Abstract
Adaptive immune responses of γδ T cells during active mycobacterial coinfection of human immunodeficiency virus-infected humans have not been studied. Macaques infected with the simian immunodeficiency virus (SIV) SIVmac were employed to determine the extent to which a coincident AIDS virus infection might compromise immune responses of mycobacterium-specific Vγ2Vδ2+ T cells during active mycobacterial infection. Control SIVmac-negative macaques developed primary and recall expansions of phosphoantigen-specific Vγ2Vδ2+ T cells after Mycobacterium bovis BCG infection and BCG reinfection, respectively. In contrast, SIVmac-infected macaques did not exhibit sound primary and recall expansions of Vγ2Vδ2+ T cells in the blood and pulmonary alveoli following BCG infection and reinfection. The absence of adaptive Vγ2Vδ2+ T-cell responses was associated with profound CD4+ T-cell deficiency and subsequent development of SIVmac-related tuberculosis-like disease in the coinfected monkeys. Consistently, Vγ2Vδ2+ T cells from coinfected monkeys displayed a reduced capacity to expand in vitro following stimulation with phosphoantigen. The reduced ability of Vγ2Vδ2+ peripheral blood lymphocytes (PBL) to expand could be restored to some extent by coculture of these cells with CD4+ T cells purified from PBL of SIV-negative monkeys. Furthermore, naïve monkeys inoculated simultaneously with SIVmac and BCG were unable to sustain expansion of Vγ2Vδ2+ T cells at the time that the coinfected monkeys developed lymphoid depletion and a fatal tuberculosis-like disease. Nevertheless, no deletion in Vδ2 T-cell receptor repertoire was identified in SIVmac-BCG-coinfected macaques, implicating an SIVmac-induced down-regulation rather than a clonal exhaustion of these cells. Thus, an SIVmac-induced compromise of the adaptive Vγ2Vδ2+ T-cell responses may contribute to the immunopathogenesis of the SIV-related tuberculosis-like disease in macaques.
Vγ2Vδ2+ T cells comprise the majority of circulating human γδ T cells and may contribute to innate and acquired immunity to microbial infections. It has been shown previously that human Vγ2Vδ2+ T cells recognize small organic phosphate antigens (9, 13, 31, 34, 41, 42) from microbes and other nonpeptide molecules such as alkylamines and aminobisphosphonates (8, 26). Moreover, expansions of γδ T cells have been reported for Mycobacterium tuberculosis and numerous other microbial infections (3, 5, 10, 15, 21-24, 35-37, 39, 40), suggesting that human γδ T cells participate in immune regulation and host defense. The broad recognition of various nonpeptide antigens by Vγ2Vδ2+ T cells and their capacity to expand in microbial infections also imply that these cells may play a role in bridging innate and acquired immunity to infections.
Recent studies of human immunodeficiency virus type 1 (HIV-1)-infected humans suggest that HIV infection can impact on γδ T-cell subset distribution (1, 2, 6, 14, 33). While a decrease in the representation of circulating Vδ2+ T cells during HIV-1 infection has been reported, an increase in Vδ1+ T cells has also been described for some HIV-1-infected individuals (1, 6, 14, 19, 33). Furthermore, in vitro studies have shown that HIV infection can inhibit the ability of Vγ2Vδ2+ T cells to proliferate or produce Th1 cytokines following stimulation with M. tuberculosis or nonpeptide antigens (20, 29, 30, 33, 45). Nevertheless, little is known about whether HIV-infected humans are able to mount phosphoantigen-specific Vγ2Vδ2+ T-cell immune responses during primary and reactivation tuberculosis. The absence of information regarding the development of Vγ2Vδ2+ T-cell responses in HIV-M. tuberculosis-coinfected persons can be attributed, at least in part, to the difficulty in recruiting the coinfected patients for sampling during active M. tuberculosis infections. It is important to elucidate the contribution of Vγ2Vδ2+ T cells to host immunity against mycobacterial infections and the defect of Vγ2Vδ2+ T-cell responses in the setting of AIDS virus infections.
We have recently demonstrated that Mycobacterium bovis BCG and M. tuberculosis infections can induce adaptive immune responses of phosphoantigen-specific Vγ2Vδ2+ T cells in normal macaques. The major expansion of Vγ2Vδ2+ T cells is temporally associated with resolution of active BCG infection as well as with protection against acutely fatal tuberculosis in BCG-vaccinated monkeys (39). The identification of these Vγ2Vδ2+ T-cell immune responses in macaques makes it possible to directly examine the impact of AIDS virus infection on the development of γδ T-cell responses during mycobacterial coinfection. We therefore made use of macaques infected with the simian immunodeficiency virus SIVmac to determine the extent to which adaptive immune responses of Vγ2Vδ2+ T cells are compromised during active mycobacterial coinfection of SIVmac-infected monkeys.
MATERIALS AND METHODS
Animals and virus.
Rhesus (Macaca mulatta) and pigtailed (Macaca nemestrina) macaques, 2 to 5 years of age, were used in these studies. These animals were maintained in accordance with the guidelines of the Committee on Animals for Harvard Medical School and the Guide for the Care and Use of Laboratory Animals (30a). For SIVmac infection, macaques were inoculated intravenously with 106 50% tissue culture infective doses of SIVmac strain 251 (SIVmac251), as described previously (11).
M. bovis BCG infection.
Macaques were infected with M. bovis BCG (Pasteur strain), as previously described (39). BCG was stored in liquid nitrogen and thawed immediately before inoculation. Three settings of BCG coinfection were established to study the immune responses of Vγ2Vδ2+ T cells in SIVmac-infected macaques. (i) Macaques were infected with SIVmac251 for 2 to 16 months and then inoculated with BCG. These groups of macaques were used for evaluating the primary immune response of BCG-specific Vγ2Vδ2+ T cells in the setting of chronic SIVmac infection. All macaques developed progressive BCG infection and died from SIVmac-related BCG disease 1 to 7 months after BCG coinfection. (ii) Naïve macaques were infected simultaneously with SIVmac and BCG to examine the impact of acute SIVmac infection on Vγ2Vδ2+ T cells in response to BCG coinfection. These monkeys died from SIV-related BCG disease within 2 months of SIVmac-BCG coinfection. (iii) Macaques were infected sequentially with BCG, followed by SIVmac, and finally BCG again (BCG → SIVmac → BCG) at 2-month intervals. This group of SIVmac-infected macaques was used to examine the memory immune responses of Vγ2Vδ2+ T cells in the setting of BCG reinfection. Due to an early stage of SIVmac infection and a high level of SIV RNA in plasma, these SIVmac-BCG-coinfected monkeys developed fatal SIV-related BCG disease 1 to 2 months after BCG reinfection. As controls, healthy, SIVmac-negative macaques were similarly inoculated with BCG to study the primary and memory responses of Vγ2Vδ2+ T cells in the settings of BCG infection and reinfection. To optimally examine immune responses of Vγ2Vδ2+ T cells, systemic BCG infection was introduced by intravenous inoculation. BCG coinfection of chronically SIVmac-infected monkeys was introduced by intravenous inoculation with 106 CFU of BCG, whereas BCG reinfection of SIVmac-infected monkeys and simultaneous SIVmac-BCG coinfection were introduced by intravenous inoculation with 108 CFU of BCG.
Isolation of lymphocyte populations from blood and pulmonary alveoli.
Peripheral blood lymphocytes (PBL) were isolated from EDTA-anticoagulated blood of the monkeys by Ficoll-diatrizoate gradient centrifugation. Lymphocytes in pulmonary alveoli were obtained from bronchoalveolar lavage (BAL) fluid. BAL was done with a pediatric bronchoscope. A total of 50 ml of sterile saline solution was instilled and recovered through the biopsy channel of the scope. The recovery rate of saline solution was about 75 to 80%. The lymphocytes in BAL fluid were collected by Ficoll-diatrizoate gradient centrifugation. Total numbers of viable lymphocytes in BAL fluid were counted under a microscope by using trypan blue staining to exclude dead cells.
Monoclonal antibodies (MAbs) and flow cytometric analysis.
The γδ T-cell receptor (TCR) repertoire of the monkeys was analyzed by whole-blood staining, as described previously (12). Anti-human γδ MAbs that cross-react with the corresponding macaque γδ+ T cells were used (12). The MAbs used were as follows: pan-anti-TCR Cδ (anti-TCRδ1); anti-human Vγ1.2, -1.3, and -1.4 (23D12); anti-human Vγ2 (7A5); anti-human Vδ1Jδ1/Jδ2 (Ts8); anti-human Vδ2 (15D); and anti-human Vδ3 (P11.5B) (all from Endogen, Woburn, Mass.). Fluorescein isothiocyanate (FITC)-conjugated anti-human CD3 (PharMingen, San Diego, Calif.) was used for costaining. Two-color staining was done as follows: Vδ1-CD3, Vδ2-CD3, Vδ3-CD3, Vγ1-CD3, Vγ2-CD3, Cδ-CD3, and Vγ2-Vδ2. PBL or cells from tissue compartments were incubated first with one of the γδ TCR MAbs. After washing, each aliquot of cells was subjected to dual staining with phycoerythrin (PE)-conjugated anti-mouse immunoglobulin G, as the secondary antibody, and FITC-conjugated anti-CD3 to identify CD3+ γδ+ T cells. To stain Vγ2Vδ2+ T cells, cells were incubated with anti-Vδ2 followed by FITC-conjugated anti-Vγ2 MAb. Two-color flow cytometric analyses were performed on an XL flow cytometer (Coulter, Hialeah, Fla.). Lymphocytes were gated by means of forward and side scatters, and up to 20,000 gated cells were analyzed. The frequency of γδ+ T cells was determined as the percentage of CD3+ T cells. Absolute numbers of Vγ+ or Vδ+ cells in the blood were calculated based on the flow data and complete blood count analyses. Complete blood counts were performed on a hematology analyzer, the Coulter T 540.
In vitro expansion of Vγ2Vδ2+ T cells in response to phosphoantigen stimulation.
The in vitro experiments were done as previously described (39). PBL obtained from macaques were stimulated in culture medium with or without partially purified prenyl pyrophosphate (Ag1) from Mycobacterium fortuitum supernatant or nonpeptidic BCG extract depleted of proteins and lipids (4, 39). On day 3, interleukin 2 (IL-2) was added, and on days 10 to 14, the cells were counted and analyzed by flow cytometry with γδ TCR-specific MAbs as described above. A 1:1,000 dilution of M. fortuitum supernatant and a 1:500 dilution of aqueous BCG extract were used for in vitro stimulation. In the reconstitution experiments, allogeneic CD4+ T cells were mixed at a 1:5 ratio with PBL obtained from SIVmac-infected monkeys. Allogeneic CD4+ T cells were purified from PBL of SIVmac-negative BCG-vaccinated monkeys by using CD4-detached immunomagnetic beads (35). As a control, a B-cell line generated from monkey PBL was used in the mixed culture. In our control experiments, we did not see any significant expansions of Vγ2Vδ2+ T cells in the culture containing allogeneic CD4+ T cells alone without BCG phosphoantigens. In addition, we saw no significant differences in numbers of Vγ2Vδ2+ T cells between the cocultures containing allogeneic CD4+ T cells from BCG-vaccinated and naïve monkeys. Furthermore, the increased frequency of Vγ2Vδ2+ T cells in CD3+ T cells appeared to represent expansion of these cells rather than a contraction of other subsets, since the stimulation with BCG phosphoantigen resulted in increased numbers of both total lymphocytes and Vγ2Vδ2+ T cells. On the other hand, the culture without BCG phosphoantigens did not lead to an increase in total cultured cells or a relative increase in Vγ2Vδ2+ T cells.
Statuses of CD4+ T-cell counts, BCG burdens, and SIVmac loads.
The clinical parameters for the present studies were evaluated as previously described (38). CD4+ T-cell populations in PBL, lymph nodes, and spleens were assessed by three-color cytometric analyses with FITC-conjugated anti-rhesus monkey CD3 (FN18; Biosource, Camarillo, Calif.), PE-conjugated anti-human CD4 (Ortho Diagnostic Systems, Raritan, N.J.), and PE-Cy5-conjugated anti-human CD8 (Dako Corporation, Carpinteria, Calif.). CD4+ PBL counts were calculated based on the results of complete blood counts and immune flow cytometry data showing the percentages of CD4+ PBL. Peripheral lymph nodes were obtained by standard biopsy procedures before and after BCG inoculation. Spleens were collected at the time that the coinfected animals died from SIV-related BCG disease. Lymph nodes and spleens were carefully teased to generate single-cell suspensions. Quantitation of BCG infection was done by measuring colony counts (38). Quantitative measurement of plasma SIV RNA was done as previously described (35).
TCR CDR3 profiles of Vδ2+ T cells.
CDR3 profiles were analyzed by Genescan-based spectrotyping as previously described (46). cDNAs were amplified by PCR for expression of the Vδ2 family gene by using a Vδ2-specific primer (5′-GGGGACCCTGCCACCCTCAAGTGC-3′) and a Cδ-specific primer (5′-CTTGGGGTAGAAGTCCTTCAC-3′). A second round of PCR was performed with a nested Vδ2 primer (5′-ATGAAAGGAGAAGCAATCAGTAAC-3′) and a Cδ primer (5′-CAGACAAGCAACATTTGTCCC-3′). The internal Cδ primer was labeled at its 5′ end with the Fam fluorophore (Applied Biosystems, Foster City, Calif.), designed as previously described (46). The first- and second-round PCR mixtures were amplified for 35 and 15 cycles, respectively, with the following conditions: 95°C for 30 s, 55°C for 30 s, and 72°C for 30 s. One microliter of each reaction product was mixed with deionized formamide and a ROCK-500 size standard and then electrophoresed on a 5% acrylamide gel on a 310 DNA sequencer (Applied Biosystems). Data were analyzed for size and fluorescence intensity with the Genescan software. These lengths were expressed as predicted numbers of amino acids.
Statistical analysis.
The Student t test and nonparametric methods, as described previously (39), were employed to examine differences in expansion of Vγ2Vδ2+ T cells between SIVmac-infected and control macaques after BCG infection and reinfection.
RESULTS
SIVmac-infected macaques were unable to develop a sound primary Vγ2Vδ2+ T-cell response during the active phase of BCG coinfection.
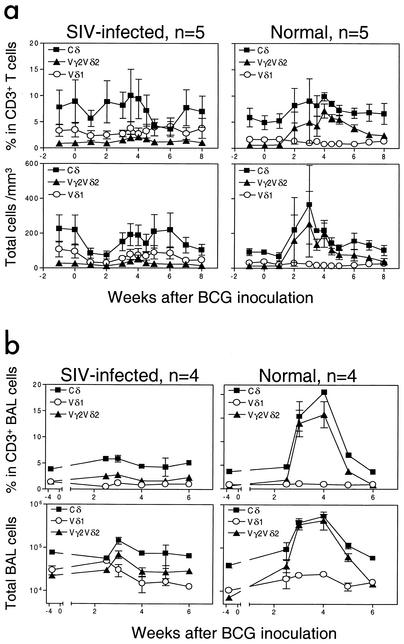
To examine the impact of AIDS virus infection on γδ T-cell response, SIVmac-infected macaques were infected with BCG and assessed for the development of Vγ2Vδ2+ T-cell immune responses. As controls, healthy macaques not infected with SIVmac were similarly infected with BCG. The control macaques showed a marked expansion of circulating Vγ2Vδ2+ T cells following BCG inoculation (Fig. 1). In contrast, the SIVmac-infected macaques did not develop a detectable expansion of those cells after BCG coinfection (Fig. 1). No significant increase in the percentage or absolute number of Vγ2Vδ2+ T cells was identified in the blood of the SIVmac-infected macaques (Fig. 1a).
FIG. 1.
BCG coinfection of SIVmac-infected rhesus macaques did not induce a primary immune response of Vγ2Vδ2+ T cells. (a) Changes in the percentages (upper panels) and absolute numbers (lower panels) of CD3+ T cells that are Vδ1+ or Vγ2Vδ2+ in the blood after BCG inoculation. Data shown are the means and standard errors of the means of values from five SIVmac-infected and five control monkeys. (b) Changes in the percentages and absolute numbers of CD3+ T cells that are Vδ1+ or Vγ2Vδ2+ in BAL fluid after BCG inoculation. P values were <0.001 for the statistical analyses of differences in numbers of circulating or pulmonary Vγ2Vδ2+ T cells between the SIVmac-infected and control groups at the time points of 3 to 5 weeks after BCG infection.
Since mycobacterium-mediated accumulation of Vγ2Vδ2+ T cells occurs in the lung (39), we also sought to determine if SIVmac infection could down-regulate the pulmonary response of these cells during the acute stage of BCG coinfection. No major expansion of Vγ2Vδ2+ T cells was noted in cells from the pulmonary alveoli of the SIVmac-infected macaques, whereas pulmonary lymphocytes from SIVmac-negative monkeys showed a remarkable increase in this γδ T-cell subpopulation (Fig. 1b). These results therefore demonstrated that chronic SIVmac infection could compromise the development of a primary immune response of macaque Vγ2Vδ2+ T cells during acute BCG coinfection.
Naïve macaques simultaneously coinfected with SIVmac and BCG did not sustain an expansion of Vγ2Vδ2+ T cells at the time that the coinfected monkeys developed a profound depletion of CD4+ T cells and fatal tuberculosis-like disease.
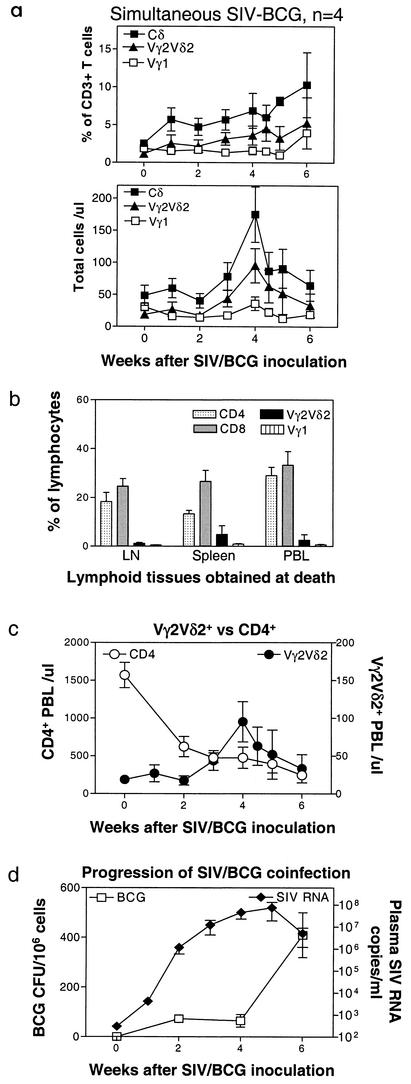
We then sought to examine whether SIVmac replication or the virus-induced CD4+ T-cell deficiency is responsible for the loss of Vγ2Vδ2+ T-cell response in the coinfected monkeys. If SIVmac replication undermines the responses of γδ T cells, an absent Vγ2Vδ2+ T-cell response should be evident at the time of the initial burst of viral replication during acute SIVmac infection. We therefore determined whether an acute SIVmac infection in naïve monkeys could impair the ability of Vγ2Vδ2+ T cells to expand in response to BCG administered concurrently. Naïve macaques were inoculated simultaneously with SIVmac and BCG and assessed for a change in representation of Vγ2Vδ2+ T cells in the peripheral blood and lymphoid tissues. In fact, some of these previously naïve macaques mounted a detectable expansion of Vγ2Vδ2+ T cells following simultaneous inoculation with SIVmac and BCG (Fig. 2a). Only a subtle increase in Vγ2Vδ2+ T cells was seen in lymph nodes after BCG infection (data not shown). Despite the occurrence of primary Vγ2Vδ2+ T-cell immune responses, the expansion of these cells was not sustained at the time that the animals developed fatal SIV-related BCG disease (Fig. 2a). Vγ2Vδ2+ T cells were not highly represented in the cells from lymph nodes and spleen (Fig. 2b). The absence of sustained increases in numbers of circulating Vγ2Vδ2+ T cells contrasted with persistently increased numbers of these cells during the progression of mycobacterial infection seen in naïve monkeys not infected with SIVmac (39).
FIG. 2.
Naïve monkeys coinfected simultaneously with SIVmac and BCG did not sustain an expansion of Vγ2Vδ2+ T cells. Data at the end points were obtained from the PBL of the naïve monkeys at the time that these animals developed a fatal tuberculosis-like disease. Necropsy showed SIV-related lymphoid depletion and granulomas in multiple organs, with high numbers of BCG CFU identified in lymph nodes of the SIVmac-BCG-coinfected monkeys (38). (a) Changes in the percentages and absolute numbers of CD3+ T cells that are Vγ1+or Vγ2Vδ2+ in the blood after BCG inoculation. Data shown are the means and standard errors of the means of values from four SIVmac-infected monkeys (two pigtailed and two rhesus). (b) Comparisons of CD4+, CD8+, Vγ1+, and Vγ2Vδ2+ T cells among lymph nodes, spleens, and PBL at the death of the coinfected monkeys. Numbers are percentages of gated lymphocytes as seen in flow cytometry. (c) Kinetic changes in numbers of CD4+ T cells in the blood and Vγ2Vδ2+ T cells in naïve monkeys following simultaneous SIVmac-BCG inoculation. (d) Plasma SIV RNA and BCG burdens in lymph nodes of naïve monkeys following simultaneous SIVmac and BCG inoculation.
Since both AIDS viruses and mycobacteria can impact on the function of the immune system, we sought to examine whether the kinetics of SIV loads, CD4+ T-cell depletion, and BCG burdens could correlate with the late decline of Vγ2Vδ2+ T cells. The decline of Vγ2Vδ2+ T cells at the late phase of SIVmac-BCG coinfection was associated with the profound decline of CD4+ PBL after simultaneous SIV-BCG coinfection (Fig. 2c). The decrease in the numbers of Vγ2Vδ2+ T cells occurred despite BCG dissemination in lymph nodes (Fig. 2d) and other organs (data not shown) at the time that the SIVmac-BCG-coinfected monkeys developed a fatal SIVmac-related BCG disease. The fatal outcome of SIVmac-BCG coinfection was characterized clinically by diarrhea and weight loss and pathologically by disseminated granulomas in multiple organs (38). The decline of Vγ2Vδ2+ T cells in the presence of high BCG loads differed from what was seen for control SIVmac-negative monkeys, where primary expansion of Vγ2Vδ2+ T cells was driven by mycobacterial loads, and a peak response was evident at the time that the monkeys developed disseminated tuberculosis (39). The generation of Vγ2Vδ2+ T-cell expansion in the SIVmac-BCG-coinfected monkeys during the early event of SIVmac replication and the absence of sustained Vγ2Vδ2+ T-cell expansion in these coinfected animals during the late phase of clinical disease despite high mycobacterial loads suggest that the generation of this γδ T-cell subpopulation may be blocked by SIVmac-mediated CD4+ T-cell deficiency rather than by viral replication.
The absence of recall Vγ2Vδ2+ T-cell expansion in the BCG reinfection of SIVmac-infected monkeys was associated with CD4+ T-cell deficiency and development of a fatal SIVmac-related BCG disease.
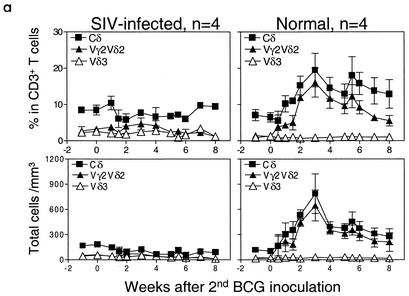
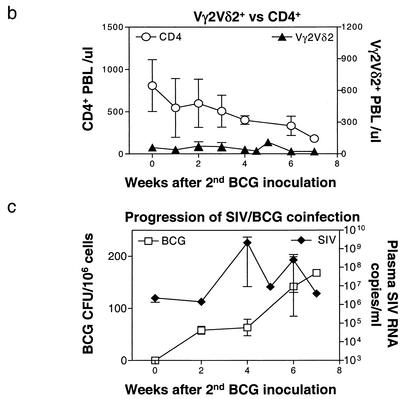
Since SIVmac-induced immune dysfunction compromised primary immune responses of Vγ2Vδ2+ T cells, we anticipated that this viral infection would also impact on a memory response of Vγ2Vδ2+ T cells during active BCG reinfection. To assess this possibility, macaques were infected first with BCG, then SIVmac, and finally BCG again at 2- to 3-month intervals. As controls, healthy macaques previously infected with BCG were reinfected with BCG again to elicit a recall response of Vγ2Vδ2+ T cells. The control SIVmac-negative monkeys developed potent memory Vγ2Vδ2+ T-cell responses following the second BCG inoculation. In these control macaques, the magnitude of this expansion of Vγ2Vδ2+ T cells following BCG reinfection was greater than that observed during the primary BCG infection (Fig. 3a). This remarkable expansion of Vγ2Vδ2+ T cells could be detected as early as 5 days and lasted for up to 7 months after the second BCG inoculation. In contrast, although the primary BCG infection of the experimental group of monkeys before SIVmac infection induced a readily demonstrated expansion of Vγ2Vδ2+ T cells (data not shown), the BCG reinfection of these same macaques following SIVmac infection did not induce a significant recall response of Vγ2Vδ2+ T cells. Only a subtle recall expansion of Vγ2Vδ2+ T cells was seen after BCG reinfection of these SIVmac-infected macaques (Fig. 3a). Interestingly, the absence of recall expansion of Vγ2Vδ2+ T cells in these animals was associated with apparent depletion of CD4+ T cells in the blood (Fig. 3b) and lymph nodes (data not shown) as well as subsequent development of a fatal SIVmac-related BCG disease. The SIVmac-related BCG disease was characterized by moribund conditions, high levels of BCG burdens in lymph nodes (Fig. 3c), and disseminated granulomas. In fact, marked suppression of purified protein derivative-specific CD4+ T-cell responses was also seen for these SIVmac-BCG-coinfected monkeys (38). These results demonstrated that SIVmac infection could profoundly compromise not only primary but also memory responses of Vγ2Vδ2+ T cells at the time that active BCG coinfection progressed to CD4+ T-cell deficiency and SIVmac-related BCG disease.
FIG. 3.
BCG reinfection of macaques previously infected with BCG and then SIVmac did not induce a potent recall immune response of Vγ2Vδ2+ T cells. (a) Changes in the percentages and absolute numbers of CD3+ T cells that are Vδ3+ or Vγ2Vδ2+ in the blood after the second BCG inoculation. Data shown are the means and standard errors of the means of values from four SIVmac-infected (one pigtailed and three rhesus) and four control rhesus monkeys. P was <0.001 for comparison of Vγ2Vδ2+ T-cell expansion between the SIVmac-infected and control groups 3 to 7 weeks after BCG reinfection. All animals developed a fatal SIVmac-related tuberculosis-like disease 1 to 2 months after BCG reinfection. (b) Kinetic changes in numbers of CD4+ T cells in the blood and Vγ2Vδ2+ T cells following BCG reinfection of SIVmac-infected monkeys. (c) Plasma SIV RNA and BCG burdens in lymph nodes of monkeys following BCG reinfection of SIVmac-infected monkeys.
Vγ2Vδ2+ T cells from SIVmac-BCG-coinfected monkeys had a reduced ability to expand in response to in vitro stimulation with nonpeptide antigens.
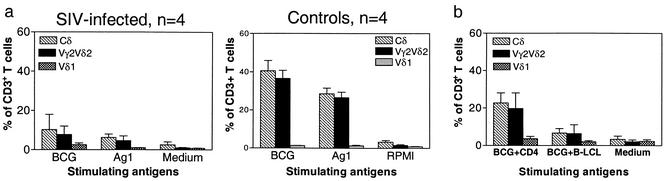
We then sought to determine whether Vγ2Vδ2+ T cells from SIVmac-BCG-coinfected monkeys had a reduced ability to proliferate in vitro after exposure to nonpeptide antigens. The Vγ2Vδ2+ T cells in PBL of control macaques infected previously with BCG alone proliferated and expanded to comprise as much as 51% of the entire T-cell population in response to the in vitro stimulation with prenyl pyrophosphate or BCG nonpeptide antigen (Fig. 4a). In contrast, the Vγ2Vδ2+ PBL obtained from the macaques coinfected with SIVmac and BCG exhibited a reduced ability to expand in response to nonpeptide antigens (Fig. 4a). Interestingly, the reduced ability of Vγ2Vδ2+ PBL from SIVmac-BCG-coinfected monkeys to expand in response to phosphoantigen stimulation was restored to some extent by coculture of these cells with CD4+ T cells purified from SIV-negative monkeys (Fig. 4b). These experiments demonstrated that SIVmac-mediated compromise of Vγ2Vδ2+ T cells was also seen in vitro and might be attributed to the virus-induced dysfunction of CD4+ T cells.
FIG. 4.
Vγ2Vδ2+ PBL from SIVmac-BCG-coinfected rhesus monkeys exhibited a reduced capacity to proliferate and expand in vitro in response to stimulation with nonpeptide antigens. PBL obtained from the macaques 4 to 6 weeks after BCG inoculation were stimulated in culture with partially purified Ag1 from M. fortuitum or BCG nonpeptide antigen. After 10 to 14 days of stimulation, cells were stained and analyzed by flow cytometry. Data shown are the means and standard errors of the means of values from four monkeys. (a) Data from SIVmac-infected macaques (left panel) and control macaques not infected with SIVmac (right panel). (b) The reduced capacity of Vγ2Vδ2+ PBL from SIVmac-BCG-coinfected monkeys to expand in vitro could be restored to some extent by coculture of these cells with CD4+ T cells but not the B-LCL cell line. P was <0.01 in comparisons of Vγ2Vδ2+ T-cell expansion between PBL cultures from SIVmac-infected monkeys and those from control animals; P was <0.05 in analyses of a difference in Vγ2Vδ2+ T-cell expansion between cultures with and without CD4+ T cells from SIV-negative monkeys.
The absence of an expansion of Vγ2Vδ2+ T cells in PBL of SIVmac-BCG-coinfected macaques was not attributable to a deletion of TCR repertoire.
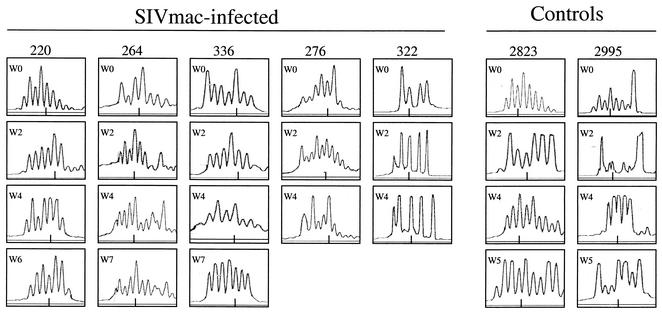
Finally, we sought to investigate whether clonal deletion-exhaustion of Vγ2Vδ2+ T cells during SIVmac-BCG coinfection led to the loss of immune responses of these cells. We examined the evolution of TCR CDR3 profiles of Vδ2+ T cells after BCG infection of the SIVmac-infected macaques. The PBL of SIVmac-infected monkeys showed no deletion of particular CDR3-bearing Vδ2+ T cells, since multiple CDR3 lengths were relatively conserved before BCG infection (Fig. 5). Following BCG infection, the Vδ2+ T cells in the SIVmac-infected macaques displayed no apparent changes in the patterns of CDR3 profiles (Fig. 5). The absence of apparent changes in CDR3 in Vδ2+ PBL was noted following both the first BCG infection and BCG reinfection in the SIVmac-infected macaques. It should be pointed out that CDR3 spectrotyping analyses of γδ T-cell repertoire could not provide detailed information regarding the deletion of a particular subspecificity, given that the Vγ2Vδ2+ TCR repertoire was diverse. Nevertheless, our results in prospective studies demonstrate that the absence of a major expansion of Vγ2Vδ2+ T cells in these monkeys was not attributable to the depletion or disruption of the Vδ2+ T-cell repertoire.
FIG. 5.
The absence of an expansion of Vγ2Vδ2+ T cells in SIVmac-BCG-coinfected macaques was not due to deletions in the TCR repertoire. Multiple CDR3 lengths could be identified in Vδ2+ T cells from the SIVmac-infected monkeys after BCG coinfection. Shown on the right are the CDR3 profiles in Vδ2+ T cells following BCG infection of the control monkeys. The numbers of nucleotides in the different CDR3 lengths were determined in control experiments (46) and are expressed as predicted numbers of amino acids. A short line at the bottom of each histogram represents the predicted CDR3 length of 13 amino acids.
DISCUSSION
The present studies of macaques demonstrate that persistent SIVmac infection can profoundly compromise both the primary and memory responses of phosphoantigen-specific Vγ2Vδ2+ T cells following a BCG challenge. These observations are consistent with the in vitro demonstration that the Vγ2Vδ2+ T cells in HIV-1-infected humans are unable to proliferate in vitro following stimulation with M. tuberculosis or nonpeptide antigens (20, 30, 33). Such findings for macaques and humans suggest that HIV infection may suppress adaptive immune responses of mycobacterium-specific Vγ2Vδ2+ T cells during primary M. tuberculosis infection or reinfection-reactivation tuberculosis.
The absence of a major expansion of Vγ2Vδ2+ T cells following BCG infection of SIVmac-infected monkeys may not be attributable to SIVmac-mediated killing of these cells resulting from virus production by BCG-activated lymphocytes. Vγ2Vδ2+ T cells of macaques do not exhibit productive SIVmac infection in vivo (data not shown), although human Vγ2Vδ2+ T-cell clones can be infected by HIV-1 in vitro (43). It is unlikely that the AIDS virus itself directly blocks Vγ2Vδ2+ T cells for the development of immune responses. This notion is supported by our demonstration in the present studies that a primary SIVmac infection fails to inhibit the expansion of Vγ2Vδ2+ T cells in previously naïve monkeys that simultaneously receive SIVmac and BCG inoculation. Finally, the loss of Vγ2Vδ2+ T-cell responses does not appear to result from a selective clonal deletion of those cells following SIVmac infection. In fact, we were unable to identify any selective deletion of a Vγ2Vδ2+ T-cell subpopulation in the macaques infected with SIVmac251 (data not shown) and SIVsmmPBj (12), although a decrease in the representation of Vδ2+ T cells has been reported previously for HIV-1-infected humans (6, 33). In addition, molecular analyses of Vδ2+ T cells in the SIVmac-infected macaques did not reveal any apparent disruption or deletion of the Vδ2+ T-cell repertoire that had expanded in response to BCG coinfection.
The present studies suggest that SIVmac-induced compromise of the immune system results in a loss of both the primary and recall expansions of Vγ2Vδ2+ T cells that occur in macaques following exposure to BCG. Two patterns of this loss of immune function were observed for SIVmac-infected monkeys. A consistent suppression of primary and recall Vγ2Vδ2+ T-cell responses during the acute phase of BCG infection was seen for the chronically SIVmac-infected monkeys. The suppression of the recall immune responses of Vγ2Vδ2+ T cells in SIVmac-BCG-coinfected macaques was particularly striking, compared to the magnitude of Vγ2Vδ2+ T-cell expansions identified in the BCG-infected control macaques not infected with SIVmac. In a second pattern of inhibition of Vγ2Vδ2+ T-cell immune responses, SIVmac-BCG coinfection of naïve monkeys resulted in an initial expansion of Vγ2Vδ2+ T cells that was not sustainable as the animals developed a BCG dissemination and tuberculosis-like disease. It was recently demonstrated with M. tuberculosis-infected SIVmac-negative monkeys that the initial expansion of Vγ2Vδ2+ T cells can be sustained during the progression of this mycobacterial infection and that the peak response of these cells can be seen even at the time that the animals develop miliary tuberculosis (39). In those previously healthy monkeys, the expanded Vγ2Vδ2+ T cells decline or return to baseline levels only when the active mycobacterial infection resolves (39). The extent of SIVmac-induced disease appears to be important in the inhibition of the adaptive immune responses of mycobacterium-specific Vγ2Vδ2+ T cells.
While the mechanisms underlying the SIVmac-induced suppression of Vγ2Vδ2+ T-cell immune responses in SIVmac-BCG-coinfected macaques are likely complex, our studies demonstrate that absence or loss of adaptive Vγ2Vδ2+ T-cell responses is associated with CD4+ T-cell deficiency. The depletion of CD4+ T cells is clearly seen coincident with absence or loss of Vγ2Vδ2+ T-cell expansion following BCG coinfection of SIVmac-infected monkeys. In fact, the inhibition of BCG-specific CD4+ T-cell immune responses is seen for these SIVmac-BCG-coinfected monkeys (38). The loss and/or dysfunction of CD4+ T cells associated with the BCG infection itself can certainly impact on the immune responses of Vγ2Vδ2+ T cells. The dramatic changes in the cytokine environment due to CD4+ T-cell deficiency are likely to compromise Vγ2Vδ2+ T-cell immune responses in SIVmac-BCG-coinfected monkeys. Our finding that CD4+ T cells from SIVmac-negative monkeys can improve the ability of Vγ2Vδ2+ T cells to expand in vitro suggests that T helper cells or cytokines produced by these cells are needed for phosphoantigen-stimulated expansion of these γδ T cells. In fact, a role for IL-2 and IL-15 in stimulating Vγ2Vδ2+ T-cell proliferation has been recently demonstrated (7, 18). A decrease in the production of these cytokines in the setting of pathogenic SIVmac-BCG coinfection might certainly impair the ability of Vγ2Vδ2+ T cells to proliferate and expand in response to nonpeptide antigen stimulation. We also cannot exclude the possibility that other specific cytokines that inhibit Vγ2Vδ2+ T-cell responses may be overproduced in the monkeys during the acute phase of SIVmac-BCG infection.
The SIVmac-induced suppression of the immune responses of Vγ2Vδ2+ T cells may contribute to the immunopathogenesis of the AIDS virus-related tuberculosis-like disease in macaques. Although the effector function of human γδ+ T cells remains poorly understood, some studies suggest that these cells are involved in the immune regulation of mycobacterial or HIV infections (16, 17, 25, 27, 28, 32). Vγ2Vδ2+ T cells have been shown previously to produce gamma interferon and tumor necrosis factor alpha, cytokines that mediate resistance to tuberculosis in mice (20, 44). Previous studies of SIVmac-negative macaques have demonstrated that a major expansion of Vγ2Vδ2+ T cells can be associated temporally with the resolution of active BCG infection (39). The rapid recall expansion of these cells following M. tuberculosis challenge coincided with resistance to acutely fatal tuberculosis in BCG-vaccinated monkeys. Thus, suppression of Vγ2Vδ2+ T-cell immune responses may result in the incomplete control of a mycobacterial coinfection in SIVmac-infected monkeys. In fact, the SIVmac-infected macaques that showed no detectable expansion of Vγ2Vδ2+ T cells after the primary BCG infection died within 1 to 7 months after BCG inoculation, with evidence of BCG dissemination or disseminated granulomas in multiple organs. In addition, the SIVmac-BCG-coinfected monkeys that did not show recall expansion of Vγ2Vδ2+ T cells after BCG reinfection died from a fatal SIVmac-related tuberculosis-like disease within 3 months after the second BCG inoculation. Furthermore, the simultaneous coinfection of naïve monkeys with SIVmac and BCG resulted in a decline or loss of Vγ2Vδ2+ T cells after their initial expansion. The absence of a sustained Vγ2Vδ2+ T-cell response in these simultaneously coinfected monkeys was associated with the development of SIVmac-related tuberculosis-like disease. These observations suggest that the suppression of Vγ2Vδ2+ T-cell immune responses during SIVmac infection may be one of the mechanisms underlying the development of SIVmac-related tuberculosis-like disease in macaques.
Acknowledgments
This work was supported by NIH RO1 grant HL64560 (to Z.W.C.).
We thank members of the Chen laboratory for technical support and Craig Morita at the University of Iowa for providing nonpeptide antigens from M. fortuitum for in vitro experiments.
REFERENCES
- 1.Agrati, C., G. D'Offizi, P. Narciso, C. Selva, L. P. Pucillo, G. Ippolito, and F. Poccia. 2001. γδ T cell activation by chronic HIV infection may contribute to intrahepatic vδ1 compartmentalization and hepatitis C virus disease progression independent of highly active antiretroviral therapy. AIDS Res. Hum. Retrovir. 17:1357-1363. [DOI] [PubMed] [Google Scholar]
- 2.Autran, B., F. Triebel, C. Katlama, W. Rozenbaum, T. Hercend, and P. Debre. 1989. T cell receptor γ/δ+ lymphocyte subsets during HIV infection. Clin. Exp. Immunol. 75:206-210. [PMC free article] [PubMed] [Google Scholar]
- 3.Balbi, B., M. T. Valle, S. Oddera, D. Giunti, F. Manca, G. A. Rossi, and L. Allegra. 1993. T-lymphocytes with γδ+Vδ2+ antigen receptors are present in increased proportions in a fraction of patients with tuberculosis or with sarcoidosis. Am. Rev. Respir. Dis. 148:1685-1690. [DOI] [PubMed] [Google Scholar]
- 4.Beckman, E. M., S. A. Porcelli, C. T. Morita, S. M. Behar, S. T. Furlong, and M. B. Brenner. 1994. Recognition of a lipid antigen by CD1-restricted αβ+ T cells. Nature 372:691-694. [DOI] [PubMed] [Google Scholar]
- 5.Bertotto, A., R. Gerli, F. Spinozzi, C. Muscat, F. Scalise, G. Castellucci, M. Sposito, F. Candio, and R. Vaccaro. 1993. Lymphocytes bearing the γδ T cell receptor in acute Brucella melitensis infection. Eur. J. Immunol. 23:1177-1180. [DOI] [PubMed] [Google Scholar]
- 6.Boullier, S., M. Cochet, F. Poccia, and M. L. Gougeon. 1995. CDR3-independent γδVδ1+ T cell expansion in the peripheral blood of HIV-infected persons. J. Immunol. 154:1418-1431. [PubMed] [Google Scholar]
- 7.Boullier, S., Y. Poquet, T. Debord, J. J. Fournie, and M. L. Gougeon. 1999. Regulation by cytokines (IL-12, IL-15, IL-4 and IL-10) of the Vγ9Vδ2 T cell response to mycobacterial phosphoantigens in responder and anergic HIV-infected persons. Eur. J. Immunol. 29:90-99. [DOI] [PubMed] [Google Scholar]
- 8.Bukowski, J. F., C. T. Morita, and M. B. Brenner. 1999. Human γδ T cells recognize alkylamines derived from microbes, edible plants, and tea: implications for innate immunity. Immunity 11:57-65. [DOI] [PubMed] [Google Scholar]
- 9.Burk, M. R., L. Mori, and G. De Libero. 1995. Human Vγ9-Vδ2 cells are stimulated in a cross-reactive fashion by a variety of phosphorylated metabolites. Eur. J. Immunol. 25:2052-2058. [DOI] [PubMed] [Google Scholar]
- 10.Caldwell, C. W., E. D. Everett, G. McDonald, Y. W. Yesus, and W. E. Roland. 1995. Lymphocytosis of γ/δ T cells in human ehrlichiosis. Am. J. Clin. Pathol. 103:761-766. [DOI] [PubMed] [Google Scholar]
- 11.Chen, Z. W., Z. C. Kou, C. Lekutis, L. Shen, D. Zhou, M. Halloran, J. Li, J. Sodroski, D. Lee-Parritz, and N. L. Letvin. 1995. T cell receptor Vβ repertoire in an acute infection of rhesus monkeys with simian immunodeficiency viruses and a chimeric simian-human immunodeficiency virus. J. Exp. Med. 182:21-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, Z. W., Y. Shen, I. C. Davis, L. Shen, N. L. Letvin, and P. N. Fultz. 2000. Down-regulation of macaque γδ+ T cells in lymphoid compartments after rectal infection with SIVsmmPBj14. J. Med. Primatol. 29:143-147. [DOI] [PubMed] [Google Scholar]
- 13.Constant, P., F. Davodeau, M. A. Peyrat, Y. Poquet, G. Puzo, M. Bonneville, and J. J. Fournie. 1994. Stimulation of human γδ T cells by nonpeptidic mycobacterial ligands. Science 264:267-270. [DOI] [PubMed] [Google Scholar]
- 14.De Paoli, P., D. Gennari, P. Martelli, G. Basaglia, M. Crovatto, S. Battistin, and G. Santini. 1991. A subset of γδ lymphocytes is increased during HIV-1 infection. Clin. Exp. Immunol. 83:187-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dieli, F., G. Sireci, C. Di Sano, E. Champagne, J. J. Fournie, and J. I. Salerno. 1999. Predominance of Vγ9/Vδ2 T lymphocytes in the cerebrospinal fluid of children with tuberculous meningitis: reversal after chemotherapy. Mol. Med. 5:301-312. [PMC free article] [PubMed] [Google Scholar]
- 16.Dieli, F., M. Troye-Blomberg, J. Ivanyi, J. J. Fournie, M. Bonneville, M. A. Peyrat, G. Sireci, and A. Salerno. 2000. Vγ9/Vδ2 T lymphocytes reduce the viability of intracellular Mycobacterium tuberculosis. Eur. J. Immunol. 30:1512-1519. [DOI] [PubMed] [Google Scholar]
- 17.Dieli, F., M. Troye-Blomberg, J. Ivanyi, J. J. Fournie, A. M. Krensky, M. Bonneville, M. A. Peyrat, N. Caccamo, G. Sireci, and A. Salerno. 2001. Granulysin-dependent killing of intracellular and extracellular Mycobacterium tuberculosis by Vγ9/Vδ2 T lymphocytes. J. Infect. Dis. 184:1082-1085. [DOI] [PubMed] [Google Scholar]
- 18.Garcia, V. E., D. Jullien, M. Song, K. Uyemura, K. Shuai, C. T. Morita, and R. L. Modlin. 1998. IL-15 enhances the response of human γδ T cells to nonpeptide microbial antigens. J. Immunol. 160:4322-4329. [PubMed] [Google Scholar]
- 19.Gioia, C., C. Agrati, R. Casetti, C. Cairo, G. Borsellino, L. Battistini, G. Mancino, D. Goletti, V. Colizzi, L. P. Pucillo, and F. Poccia. 2002. Lack of CD27-CD45RA-Vγ9Vδ2+ T cell effectors in immunocompromised hosts and during active pulmonary tuberculosis. J. Immunol. 168:1484-1489. [DOI] [PubMed] [Google Scholar]
- 20.Gougeon, M. L., F. Poccia, and S. Boullier. 2000. Human γδ T lymphocytes in HIV disease: effector functions and control by natural killer cell receptors. Springer Semin. Immunopathol. 22:251-263. [DOI] [PubMed] [Google Scholar]
- 21.Hara, T., Y. Mizuno, K. Takaki, H. Takada, H. Akeda, T. Aoki, M. Nagata, K. Ueda, G. Matsuzaki, Y. Yoshikai, et al. 1992. Predominant activation and expansion of Vγ9-bearing γδ T cells in vivo as well as in vitro in Salmonella infection. J. Clin. Investig. 90:204-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ho, M., P. Tongtawe, J. Kriangkum, T. Wimonwattrawatee, K. Pattanapanyasat, L. Bryant, J. Shafiq, P. Suntharsamai, S. Looareesuwan, H. K. Webster, and J. F. Elliott. 1994. Polyclonal expansion of peripheral γδ T cells in human Plasmodium falciparum malaria. Infect. Immun. 62:855-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ito, M., N. Kojiro, T. Ikeda, T. Ito, J. Funada, and T. Kokubu. 1992. Increased proportions of peripheral blood γδ T cells in patients with pulmonary tuberculosis. Chest 102:195-197. [DOI] [PubMed] [Google Scholar]
- 24.Jouen-Beades, F., E. Paris, C. Dieulois, J. F. Lemeland, V. Barre-Dezelus, S. Marret, G. Humbert, J. Leroy, and F. Tron. 1997. In vivo and in vitro activation and expansion of γδ T cells during Listeria monocytogenes infection in humans. Infect. Immun. 65:4267-4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kennedy, H. E., M. D. Welsh, D. G. Bryson, J. P. Cassidy, F. I. Forster, C. J. Howard, R. A. Collins, and J. M. Pollock. 2002. Modulation of immune responses to Mycobacterium bovis in cattle depleted of WC1+ γδ T cells. Infect. Immun. 70:1488-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kunzmann, V., E. Bauer, and M. Wilhelm. 1999. γ/δ T-cell stimulation by pamidronate. N. Engl. J. Med. 340:737-738. [DOI] [PubMed] [Google Scholar]
- 27.Ladel, C. H., J. Hess, S. Daugelat, P. Mombaerts, S. Tonegawa, and S. H. Kaufmann. 1995. Contribution of α/β and γ/δ T lymphocytes to immunity against Mycobacterium bovis bacillus Calmette-Guerin: studies with T cell receptor-deficient mutant mice. Eur. J. Immunol. 25:838-846. [DOI] [PubMed] [Google Scholar]
- 28.Lehner, T., E. Mitchell, L. Bergmeier, M. Singh, R. Spallek, M. Cranage, G. Hall, M. Dennis, F. Villinger, and Y. Wang. 2000. The role of γδ T cells in generating antiviral factors and β-chemokines in protection against mucosal simian immunodeficiency virus infection. Eur. J. Immunol. 30:2245-2256. [DOI] [PubMed] [Google Scholar]
- 29.Martini, F., F. Poccia, D. Goletti, S. Carrara, D. Vincenti, G. D'Offizi, C. Agrati, G. Ippolito, V. Colizzi, L. P. Pucillo, and C. Montesano. 2002. Acute human immunodeficiency virus replication causes a rapid and persistent impairment of Vγ9Vδ2 T cells in chronically infected patients undergoing structured treatment interruption. J. Infect. Dis. 186:847-850. [DOI] [PubMed] [Google Scholar]
- 30.Martini, F., R. Urso, C. Gioia, A. De Felici, P. Narciso, A. Amendola, M. G. Paglia, V. Colizzi, and F. Poccia. 2000. γδ T-cell anergy in human immunodeficiency virus-infected persons with opportunistic infections and recovery after highly active antiretroviral therapy. Immunology 100:481-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30a.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, D.C.
- 31.Pfeffer, K., B. Schoel, H. Gulle, S. H. Kaufmann, and H. Wagner. 1990. Primary responses of human T cells to mycobacteria: a frequent set of γ/δ T cells are stimulated by protease-resistant ligands. Eur. J. Immunol. 20:1175-1179. [DOI] [PubMed] [Google Scholar]
- 32.Poccia, F., L. Battistini, B. Cipriani, G. Mancino, F. Martini, M. L. Gougeon, and V. Colizzi. 1999. Phosphoantigen-reactive Vγ9Vδ2 T lymphocytes suppress in vitro human immunodeficiency virus type 1 replication by cell-released antiviral factors including CC chemokines. J. Infect. Dis. 180:858-861. [DOI] [PubMed] [Google Scholar]
- 33.Poccia, F., S. Boullier, H. Lecoeur, M. Cochet, Y. Poquet, V. Colizzi, J. J. Fournie, and M. L. Gougeon. 1996. Peripheral Vγ9/Vδ2 T cell deletion and anergy to nonpeptidic mycobacterial antigens in asymptomatic HIV-1-infected persons. J. Immunol. 157:449-461. [PubMed] [Google Scholar]
- 34.Poquet, Y., F. Halary, E. Champagne, F. Davodeau, M. L. Gougeon, M. Bonneville, and J. J. Fournie. 1996. Human γδ T cells in tuberculosis. Res. Immunol. 147:542-549. [DOI] [PubMed] [Google Scholar]
- 35.Raziuddin, S., A. W. Telmasani, M. el-Hag el-Awad, O. al-Amari, and M. al-Janadi. 1992. γδ T cells and the immune response in visceral leishmaniasis. Eur. J. Immunol. 22:1143-1148. [DOI] [PubMed] [Google Scholar]
- 36.Russo, D. M., R. J. Armitage, M. Barral-Netto, A. Barral, K. H. Grabstein, and S. G. Reed. 1993. Antigen-reactive γδ T cells in human leishmaniasis. J. Immunol. 151:3712-3718. [PubMed] [Google Scholar]
- 37.Scalise, F., R. Gerli, G. Castellucci, F. Spinozzi, G. M. Fabietti, S. Crupi, L. Sensi, R. Britta, R. Vaccaro, and A. Bertotto. 1992. Lymphocytes bearing the γδ T-cell receptor in acute toxoplasmosis. Immunology 76:668-670. [PMC free article] [PubMed] [Google Scholar]
- 38.Shen, Y., D. Zhou, L. Chalifoux, L. Shen, M. Simon, X. Zeng, X. Lai, Y. Li, P. Sehgal, N. L. Letvin, and Z. W. Chen. 2002. Induction of an AIDS virus-related tuberculosis-like disease in macaques: a model of simian immunodeficiency virus-mycobacterium coinfection. Infect. Immun. 70:869-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen, Y., D. Zhou, L. Qiu, X. Lai, M. Simon, L. Shen, Z. Kou, Q. Wang, L. Jiang, J. Estep, R. Hunt, M. Clagett, P. K. Sehgal, Y. Li, X. Zeng, C. T. Morita, M. B. Brenner, N. L. Letvin, and Z. W. Chen. 2002. Adaptive immune response of Vγ2Vδ2+ T cells during mycobacterial infections. Science 295:2255-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sumida, T., T. Maeda, H. Takahashi, S. Yoshida, F. Yonaha, A. Sakamoto, H. Tomioka, and T. Koike. 1992. Predominant expansion of Vγ9/Vδ2 T cells in a tularemia patient. Infect. Immun. 60:2554-2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanaka, Y., C. T. Morita, E. Nieves, M. B. Brenner, and B. R. Bloom. 1995. Natural and synthetic non-peptide antigens recognized by human γδ T cells. Nature 375:155-158. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka, Y., S. Sano, E. Nieves, G. De Libero, D. Rosa, R. L. Modlin, M. B. Brenner, B. R. Bloom, and C. T. Morita. 1994. Nonpeptide ligands for human γδ T cells. Proc. Natl. Acad. Sci. USA 91:8175-8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wallace, M., A. M. Scharko, C. D. Pauza, P. Fisch, K. Imaoka, S. Kawabata, K. Fujihashi, H. Kiyono, Y. Tanaka, B. R. Bloom, and M. Malkovsky. 1997. Functional γδ T-lymphocyte defect associated with human immunodeficiency virus infections. Mol. Med. 3:60-71. [PMC free article] [PubMed] [Google Scholar]
- 44.Wang, L., H. Das, A. Kamath, and J. F. Bukowski. 2001. Human Vγ2Vδ2 T cells produce IFN-γ and TNF-α with an on/off/on cycling pattern in response to live bacterial products. J. Immunol. 167:6195-6201. [DOI] [PubMed] [Google Scholar]
- 45.Wesch, D., D. Kabelitz, K. Friese, and K. Pechhold. 1996. Mycobacteria-reactive γδ T cells in HIV-infected individuals: lack of Vγ9 cell responsiveness is due to deficiency of antigen-specific CD4 T helper type 1 cells. Eur. J. Immunol. 26:557-562. [DOI] [PubMed] [Google Scholar]
- 46.Zhou, D., Y. Shen, L. Chalifoux, D. Lee-Parritz, M. Simon, P. K. Sehgal, L. Zheng, M. Halloran, and Z. W. Chen. 1999. Mycobacterium bovis bacille Calmette-Guerin enhances pathogenicity of simian immunodeficiency virus infection and accelerates progression to AIDS in macaques: a role of persistent T cell activation in AIDS pathogenesis. J. Immunol. 162:2204-2216. [PubMed] [Google Scholar]