 kb with hairpin structures at each extremity (12). Two major open reading frames (ORFs) extend through almost the entire genome of B19 virus (23). A nonstructural protein (NS1) on the left side of the genome with a molecular mass of 70 to 77 kDa appears as two bands on Westernblot analysis (17). NS1 has been found to be essential for replication of viral DNA, and for the regulation of its own viral promoters (6). NS1 contains a consensus sequence for purine nucleotide binding in the middle part (3). This consensus is predicted to be an ATP- or GTP-binding site, which is associated with ATPase and DNA helicase activities (3). NS1 is also known to be cytotoxic for erythroid cells and is possibly related to the pathogenesis of B19 virus infection (19). Our previous studies demonstrated that NS1 expression induced apoptosis in erythroid linage cells both in vivo and in vitro (7, 15, 30); however, the molecular mechanism by which NS1 mediates apoptosis has not been clarified.
We previously demonstrated that B19 virus infection induces growth inhibition of erythroid cells immediately after infection, with almost all of B19 virus-infected cells arresting at the G2 phase of the cell cycle (17). This was accompanied by an accumulation of mitotic cyclins such as cyclin A and cyclin B (17). In minute virus of mice (MVM), a rodent parvovirus, however, virus-infected cells were shown to fall into G1- and S-phase arrest, as well as G2 arrest, and these multistep cell cycle arrests were induced by expression of NS1 of the MVM (11). In the present study, we show that B19 virus-infected cells also falls into not only G2 arrest but also G1 arrest, and this G1 arrest is mediated by NS1.
MATERIALS AND METHODS
Virus and cells.
UT7/Epo-S1 cells, which are highly susceptible for B19 virus (17), were propagated in RPMI 1640 medium containing 10% fetal calf serum and 2 U of recombinant erythropoietin (Epo; a gift from Kirin Brewery Pharmaceutical Research Laboratory, Tokyo, Japan)/ml. Human serum containing human parvovirus B19 antigen was used as a B19 virus seed, of which the virus titer was calculated by anti-VP1/2 immunostaining assays to be 108 infectious units per ml. UT7/Epo-S1 cells were inoculated with the multiplicity of infection (MOI) of 10- and 20-fold diluted virus seed at 106 cells/ml in Isocove modified Dulbecco medium (IMDM) and incubated for 2 h at 4°C for virus adsorption. The cells were diluted to 2 × 105 cells/ml in IMDM containing 10% fetal calf serum and 2 U of Epo/ml and then cultured at 37°C and 2% CO2 in air.
UV inactivation of B19 virus.
B19 virus was inactivated by spreading 1 ml of 20-fold diluted B19 virus-positive serum in a 3-cm tissue culture dish. The virus was then UV-irradiated in a Stratalinker 1800 (Stratagene, La Jolla, Calif.) for total doses of 5, 10, 25, 50, and 100 J/cm2. NS1 expression was monitored at 24 h postinfection by Western blot analysis.
Plasmid construction and transfection.
Based on the B19 virus genomic sequence (23), DNA containing p6 promoter and NS1 ORF (pMP6-NS1) or only p6 promoter (pMP6), as a control, was amplified from pGEM1-B19 genomic DNA by PCR by using a 5′ primer (5′-TTCCGAATTCGTCACAGGAAATGACGTAATTGT-3′) and a 3′ primer (5′-GCCACTCGAGTTACTCATAATCTACAAAGCTTTGCA-3′) for pMP6-NS1 or 5′-GAAGAAGCTTTCTAAATAGCTCCATGTTAGGAT-3′ for pMP6. The primers used for PCR contain EcoRI and XhoI recognition sequences for subsequent cloning. Amplified DNA was ligated into the EcoRI and XhoI cloning site of the pMX-IRES-GFP vector (14). DNA sequence analysis of the complete ORF confirmed the correct sequence. Transient transfections to 293T cells were performed with FuGene 6 (Roche Diagnostics, Indianapolis, Ind.) and to UT7/Epo-S1 cells were performed with “amaxa” electroporation gene transfer tool (Amaxa GmbH, Berlin, Germany).
Cell cycle analysis.
Cell cycle analyses were performed as described previously (9). In brief, cells were infected with B19 virus or transfected with NS1 expression plasmids and cultured for indicated times. After two washes with phosphate-buffered saline, the cells were suspended in propidium iodide (PI) solution (50 μg of PI/ml, 0.1% sodium citrate, 0.2% NP-40, 0.25 mg of RNase/ml) and incubated for 30 min at 4°C. PI-positive cells at the G0/G1 and G2/M fractions were counted by FACSCan (BD Bioscience, Inc.). To detect G1-arrested cells, the cells were treated with 20 μM paclitaxel, a mitotic inhibitor (Wako Pure Chemical Industries, Ltd.) for 24 h before staining with PI. To induce G1 arrest through DNA damage, cells were irradiated with X-ray by SOFTEX M1005w (SOFTEX Co., Ltd., Tokyo, Japan) at 3.68 Gy/min for 162 s at room temperature under ambient conditions.
Western blotting.
Western blot analyses were carried out as described elsewhere (2). In brief, cells were lysed in an aliquot volume of whole-cell extraction buffer (10 mM NaHPO4, 1 mM EDTA, 1 mM dithiothreitol, 400 mM KCl, 10% glycerol, 5 μg of aprotinin/ml, 10 μg of leupeptin/ml, 2 μM pepstatin, 1 mM phenylmethylsulfonyl fluoride, 5 mM NaF, 1 mM Na3VO4). After freeze and thaw cycles, cell lysates were microcentrifuged at 14,000 rpm for 20 min to remove the cell debris. After 10 μg of proteins from the supernatants were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 10 or 12% polyacrylamide gels, the proteins were transferred onto nitrocellulose membrane by electroblotting for 1.5 h at 40 V in a ATTO semidry blotting system. The membrane was then incubated for 1 h in blocking buffer (Tris-buffered saline solution containing 1% Tween 20 and 5% bovine serum albumin) and further incubated for 3 h at room temperature with the anti-NS1 monoclonal antibody (MAb; ParC-NS1) specific for the NS1 C-terminal half of B19 virus (17), mouse anti-α-tubulin MAb (Sigma Aldrich Fine Chemicals, St. Louis, Mo.), or mouse anti-p21 MAb (Santa Cruz Biotechnology, Santa Cruz, Calif.). Bound antibodies were then proved with horseradish peroxidase-conjugated anti-mouse antibody, washed extensively, and revealed by using a sensitive enhanced chemiluminescence detection system (ECL Detection Kit; Amersham Bioscience Corp., Piscataway, N.J.).
[3H]thymidine incorporation assay.
UT7/Epo-S1 cells were transiently transfected with 5 μg of the pMP6-NS1 or pMP6 plasmid by electroporation. The cells (105 per well) were cultured for 24 h and assayed for [3H]thymidine incorporation for the last 4 h of cultivation as described previously (2).
RESULTS
B19 virus infection induces G1 arrest.
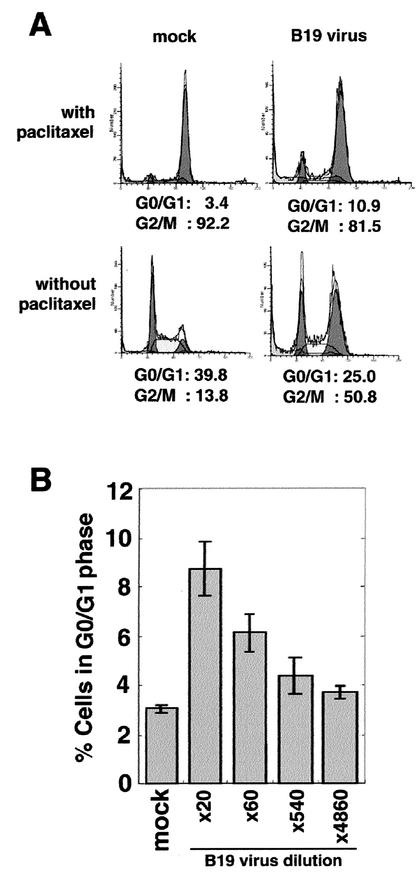
We previously established a highly susceptible cell line, UT7/Epo-S1, for B19 virus infection, in which more than 40% of cells were positive for viral antigens detected with anti-VP1/2 immunostaining at 96 h postinfection (17). B19 virus infection to UT7/Epo-S1 cells induced an increase (from 13.8 to 50.8%) of the cell population at the G2/M cell cycle phase (Fig. 1A, lower panels) as shown previously. However, we were unable to exclude the possibility that a minor cell population of the B19 virus-infected cells arrests at the G1 phase. To examine this possibility, we first established a detection system for virus-induced G1 arrest. Since G1-phase cells are the major cell population (39.8%) in the normal cell culture (Fig. 1A, lower-right panel), we eliminated the normal G1 phase cells in order to detect any minor G1-arrested cells in B19 virus-infected cell cultures. For this purpose, we used a low dose (20 μM) of paclitaxel, a mitotic inhibitor, which induces mitotic arrest of cells in the normal cell cycle by promoting and stabilizing the assembly of microtubules (26). UT7/Epo-S1 cells were treated with paclitaxel at 24 h after B19 virus infection. After a further 24 h cultivation, the cells were stained with PI and analyzed by flow cytometry. Almost all (92.2%) of the mock-infected cells accumulated in the G2/M phase and only 3.4% cells remained in the G0/G1 phase (Fig. 1A, upper-left panel). In the B19 virus-infected cell culture, however, cells in the G0/G1 phase were increased up to 10.9% (Fig. 1A, upper-right panel). These results indicate that B19 virus infection induces not only G2 arrest but also G1 arrest in UT7/Epo-S1 cells. The B19 virus-induced G1 arrest was confirmed to be dependent on the viral dose (Fig. 1B).
FIG. 1.
B19 virus infection induces both G1 and G2 arrests. (A) UT7/Epo-S1 cells were infected with 20-fold-diluted B19 virus or mock infected, and at 24 h postinfection they were left treated or treated with a mitotic inhibitor, paclitaxel, for 24 h. Subsequently, they were stained with PI for detection of DNA content and then loaded onto a fluorescence-activated cell sorting (FACS) caliber apparatus. (B) UT7/Epo-S1 cells were infected with various doses of B19 virus or mock infected and then treated with paclitaxel and PI in a manner similar to that for panel A. The percentages of G0/G1- and G2/M-phase cells were calculated.
NS1 expression is not essential for B19 virus-induced G2 arrest.
Since B19-NS1 has been shown to induce G2 arrest in UT7/Epo cells (24), we further addressed whether NS1 is responsible for the G2 arrest or not. To inactivate the expression of NS1, B19 virus was irradiated with various doses of UV and then infected to UT7/Epo-S1 cells. The cells infected with UV-irradiated B19 virus were treated with paclitaxel to assess the G1 arrest. The induction of G1 arrest was significantly suppressed by the following level of UV irradiation; 100 J of UV irradiation/m2 completely suppressed the B19 virus-induced G1 arrest at the level of mock-infected cells (Fig. 2A). The UV irradiation was confirmed to abolish NS1 expression in a dose-dependent manner (Fig. 2C). These results suggest that the B19 virus-induced G1 arrest requires an intact B19 viral genome. In contrast to the G1 arrest, unexpectedly, the B19 virus-induced G2 arrest was little affected or rather slightly enhanced by UV irradiation of B19 virus (Fig. 2B). Even up to 100 J/m2 of UV irradiation, appreciable G2 arrest still occurred (Fig. 2B). These results suggest that B19 virus lacking the expression of NS1 still harbors an activity to induce G2 arrest in UT7/Epo-S1 cells.
FIG. 2.
UV-irradiated virus can induce G2 arrest but not G1 arrest. B19 viruses in human serum were irradiated with various doses of UV and then infected to UT7/Epo-S1 cells. After 24 h postinfection, the cells were treated (A) or not treated (B) with paclitaxel for 24 h. They were then stained with PI for detection of DNA content and loaded on a FACS caliber. Percentages of the cells at G0/G1 phase (A) and G2/M phase (B) were plotted. A part of the cells treated with paclitaxel in panel B were lysed in whole-cell extraction buffer and separated by SDS-PAGE. (C) After membrane transfer, proteins were detected by anti-NS1 and anti-α-tubulin MAbs.
Caffeine is known to have a pharmacological activity to release DNA damage leading to G2 arrest (5). We then assessed an effect of caffeine on the B19 virus-induced G2 arrest. UT7/Epo-S1 cells were infected with B19 virus or treated with 10 Gy of X-ray irradiation to induce G2 arrests and simultaneously treated with various doses up to 5 mM caffeine. The caffeine treatment significantly decreased the G2/M-phase populations in the cells infected with B19 virus and irradiated with X-rays (Fig. 3A). Although NS1 expression in the B19 virus-infected cells was also suppressed by caffeine treatment (Fig. 3B), an appreciable amount (50%) of NS1 was still detectable in the cells treated with 5 mM caffeine (Fig. 3C). These results suggest that B19 virus induces G2 arrest through a mechanism similar to that of the G2 arrest induced by X-ray-mediated DNA damage. Furthermore, these data support the possibility that the main pathway of B19-induced G2 arrest is not mediated by NS1.
FIG. 3.
G2 checkpoint inhibitor (caffeine) treatment abrogates B19 virus-induced G2 arrest. B19 virus-infected, mock-infected, or X-ray (10 Gy)-irradiated UT7/Epo-S1 cells were treated with or without various doses of caffeine. (A) After 48 h of cultivation, the cells were stained with PI, and the percentages of G2/M-phase cells were measured by a FACS caliber. The remainder cells were lysed in whole-cell extraction buffer and separated by SDS-PAGE. (B) After membrane transfer, proteins were detected by using anti-NS1 and anti-α-tubulin MAbs. (C) Immunoblotted bands of NS1 in panel B were measured with a densitometer, and their densities were represented as relative amounts.
NS1 expression induces G1 cell cycle arrest.
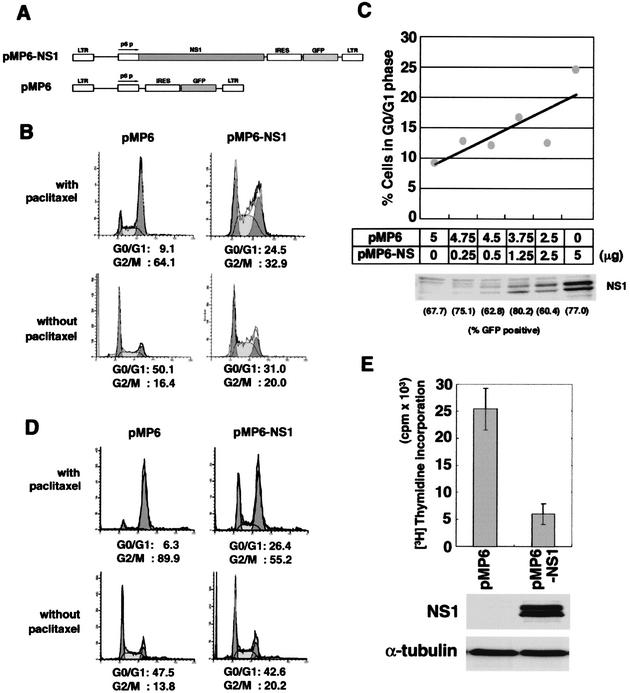
To determine the role of NS1 in G1 arrest, we performed an NS1 transfection assay by using an NS1 expression vector. To determine the transfection efficiency, we connected an internal ribosome entry site (IRES) and a GFP sequence downstream of the NS1 gene (Fig. 4A). After a 24-h transfection of NS1, UT7/Epo-S1 cells were treated with paclitaxel to analyze for G1 cell cycle arrest. Both transfection efficiencies of NS1-GFP and GFP control vectors were calculated as 60 to 80% (data not shown). G0/G1 phase cells were significantly increased from 9.1 to 24.5% upon NS1 transfection in the paclitaxel-treated culture (Fig. 4B, upper panels). However, cells at the G2/M-phase slightly increased from 16.4 to 20% upon NS1 transfection in the paclitaxel-nontreated culture (Fig. 4B, bottom panels). These results indicate that NS1 expression induces G1 arrest, and NS1 alone is not sufficient to induce G2 arrest. The NS1-induced G1 arrest was confirmed to be dependent on NS1 plasmid dose (Fig. 4C). Similar results were obtained by using 293T cells (Fig. 4D). 293T is a human embryonic kidney cell line transformed by both adenovirus E1 and simian virus 40 large T antigen (18). These viral oncogene products are able to inactivate both p53 and Rb suppressor oncogene products, which are known to be negative regulators of cell cycle progression (8). Hence, the NS1-mediated G1 arrest is thought to occur via a p53- and Rb-independent pathway. We also confirmed that NS1 transfection suppresses the UT7/Epo-S1 cell growth as measured by [3H]thymidine incorporation (Fig. 4E).
FIG. 4.
NS1 expression induces cell cycle arrest at the G1 phase. (A) Schematic structure of NS1 expressing and control vector constructs. UT7/Epo-S1 (B) or 293T cells (D) were transfected with pMP6 (left panels) or pMP6-NS1 (right panels) and incubated for 24 h. They were then treated (upper panels) or untreated (lower panels) with a mitotic inhibitor, paclitaxel, for 24 h. Subsequently, they were stained with PI for detection of DNA content and loaded onto a FACS caliber. (C) The percentages of UT7/Epo-S1 cells at the G1 phase were plotted after transfection of pMP6 and pMP6-NS1 plasmids at various ratios, and their NS1 expression levels were detected by Western blotting. (E) The transfected UT7/Epo-S1 cells with pMP6 or pMP6-NS1 plasmids were assayed for [3H]thymidine incorporation (upper panel). UT7/Epo-S1 cells were transiently transfected with 5 μg of the pMP6-NS1 or pMP6 plasmid by electroporation, and then the cells (105 per well) were cultured for 24 h and assayed for [3H]thymidine incorporation for the last 4 h of cultivation. The expression levels of NS1 and α-tubulin were detected by Western blotting with their specific antibodies (lower panels).
NS1 expression induces upregulation of p21/WAF1.
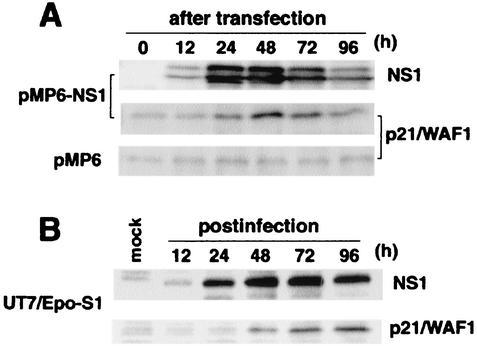
We assessed the effect of NS1 expression on several cellular proteins contributing to G1-S transition, such as p21/WAF1, p27/Kip1, Cdk2, Cdk4, Cdk6, cyclin D1, and cyclin D3. 293T cells were transfected with NS1, treated with paclitaxel, and then assayed for expression of these cellular proteins by Western blots. Expression of NS1 became detectable after 12 h and peaked at 24 h after NS1 transfection, and expression levels of p21/WAF1, a cyclin-dependent kinase inhibitor, was detected at 24 h and peaked at 48 h after NS1 transfection (Fig. 5A). The expression levels of the other cellular proteins were not significantly changed (data not shown). Similar results were obtained with B19 virus-infected UT7/Epo-S1 cells. NS1 expression was detected from 12 h postinfection and peaked at 48 to 72 h postinfection in B19 virus-infected UT7/Epo-S1 cells, and the expression level of p21/WAF1 was also upregulated from 24 h postinfection and reached a plateau at between 72 and 96 h postinfection (Fig. 5B). These results suggest that NS1 contributes to accumulation of p21/WAF1, which may result in G1 arrest.
FIG. 5.
Both B19 virus infection and NS11 transfection induce expression of p21/WAF1. Expression levels of p21/WAF1 and NS1 were analyzed sequentially in 293T cells transfected with pMP6 or pMP6-NS1 plasmids (A) and in UT7/Epo-S1 cells infected with B19 virus (B) by Western blotting with anti-NS1 and anti-p21/WAF1 MAbs, respectively.
DISCUSSION
We previously reported that B19 virus infection induces cell cycle arrest at the G2 phase in UT7/Epo-S1, an erythroid cell line (17). Upon further investigation of the mechanism of B19 virus-induced G2 arrest, we found that B19 virus has the ability to induce not only G2 arrest but also G1 arrest. We utilized a low dose of paclitaxel to detect G1 arrest induced by B19 virus infection. The present study demonstrated that the B19 virus-induced G1 arrest requires expression of NS1, a nonstructural viral protein of the B19 virus. We also show that the B19 virus-induced G2 arrest is independent of NS1 expression. We and others have shown that NS1 expression induces cell death with apoptotic features in erythroid cells in vivo and in vitro, suggesting that NS1 is a viral apoptotic factor (7, 15, 19, 24). However, there was an obvious time lag between NS1 expression and the appearance of apoptotic features in B19 virus-infected UT7/Epo-S1 cells; NS1 expression dramatically increased by 48 h of infection and then gradually decreased, whereas apoptotic cells gradually increased up to at least day 6 of postinfection (17). These observations suggest that NS1 is not directly involved in the regulatory pathway for apoptosis induction. Since we here revealed that NS1 induces G1 arrest, it is possible that the B19-induced apoptosis may be mediated by NS1 through induction of G1 arrest.
CDK inhibitor p21/WAF1, a negative regulator for G1-S transition in the cell cycle, was detected by immunostaining giant proerythroblasts in bone marrow derived from patients with pure red cell aplasia containing B19 viral DNA and/or immunoglobulin M (IgM) antibodies in their sera (22), suggesting that B19 virus infection is possibly involved in p21/WAF1 expression. In the present study, we demonstrated that the expression of p21/WAF1 is upregulated in NS1-transfected cells, as well as B19 virus-infected cells. These results suggest that NS1 of B19 virus has ability to promote expression of p21/WAF1, which should result in G1 arrest. In the case of MVM, a rodent parvovirus, p21/WAF1 was also upregulated in cells expressing MVM NS1 (11). Although MVM is known to induce cell cycle arrests at the G1, S, and G2 phases, MVM did not induce G1 arrest in p21/WAF1-null cells (11). These observations suggest the presence of a common mechanism for the G1 arrests between cells infected with B19 virus and MVM. We propose that the NS1-mediated p21/WAF1-inducing pathway is responsible. p21/WAF1 was originally identified as a mediator of p53-induced growth arrest (13), and p53 is known to be directly involved in transcriptional activation of p21/WAF1. However, since p21/WAF1 was reportedly induced even in p53-deficient cells, there may be a p53-independent pathway for transcriptional activation of p21/WAF1 (21). In fact, we detected here the induction of p21/WAF1 in NS1-transfected 293T cells, in which p53 is inactivated by simian virus 40 large T and adenovirus E1 proteins (8, 18). These observations suggest that parvoviral NS1 is involved in upregulation of p21/WAF1 transcription via the p53-independent pathway.
An interesting study recently reported that an inactivated adeno-associated virus (AAV) induced p53 expression, whereas empty viral capsids did not (20). Furthermore, terminal hairpin DNAs of AAV genome were shown to induce apoptosis in p53-deficient cells by microinjection techniques (20). In that report, AAV was also shown to induce G2 arrest in both p53+/+ and p53−/− cells (20). Although the mechanism of p53-independent apoptosis induction is still obscure, it was hypothesized that nonreplicating viral DNA by itself, with hairpin structures at both ends, can elicit a DNA damage response, leading to apoptosis and G2 arrest (20). Our data are consistent at least with the induction of G2 arrest by AAV. While UV irradiation extremely reduced the infectivity of B19 virus and suppressed the expression of NS1, the capacity to induce G2 arrest was not significantly different between the nonirradiated and irradiated B19 viruses (Fig. 2). These results suggest that the G2 arrest after B19 virus infection is caused by the entry of viral single-stranded DNA with terminal hairpin structures and not by the expression of viral genes that include NS1. Furthermore, when the B19 virus-infected UT7/Epo-S1 cells were treated with caffeine, an inhibitor for a DNA damage response, the virus-induced G2 arrest was abrogated, despite significant expression of NS1 in these caffeine-treated cells. These results support the hypothesis that hairpin DNA plays an important role in the induction of G2 arrest, and NS1 expression is dispensable for it. In the B19 virus-infected UT7/Epo-S1 cell, >50% cells were arrested at G2 phase (Fig. 1A, lower-right) (17), whereas only 7% cells were arrested at G1 phase (Fig. 1A, upper right). This difference in the cell cycle population may be explained by the sequential induction of cell cycle arrest. Initially, the hairpin DNA induces G2 arrest prior to viral gene expression immediately after B19 virus infection, and then NS1 expression may induce G1 arrest in a small population of cells that have escaped from G2 arrest.
Our present study suggests that the signal cascade for cellular DNA repair checkpoint plays a critical role in the B19 virus-induced G2 arrest. According to our current understanding of the G2 checkpoint signal, DNA damage activates ATM (ataxia telangiectasia mutated) and ATR (ATM- and Rad3-related) members of the phosphoinositide kinase family (1). The activation of ATM and ATR induces activations of their downstream protein kinases, Chk1 and Chk2, which are able to phosphorylate cdc25C on serine 216 residue (10). This phosphorylation is thought to suppress the phosphatase activity of cdc25C and to promote the association of cdc25C with 14-3-3 proteins, which result in inactivation of cdc2 (25). Chk1 and Chk2 also phosphorylate and activate Wee1, a kinase that catalyzes cdc2 to be inactivated (10). Caffeine is known to have an in vitro inhibitory activity for ATM and ATR protein kinases (5). This agent is thus capable of releasing the G2 arrest in B19 virus-infected cells, as well as X-ray-irradiated cells (Fig. 3), even as the expression levels of ATM and ATR of B19 virus or mock-infected UT7/Epo-S1 cells are not significantly different (data not shown). Similar results were shown in AAV-infected cells; AAV induced the G2 arrest in the ATM (+/+) cells but not in the ATM (−/−) cells, and caffeine treatment abrogated the G2 arrest induced by AAV (20). These data suggest that ATM and/or ATR kinase activities were indispensable for the induction of G2 arrest in cells infected with B19 virus as well as AAV. ATM is known to directly phosphorylate p53 at serine 15 residue (10), and the serine 15 phosphorylation of p53 was upregulated in B19 virus-infected UT7/Epo-S1 cells (data not shown), suggesting the possibility that the ATM kinase is activated by B19 virus infection.
We previously suggested that B19-induced G2 arrest is mediated by impairment of nuclear translocation of cdc2-cyclin B1 complexes (17). There is accumulating evidence that translocation of cdc2-cyclin B1 complexes to the nucleus is regulated by phosphorylation of the serine residue in the middle of nuclear export signal (NES) sequence of cyclin B1 (27). Polo-like kinase 1(Plk1), which is synthesized and activated during G2/M phase, was identified as a protein kinase that directly phosphorylates a serine residue (S147) in the NES of cyclin B1 and targets it to the nucleus during prophase in vertebrate cells (28). Furthermore, Plk1 was previously shown to be inactivated in cells arrested at G2 phase upon DNA damage (29). The inhibition of Plk1 was efficiently blocked by treatment with caffeine, and the Plk1 kinase activity was not inhibited in the ATM-null (−/−) cells by DNA damage (29), indicating that Plk1, which controls the nuclear localization of cdc2-cyclin B1, acts downstream of ATM and ATR kinases. Hence, the cytoplasmic accumulation of cdc2-cyclin B1 complexes in the B19 virus-infected cells may be mediated through the activation of ATM and ATR kinases.
As described above, it is clear that B19 virus NS1 expression is not essential for the induction of G2 arrest. However, NS1 has been reported to enhance G2 arrest (24). In the present study, we also detected a slight increase of the G2-arrested cell population in NS1-transfected cell culture (Fig. 4). These observations suggest the possibility that NS1 contributes to induction of multistep cell cycle arrests that include G1 and G2 arrests.
We here provide evidence for the molecular mechanisms of B19 virus-induced cell cycle arrests. Little is still known about the cascade from the cell cycle arrest to apoptosis upon B19 virus infection. Analysis of NS1 and other viral factors mediating apoptosis induction will give us much information to understand the pathological consequence of B19 virus infection in human.
Acknowledgments
We thank L. C. Ndhlovu for critically reviewing the manuscript. We thank Toshio Kitamura, Institute of Medical Science, The University of Tokyo, Tokyo, Japan, for providing pMX-IRES-GFP. We gratefully acknowledge the gift of erythropoietin from Kirin Brewery Pharmaceutical Research Laboratory, Tokyo, Japan.
REFERENCES
- 1.Abraham, R. T. 2001. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes. Dev. 15:2177-2196. [DOI] [PubMed] [Google Scholar]
- 2.Asao, H., Y. Sasaki, T. Arita, N. Tanaka, K. Endo, H. Kasai, T. Takeshita, Y. Endo, T. Fujita, and K. Sugamura. 1997. Hrs is associated with STAM, a signal-transducing adaptor molecule: its suppressive effect on cytokine-induced cell growth. J. Biol. Chem. 52:32785-32791. [DOI] [PubMed] [Google Scholar]
- 3.Astell, C. R., C. D. Mol, and W. F. Anderson. 1987. Structural and functional homology of parvovirus and papovavirus polypeptides. J. Gen. Virol. 68:885-893. [DOI] [PubMed] [Google Scholar]
- 4.Ball, K. L. 1997. p21: structure and functions associated with cyclin-CDK binding. Prog. Cell Cycle Res. 3:125-134. [DOI] [PubMed] [Google Scholar]
- 5.Blasina, A., B. D. Price, G. A. Turenne, and C. H. McGowan. 1999. Caffeine inhibits the checkpoint kinase ATM. Curr. Biol. 9:1135-1138. [DOI] [PubMed] [Google Scholar]
- 6.Brown, K. E., N. S. Young, and J. M. Liu. 1994. Molecular, cellular and clinical aspects of parvovirus B19 infection. Crit. Rev. Oncol. Hematol. 16:1-31. [DOI] [PubMed] [Google Scholar]
- 7.Chisaka, H., E. Morita, K. Murata, N. Ishii, N. Yaegashi, K. Okamura, and K. Sugamura. 2002. A transgenic mouse model for non-immune hydrops fetalis induced by the NS1 gene of human parvovirus B19. J. Gen. Virol. 83:273-281. [DOI] [PubMed] [Google Scholar]
- 8.Clertant, P., and I. Seif. 1984. A common function for polyoma virus large-T and papillomavirus E1 proteins? Nature 311:276-279. [DOI] [PubMed] [Google Scholar]
- 9.Darzynkiewicz, Z., S. Bruno, G. Del Bino, W. Gorczyca, M. A. Hotz, P. Lassota, and F. Traganos. 1992. Features of apoptotic cells measured by flow cytometry. Cytometry 13:795-808. [DOI] [PubMed] [Google Scholar]
- 10.Dasika, G. K., S. C. Lin, S. Zhao, P. Sung, A. Tomkinson, and E. Y. Lee. 1999. DNA damage-induced cell cycle checkpoints and DNA strand break repair in development and tumorigenesis. Oncogene 18:7883-7899. [DOI] [PubMed] [Google Scholar]
- 11.De Beeck, A. O., J. Sobczak-Thepot, H. Sirma, F. Bourgain, C. Brechot, and P. Caillet-Fauquet. 2001. NS1- and minute virus of mice-induced cell cycle arrest: involvement of p53 and p21cip1. J. Virol. 75:11071-11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deiss, V., J. D. Tratschin, M. Weitz, and G. Siegl. 1990. Cloning of the human parvovirus B19 genome and structural analysis of its palindromic termini. Virology 175:247-254. [DOI] [PubMed] [Google Scholar]
- 13.el-Deiry, W. S., T. Tokino, V. E. Velculescu, D. B. Levy, R. Parsons, J. M. Trent, D. Lin, W. E. Mercer, K. W. Kinzler, and B. Vogelstein. 1993. WAF1, a potential mediator of p53 tumor suppression. Cell 75:817-825. [DOI] [PubMed] [Google Scholar]
- 14.Hozumi, K., R. Ohtsuka, D. Suzuki, K. Ando, M. Ito, T. Nishimura, M. Merkenschlager, and S. Habu. 2000. Establishment of efficient reaggregation culture system for gene transfection into immature T cells by retroviral vectors. Immunol. Lett. 71:61-66. [DOI] [PubMed] [Google Scholar]
- 15.Moffatt, S., N. Yaegashi, K. Tada, N. Tanaka, and K. Sugamura. 1998. Human parvovirus B19 nonstructural (NS1) protein induces apoptosis in erythroid lineage cells. J. Virol. 72:3018-3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morita, E., and K. Sugamura. 2002.. Human parvovirus B19-induced cell cycle arrest and apoptosis. Semin. Immunopathol. 24:187-199. [DOI] [PubMed]
- 17.Morita, E., K. Tada, H. Chisaka, H. Asao, H. Sato, N. Yaegashi, and K. Sugamura. 2001. Human parvovirus B19 induces cell cycle arrest at G2 phase with accumulation of mitotic cyclins. J. Virol. 75:7555-7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Numa, F., K. Hirabayashi, N. Tsunaga, H. Kato, K. Rourke, H. Shao, C. Stechmann-Lebakken, J. Varani, A. Rapraeger, and V. M. Dixit. 1995. Elevated levels of syndecan-1 expression confer potent serum-dependent growth in human 293T cells. Cancer Res. 55:4676-4680. [PubMed] [Google Scholar]
- 19.Ozawa, J. K., Ayub, S. Kajigaya, T. Shimada, and N. Young. 1988. The gene encoding the nonstructural protein of B19 (human) parvovirus may be lethal in transfected cells. J. Virol. 62:2884-2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raj, K., P. Ogston, and P. Beard. 2001. Virus-mediated killing of cells that lack p53 activity. Nature 412:914-917. [DOI] [PubMed] [Google Scholar]
- 21.Rowan, S., R. L. Ludwig, Y. Haupt, S. Bates, X. Lu, M. Oren, and K. H. Vousden. 1996. Specific loss of apoptotic but not cell-cycle arrest function in a human tumor derived p53 mutant. EMBO J. 15:827-838. [PMC free article] [PubMed] [Google Scholar]
- 22.Sadahira, Y., T. Sugihara, and Y. Yawata. 2001. Expression of p53 and Ki-67 antigen in bone marrow giant proerythroblasts associated with human parvovirus B19 infection. Int. J. Hematol. 74:147-152. [DOI] [PubMed] [Google Scholar]
- 23.Shade, R. O., M. C. Blundell, S. F. Cotmore, P. Tattersall, and C. R. Astell. 1986. Nucleotide sequence and genome organization of human parvovirus B19 isolated from the serum of a child during aplastic crisis. J. Virol. 58:921-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sol, N., J. Le Junter, I. Vassias, J. M. Freyssinier, A. Thomas, A. F. Prigent, B. B. Rudkin, S. Fichelson, and F. Morinet. 1999. Possible interactions between the NS-1 protein and tumor necrosis factor alpha pathways in erythroid cell apoptosis induced by human parvovirus B19. J. Virol. 73:8762-8770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor, W. R., and G. R. Stark. 2001. Regulation of the G2/M transition by p53. Oncogene 20:1803-1815. [DOI] [PubMed] [Google Scholar]
- 26.Thompson, W. C., L. Wilson, and D. L. Purich. 1981. Taxol induces microtubule assembly at low temperature. Cell Motil. 1:445-454. [DOI] [PubMed] [Google Scholar]
- 27.Toyoshima, F., T. Moriguchi, A. Wada, M. Fukuda, and E. Nishida. 1998. Nuclear export of cyclin B1 and its possible role in the DNA damage-induced G2 checkpoint. EMBO J. 17:2728-2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toyoshima-Morimoto, F., E. Taniguchi, N. Shinya, A. Iwamatsu, and E. Nishida. 2001. Polo-like kinase 1 phosphorylates cyclin B1 and targets it to the nucleus during prophase. Nature 410:215-220. [DOI] [PubMed] [Google Scholar]
- 29.van Vugt, M. A., V. A. Smits, R. Klompmaker, and R. H. Medema. 2001. Inhibition of Polo-like kinase-1 by DNA damage occurs in an ATM- or ATR-dependent fashion. J. Biol. Chem. 276:41656-41660. [DOI] [PubMed] [Google Scholar]
- 30.Yaegashi, N., T. Niinuma, H. Chisaka, S. Uehara, K. S. Moffatt, Tada, M. Iwabuchi, Y. Matsunaga, M. Nakayama, C. Yutani, Y. Osamura, E. Hirayama, K. Okamura, K. Sugamura, and A. Yajima. 1999. Parvovirus B19 infection induces apoptosis of erythroid cells in vitro and in vivo. J. Infect. 39:68-76. [DOI] [PubMed] [Google Scholar]