Abstract
Geminiviruses package single-stranded circular DNA and replicate via double-stranded DNA intermediates. During the past decade, increasing evidence has led to the general acceptance that their replication follows a rolling-circle replication mechanism like bacteriophages with single-stranded DNA. In a recent study, we showed that this is also true for Abutilon mosaic geminivirus (AbMV), but that this particular virus may also use a recombination-dependent replication (RDR) route in analogy to T4 phages. Because AbMV is a special case, since it has been propagated on ornamental plants for more than a hundred years, it was interesting to determine whether RDR is common among other geminiviruses. We analyzed geminiviruses from different genera and geographic origins by using BND cellulose chromatography in combination with an improved high resolution two-dimensional gel electrophoresis, and we conclude that multitasking in replication is widespread, at least for African cassava mosaic, Beet curly top, Tomato golden mosaic, and Tomato yellow leaf curl virus.
Geminiviruses have spread worldwide during the past three decades, causing severe diseases in important crop plants (23, 35). Several factors have promoted the epidemics: transportation of plant material, increase and diversification of insect vector populations, and recombination between geminiviruses that coinfect host plants. Geminiviruses may contain monopartite or bipartite genomes (45), and consequently recombination may occur in two different ways: by exchange of viral chromosomes (interchromosomal recombination, or “pseudorecombination” as plant virologists call this type of reassortment) (54, 55, 61, 62) or by crossover of chromosomes (intrachromosomal recombination) (5, 6, 17, 19, 38, 46, 68, 69). Moreover, geminiviruses are able to adopt satellite-like DNA circles which have an additional impact on pathogenesis (7, 12, 47). In comparison to RNA-containing plant viruses, geminiviruses are relatively prone to recombination and harbor frequent footprints of recombination events within their genomes (38). This phenomenon is conceivable on the basis of a recombination-dependent replication mode of geminiviruses as was proposed recently (26).
Geminiviruses package single-stranded circular DNA in twin-shaped capsids (67) and complement their DNA by using RNA-primed DNA polymerization (49). The resulting double-stranded circular DNA is packed into minichromosomes (1, 42). New rounds of replication were originally identified by two-dimensional gel electrophoresis (48), and these results have founded a rolling-circle replication (RCR) model for geminivirus multiplication (reviewed in reference 22) in analogy to the replication of bacteriophages with single-stranded circular DNA (32). A single viral protein (“AC1,” also known as “AL1” or “C1” for different virus species or “Rep” for replication-associated protein to indicate its function) is necessary and sufficient for initiation by nicking the viral origin of replication (22). The RCR mode was confirmed by direct electron-microscopic visualization of its intermediates for Abutilon mosaic virus (AbMV) (26), but these experiments, as well as an improved analysis of viral DNA by two-dimensional gel electrophoresis, identified additional viral DNA intermediates that are more compatible with a recombination-dependent replication (RDR) mode analogous to that of bacteriophage T4 (36, 37). AbMV has a special history among geminiviruses because it has been propagated on ornamental plants vegetatively without selection by insect transmission for more than a hundred years (64). Therefore, the appearance of RDR-implying intermediates might be a special adaptation to the particular host and transmission manner. A first indication that this is not the case resulted from the observation that RDR intermediates are the prominent, if not the exclusive forms after agroinfection of leaf discs of Nicotiana benthamiana plants (26). The following results show that AbMV, as well as distantly related geminiviruses, produces both types of intermediates also upon agroinoculation in systemically infected plants, suggesting that geminiviruses can multitask in replication. Thus, they probably exploit the replication-recombination-repair connection (63) to replicate more efficiently and to overcome host plant defense.
MATERIALS AND METHODS
Plants and viruses.
Abutilon mosaic virus (AbMV), African cassava mosaic virus (ACMV), and Tomato golden mosaic virus (TGMV) were agroinoculated on Nicotiana benthamiana DOMIN plants as described earlier (65), and Beet curly top virus (BCTV) was cultivated as described earlier (18). Infectious clones of Tomato yellow leaf curl virus (TYLCV) were provided by E. Bejarano, Malaga, Spain. All of the clones were prick inoculated in parallel onto seedlings in the four-leaf stage. After the onset of symptom development, the first systemically infected leaves were collected for analysis at 21 days postinoculation.
DNA extraction and purification.
Plant nucleic acids enriched for viral DNA were prepared as described previously (26), except that the concentration of polyethylene glycol was reduced to 8% (“crude extract”). This nucleic acid pool was further separated into two fractions by chromatography on benzoylated naphthoylated DEAE (BND) cellulose (Sigma B6385) as described previously (11): the “wash fraction,” enriched in double-stranded DNA (dsDNA), and the “eluate fraction,” enriched in single-stranded DNA (ssDNA) or dsDNA associated with ssDNA. A total of 10 to 20 μg of DNA in 180 μl of buffer were applied to a Micro Bio-Spin chromatography column 100 (Bio-Rad) containing 200 μl (wet gel bed) of BND cellulose. Washing and elution was performed according to the method of Dijkwel et al. (11) by using centrifugational force for the solution flow (3,000 rpm in an Eppendorf centrifuge: 60 s for the sample application, twice for 5 s, and once 10 s for 200-μl washings each; twice for 5 s and once for 10 s for 200 μl of elution buffer each). Wash as well as eluate fractions were pooled separately, supplied with carrier RNA to 40 μg/ml, precipitated with ethanol, and dissolved in 50 μl of buffer (10 mM Tris-HCl, pH 7.5; 1 mM EDTA).
Gel analysis and hybridization.
A total of 100 ng of DNA of the crude extract or 10 μl of either wash or eluate fractions after BND chromatography were processed for two-dimensional electrophoresis, hybridized with the corresponding viral DNA probes (18, 65), and detected by digoxigenin chemiluminescence as described previously (26). Probes were generated from full-length genomic clones of viral DNA: for AbMV, we used complete DNA A; for ACMV, we used a mixture of DNA A (nucleotides [nt] 221 to 2581) and DNA B (nt 245 to 2550); for TGMV, we used a mixture of complete DNA A and B; for BCTV, we used a fragment (nt 698 to 1462); and for TYLCV, we used the complete DNA.
RESULTS
To investigate whether several modes of replication are widespread among geminiviruses, we chose distantly related members of the family exemplifying various characteristics. AbMV (from the Central Americas), TGMV (from the South Americas), and ACMV (from Africa) belong to the genus Begomovirus and contain a bipartite genome; TYLCV (from the Mediterranean coast) is one of the few exceptions among begomoviruses with a monopartite genome, and BCTV (from the United States) is the type member of the genus Curtovirus with a monopartite genome. Whereas the first four viruses are transmitted by the whitefly Bemisia tabaci Genn, the last one is vectored by a leafhopper (Circulifer tenellus Baker [8]). To further analyze whether the route of infection or the host plants have an influence on the appearance of certain viral DNA intermediates in comparison to former results (26), all viral clones were agroinfected on N. benthamiana in parallel, and systemically infected leaves were processed for further analysis.
Using crude nucleic acid extracts, the same pattern of viral DNA intermediates was detected for AbMV as observed previously (26), but the resolution was worse for the other geminiviruses since they contained a larger amount of end products of replication (data not shown), reflecting their higher titers in plants. A further purification on BND cellulose solved this problem for all of the geminiviruses and improved the resolution of AbMV intermediates, allowing the assignment of additional details. BND cellulose enriches dsDNA in the wash fraction and ssDNA or dsDNA with single-stranded parts or moieties, since they occur during replication, in the eluate fraction.
The subsequent two-dimensional electrophoresis separates the DNAs in the first dimension essentially according to their molecular masses. In the second dimension, their conformation is emphasized due to a differential binding of chloroquine to dsDNA and ssDNA, as well as by the resolution of circular dsDNA into topoisomers due by intercalating chloroquine as outlined in detail for AbMV (26) and for simian virus 40 (40, 41, 53). The assignment of certain viral DNA forms to most of the spots, comma-like structures, lines, and arcs after blot hybridization has been discussed and checked by electron microscopic controls extensively (26) and will only be summarized here (see Fig. 4).
FIG. 4.
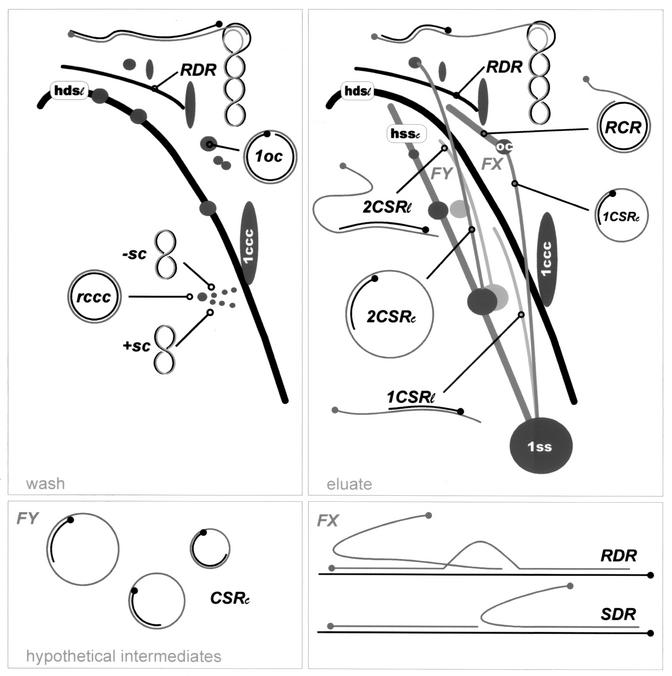
Schematic summary of viral DNA forms observed with different viruses to variable extents. Abbreviations are mostly as in Fig. 1 and “FX” for field X and “FY” for field Y. The following representation codes corresponding to the hybridization signals were used for intermediate DNA forms: black arcs for dsDNA and gray lines or arcs for ssDNA or ssDNA connected to dsDNA. For schematic drawings of replicative intermediates, gray rings or lines show viral sense DNA, and black ones the corresponding complementary strands. Dots at the ends of lines mark the 5′ termini; the other ends mark the 3′ termini. Whereas panel “wash” was derived from Fig. 1a and other similar blots (data not shown), panel “eluate” represents the summary of the best visible signals of Fig. 1b, 2a, and 3a and b. Hypothetical intermediates (FY and FX) cannot be discriminated with the two-dimensional technique because replicative intermediates with heterogeneous circular ssDNA templates would result in a field of hybridizing material (FY) above the line of hss, whereas replicative intermediates starting from heterogeneous dsDNA would produce a field of hybridizing material (FX) above the hds arc, irrespective of whether they were produced by an RDR mechanism or by strand displacement replication (SDR). Such fields of hybridizing material are obvious in most blots, but it is impossible to validate these signals against smears of incompletely resolved DNA. FX-like molecules, however, have been frequently observed by electron microscopy (26).
All viral forms have been detected in all of the virus infections tested. Changes in the relative amounts of certain DNA forms for different viruses are not virus specific because their variation lies within the range of AbMV intermediates during leaf development (26). As the chosen viruses have considerably distinct effects on the pathogenesis, it is not possible to normalize the sampling for a common developmental leaf stage. Therefore, mixed samples of young growing leaves were used.
Effect of BND chromatography on the resolution of viral DNA forms.
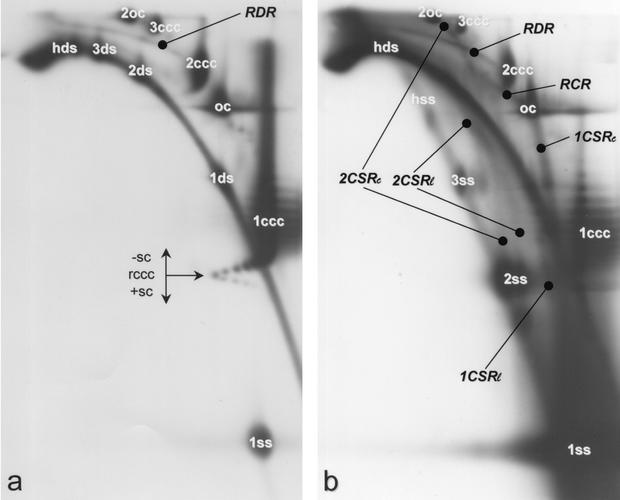
The separation of viral DNA forms is exemplified for AbMV (Fig. 1) and is essentially the same for the other viruses. The wash fraction (Fig. 1a) emphasizes dsDNA and the eluate fraction (Fig. 1b) ssDNA. For a better orientation, see the schematic representations in Fig. 4.
FIG. 1.
Two-dimensional gel analysis of AbMV DNA forms detected by DNA A probe after BND chromatography resulting in a “wash fraction” (a) and in an “eluate fraction” (b). The following DNA conformations are well resolved: single-stranded (ss), linear double-stranded (ds), open circular dsDNA (oc), and closed circular DNA (ccc). Numbers 1, 2, or 3 refer to monomer, dimer, or trimer genomic lengths; higher-order multimers are visible but have not been marked. Lines and arcs that indicate replicative intermediates are interpreted as heterogeneous linear dsDNA (hds) or heterogeneous circular ssDNA (hss), complementary strand replication (CSRl or CSRc) for linear or circular templates, RCR, and RDR products. Topoisomer conformations, as they had been prior to electrophoresis, in panel a are as follows: arrows point to relaxed closed circular conformation (rccc) and the directions of increasing negative (-sc) or positive (+sc) superhelicity. Note that 2ss and 3ssDNAs in panel b have been each separated into two spots representing circular (left spot) and linear (right spot) ssDNA. Correspondingly, 2CSR intermediates are doubled and lead to the 2oc spot (2CSRc) or approach the hds arc (CSRl) as expected for circular and linear replicating molecules, respectively. For further explanation, see also Fig. 4.
Simple forms.
As elaborated earlier (26), discrete DNA without a superhelical twist forms oval spots, superhelical topoisomers parallel patterns of lines which can fuse to comma-like structures, growing ssDNA forms straight lines, and dsDNA arcs in 2D gels (Fig. 1). After BND chromatography, most of the covalently closed circular DNA (cccDNA) has been found in the wash fraction (Fig. 1a), most of the ssDNA in the eluate fraction (Fig. 1b), but the separation was not complete under the chosen conditions. Nevertheless, some features of DNA forms were better resolved than before (26) with the additional purification step: (i) dimeric and trimeric ssDNA (Fig. 1b, 2ss and 3ss) were separated into their circular and linear forms; (ii) relaxed closed circular DNA (rccc) and its topoisomers with positive (+sc) and negative (−sc) superhelical twists were clearly visible (as in Fig. 1a) in all analyzed samples from different viruses (data not shown). The rccc and +sc forms probably indicate the final products of complementary strand replication (1CSRc; Fig. 4) of circular molecules before they were loaded with histones. The adjacent −sc forms reflect stepwise chromatin formation in vivo, since each negative superhelical turn represents the addition of one nucleosome (9).
Intermediates that indicate replication.
Replicative intermediates are most prominent in the “eluate fraction” (Fig. 1b) in dependence of their proportion of ssDNA. Only a smaller part of the RDR intermediates (see below) was found in the “wash fraction” (Fig. 1a).
CSR.
A line starting from ssDNA (1ss), leading to ocDNA (oc), and representing complementary strand synthesis (CSR) of circular molecules of one genomic length (Fig. 1b, 1CSRc [26]) was found in the eluate fractions of all tested viruses (Fig. 2 and 3). A second line (1CSRl, Fig. 4) starting from ssDNA and approximating the double-stranded linear hdsDNA at the position of one genomic length could be resolved for AbMV (Fig. 1b), ACMV (Fig. 2a), BCTV (3b), and most prominent for TYLCV (Fig. 3a). The TYLCV case is exceptional, because many spots are arranged on these lines, whereas only a single or a few spots were present with the other viruses. This difference might suggest that the process of CSR is discontinuous for TYLCV and continuous for the other geminiviruses. Whether the presence of linear and circular CSR intermediates instead of one reflects different biological properties might be questioned. Changes might also occur due to different breakage frequencies of circular ssDNA during isolation.
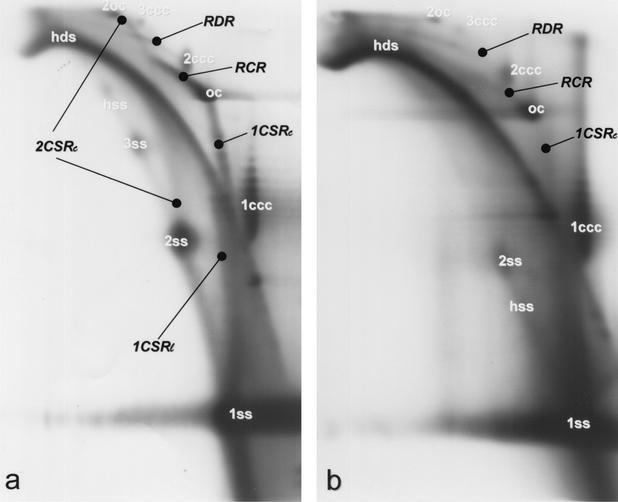
FIG. 2.
Analysis of BND cellulose eluate fractions (technique and abbreviations as described in Fig. 1) of two begomoviruses with a bipartite genome, ACMV (a) and TGMV (b). Detection was performed with ACMV or TGMV DNA A-probes, respectively.
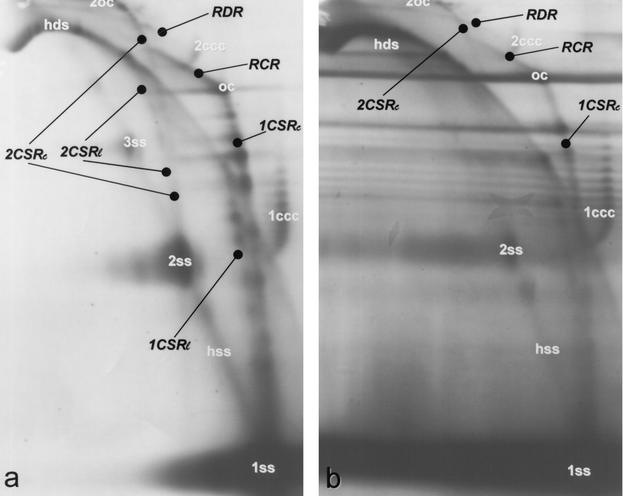
FIG. 3.
Analysis of the BND cellulose eluate fractions (technique and abbreviations are as described in Fig. 1) of a begomovirus (TYLCV) (a) and a curtovirus (BCTV) (b). Detection was performed with the respective DNA probes. The horizontal streaking in panel b is due to the slightly overloaded samples to reveal the replicative intermediates.
After chromatographic enrichment of ssDNA, new intermediates of CSR (Fig. 1b, 2a, and 3a; Fig. 4, 2CSRc and 2CSRl) were resolved. Two lines starting from dimeric circular and linear ssDNA (Fig. 4), which formed two discrete spots for AbMV (Fig. 1b, 2ss), reached either dimeric open circular (2oc; 2CSRc), or approached hdsDNA (2CSRl) at dimeric genome length were particularly visible for TYLCV (Fig. 3a) but also present for AbMV (Fig. 1b) and ACMV (Fig. 2a). Again, the relative amount of the linear molecules might rely on the purification efficiency. In both cases, these lines indicate that the replication cycle can also use dimeric and not only monomeric ssDNA as a template during systemic infection. Because the heterogeneous ssDNA crossed the origin spots of 2CSRc rather than of 2CSRl, as well as the corresponding left spot of 3ss, we conclude that the hss is, at least in the case of AbMV (Fig. 1b), composed of heterogeneous circular molecules (Fig. 4, hssc). This DNA could be the template of a population of heterogeneous molecules in complementary strand synthesis which, however, cannot be detected with certainty in two-dimensional gel electrophoresis because these intermediates would result in a field of hybridizing material (see Fig. 4, FY).
RCR.
RCR intermediates were exclusively found in the eluate fraction for all tested geminiviruses, as exemplified for AbMV in Fig. 1 (see also Fig. 2, 3, and 4). They start from ocDNA and extend into a straight line with the predominant length of one genomic copy as reported earlier (26). In the original blot, very faint prolongations of the line might hint at a rare incomplete termination of RCR, leading to multimer production by RCR. This feature was more prominent for TGMV (Fig. 2b) than for the other begomoviruses. A strand displacement replication (SDR; see Fig. 4), which is functionally analogous to RCR, might also start from heterogeneous linear dsDNA (hdsDNA) if this DNA harbors at a minimum one origin of replication. The products of such a replication should also appear in the chromatographic eluate fractions. In two-dimensional gel analysis they should form a field of hybridization signals (rather than arcs or lines) above the hdsDNA arc (Fig. 4, FX). A background hybridization of this kind was usually more prominent in eluate fractions than in wash fractions (compare, for example, Fig. 1b and a), but this appearance might also be due to other experimental factors or other replicative intermediates.
RDR.
Recombination-dependent replication (RDR) was indicated by heterogeneous linear double-stranded DNA (hdsDNA) and by an arc that approaches a pattern of superhelical topoisomers (26). In particular, the physical link between an hdsDNA with cccDNA was interpreted to be the result of a homologous recombination step to initiate replication. Both forms were visible in the wash, as well as in the eluate fraction (Fig. 1). The link between linear dsDNA and cccDNA was only resolved for the dimeric supercoiled forms (2ccc) because the respective monomeric ones, which have been resolved by electron microscopy, run too close to hdsDNA as reported previously (26). The relatively high amount of cccDNA (monomeric or dimeric) retained in the eluate fraction, however, corresponds to the interpretation that supercoiled DNA might be linked to linear dsDNA via a short ssDNA in RDR intermediates. A mere adsorptive attachment of linear dsDNA and cccDNA should result in the appearance of most, if not all of the complexes in the wash fraction. RDR-indicating intermediates were detected in all tested viruses at the dimeric level (Fig. 1b, 2, and 3). Quantitative differences in the amount of these forms should not be attributed to a certain virus but range within the variability of the infection process (unpublished data). In the case of BCTV (Fig. 3b), the gel had to be slightly overloaded to detect all forms; for the other viruses, all of the main intermediates were readily found after chromatographic purification in several independent hybridization blots.
Few unassigned signals remain in the blots of the wash fraction (Fig. 1a). Two or more single spots below ocDNA in the triangle of ocDNA, dsDNA, and cccDNA appeared reproducibly (see also reference 26), but interpretation is lacking so far.
DISCUSSION
The results extend our knowledge about multitasking in the replication of geminiviruses in several aspects. First, multitasking is not a peculiarity of AbMV, e.g., as a consequence of a special adaptation to the vegetatively propagated host but a widespread phenomenon among geminiviruses that infect dicots. Second, dsDNA intermediates are also recycled from dimeric ssDNA templates to form dimeric ocDNA and presumably dimeric cccDNA after nucleosome loading. Third, a small population of cccDNA with positive superhelicity was found with all of the viruses (Fig. 1a, +sc) that is gradually transformed to molecules with negative superhelicity (Fig. 1a, rccc and −sc) and allows the characterization of the viral chromatin structure.
An essential technical improvement for the analysis of replicative intermediates has been achieved by the combination of BND chromatography and two-dimensional gel electrophoresis, which allows the discrimination of various aspects of replication at a single glance, including the possibility to distinguish the chromatin structure of some intermediates. This combination of techniques, first introduced by Saunders et al. (48) and optimized here, has the potential to change our ideas about the dynamics of geminiviral multiplication. In the past, geminivirus research has focused on the discrete bands of viral DNA (ssDNA, cccDNA, and ocDNA), and “background” hybridization has been usually eliminated. The new technical view on the same process shows that the “smears” of hybridization signals are by no means neglible but contribute in a considerable amount to the total viral DNA and are very informative about the activity of the virus.
The results of BND chromatography further support the interpretation of the signals representing intermediate forms in former two-dimensional gel analysis, as also substantiated by electron microscopy (26). As expected, RCR and CSR intermediates were exclusively found in the eluate fractions due to their high proportion of ssDNA stretches. However, the RDR-indicating intermediates that are proposed to contain mainly linear and closed circular dsDNA also seem to possess some ssDNA stretches; otherwise, it could not be explained why so much of this material was retained on the column.
Although the presence of the particular RDR intermediates strongly suggests a RDR mechanism, the final proof is still lacking. Runon replication experiments with purified DNA (unpublished data) failed to show incorporation of nucleotides into the putative RDR intermediates, whereas both RCR and CSR intermediates were further processed under these in vitro conditions. However, this difference was not unexpected, since RDR intermediates should suffer from torsional stress during replication, which must be overcome, presumably by chromatin structure and topoisomerases. Future work will therefore concentrate on the analysis of intermediates with respect to their chromatin structure (1, 42).
The role of viral proteins in the geminiviral replication process has been studied in detail for several aspects, but whether they are involved in a recombination-dependent mechanism is unknown. Host cells have evolved instruments of proteins for RDR, mainly to repair broken chromosomes. The known number of proteins participating in this process has considerably increased during the last few years, mostly for yeasts (3, 4). Viruses might profit from these functions but also evolve their own recombination tools. The classic example for virus-encoded RDR proteins was shown for T4 phage, of which two proteins (uvsX and uvsY) cooperate to recombine two DNA strands (27). With accumulating sequence information it became clear that host and viral proteins involved in recombination are widespread and belong to common protein families (10).
Four genes encoded by the complementary sense of DNA A in case of dicots-infecting and two spliced open reading frames in the case of monocots-infecting geminiviruses have been implicated in the regulation of replication or transcription (for review, see references 22 and 39). Among them Rep is a multifunctional protein belonging to a family of RCR initiator proteins (25, 31). They are key enzymes with nicking-closing activity to start and complete RCR (22), regulate their own transcription (13, 14), and recruit host proteins to initiate DNA synthesis by inducing S phase (2, 21, 29, 30, 66). An additional helicase activity has been predicted from sequence motives (20) in the second half of the protein which could be helpful as well in RCR as in RDR. AC2 (also known as AL2) functions in transcription regulation (24, 50, 56, 57-59) and silencing suppression (60). AC3 enhances replication presumably by recruiting further host proteins for the viral replication machinery (15, 51, 52, 58). The fourth open reading frame (ac4 [also known as al4], c4) has been discussed in various contexts, from transcriptional regulation, symptom expression, to movement (28, 33, 34, 44), but in bipartite begomoviruses it is questionable whether AC4 has a function at all (43). Since transfected DNA A alone can give rise to RDR intermediates (26) and since mutants in AC1 or AC2/3 recombined and reverted to wild-type very rapidly under experimental conditions (16), our first guess was that AC2 and AC3 might cooperate in RDR in analogy to UvsX and UvsY of T4 phage, perhaps in conjunction with Rep. Mutants in both AC2 and AC3, however, are still capable of inducing RDR intermediates, although to a lesser extent (26). Therefore, these proteins are not necessary for RDR, although they are perhaps helpful. Currently, a possible function of Rep in recombination is not testable, because no mutant is available that discriminates between its basic function in RCR and RDR. In conclusion, it still remains to be shown whether a geminiviral RDR mechanism completely relies on host factors or is promoted by a viral protein.
In the present study, samples were taken from the first stages of systemic infection and expanding leaves. Under these conditions, the various intermediate DNA molecules were always found simultaneously with only few variations in their relative amounts as observed earlier (26). This coincidence is in contrast to the situation with the model case of T4 phages, where RCR is an early process and RDR is a late process during infection. For an expanding foliage leaf, however, it may be anticipated that several different cellular infection events occur one after the other, giving rise to a population of cells that behave asynchronously. The results presented here exclude that this concert of events is a peculiarity of vegetatively propagated Abutilon plants. It was also observed at the earliest stage after agroinfection with cloned DNA.
Whereas the anticipated early and late stages of replication appeared simultaneously during systemic infection, we detected only RDR but no RCR intermediates at the earliest detectable stage of local infection in leaf-disk assays by using agroinfection—against our expectation (26). However, a final decision on whether RCR or RDR is the early event on the cellular level remains to be made, because the earliest hours during the first 2 days of infection have escaped detection by Southern hybridization thus far.
Acknowledgments
We thank C. Wege and R. Ghosh for helpful discussions, E. Bejarano and J. Stanley for providing plasmids, and S. Kober for skillful technical assistance.
REFERENCES
- 1.Abouzid, A. M., T. Frischmuth, and H. Jeske. 1988. A putative replicative form of the Abutilon mosaic virus (gemini group) in a chromatin-like structure. Mol. Gen. Genet. 212:252-258. [Google Scholar]
- 2.Ach, R. A., T. Durfee, A. B. Miller, P. Taranto, L. Hanley-Bowdoin, P. C. Zambryski, and W. Gruissem. 1997. RRB1 and RRB2 encode maize retinoblastoma-related proteins that interact with a plant D-type cyclin and geminivirus replication protein. Mol. Cell. Biol. 17:5077-5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumann, P., and S. C. West. 1998. Role of the human RAD51 protein in homologous recombination and double-stranded break repair. Trends Biochem. Sci. 23:247-251. [DOI] [PubMed] [Google Scholar]
- 4.Beernink, H. T. H., and S. W. Morrical. 1999. RMPs: recombination/replication mediator proteins. Trends Biochem. Sci. 24:385-389. [DOI] [PubMed] [Google Scholar]
- 5.Berrie, L. C., E. P. Rybicki, and M. E. C. Rey. 2001. Complete nucleotide sequence and host range of South African cassava mosaic virus: further evidence for recombination amongst begomoviruses. J. Gen. Virol. 82:53-58. [DOI] [PubMed] [Google Scholar]
- 6.Briddon, R. W., I. D. Bedford, J. H. Tsai, and P. G. Markham. 1996. Analysis of the nucleotide sequence of the treehopper-transmitted geminivirus, tomato pseudo-curly top virus, suggests a recombinant origin. Virology 219:387-394. [DOI] [PubMed] [Google Scholar]
- 7.Briddon, R. W., S. Mansoor, I. Bedford, M. S. Pinner, K. Saunders, J. Stanley, Y. Zafar, K. A. Malik, and P. G. Markham. 2001. Identification of DNA components required for induction of cotton leaf curl disease. Virology 285:234-243. [DOI] [PubMed] [Google Scholar]
- 8.Briddon, R. W., M. S. Pinner, J. Stanley, and P. G. Markham. 1990. Geminivirus coat protein gene replacement alters insect specificity. Virology 177:85-94. [DOI] [PubMed] [Google Scholar]
- 9.Clark, D. J. 1998. Counting nucleosome cores on circular DNA by using topoisomerase I, p. 139-152. In H. Gould (ed.), Chromatin: a practical approach. Oxford University Press, Oxford, England.
- 10.Connelly, J. C., and D. R. F. Leach. 2002. Tethering on the brink: the evolutionarily conserved Mre 11-Rad 50 complex. Trends Biochem. Sci. 27:410-418. [DOI] [PubMed] [Google Scholar]
- 11.Dijkwel, P. A., J. P. Vaughn, and J. L. Hamlin. 1991. Mapping of replication initiation sites in mammalian genomes by two-dimensional gel analysis: stabilization and enrichment of replication intermediates by isolation on the nuclear matrix. Mol. Cell. Biol. 1:3850-3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dry, I. B., L. R. Krake, J. E. Rigden, and M. A. Rezaian. 1997. A novel subviral agent associated with a geminivirus: the first report of a DNA satellite. Proc. Natl. Acad. Sci. USA 94:7088-7093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eagle, P. A., and L. Hanley-Bowdoin. 1997. cis Elements that contribute to geminivirus transcriptional regulation and the efficiency of DNA replication. J. Virol. 71:6947-6955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eagle, P. A., B. M. Orozco, and L. Hanley-Bowdoin. 1994. A DNA sequence required for geminivirus replication also mediates transcriptional regulation. Plant Cell 6:1157-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elmer, J. S., L. Brand, G. Sunter, W. Gardiner, D. M. Bisaro, and S. G. Rogers. 1988. Genetic analysis of tomato golden mosaic virus. II. The conserved AL1 ORF product is essential for replication. Nucleic Acids Res. 16:7043-7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans, D., and H. Jeske. 1993. Complementation and recombination between mutants of complementary sense genes of DNA A of Abutilon mosaic virus. Virology 197:492-496. [DOI] [PubMed] [Google Scholar]
- 17.Fondong, V. N., J. S. Pita, M. E. C. Rey, A. de Kockko, R. N. Beachy, and C. M. Fauquet. 2000. Evidence of synergism between African cassava mosaic virus and a new double-recombinant geminivirus infecting cassava in Cameroon. J. Gen. Virol. 81:287-297. [DOI] [PubMed] [Google Scholar]
- 18.Frischmuth, T., M. Engel, and H. Jeske. 1997. Beet curly top virus DI DNA-mediated resistance is linked to its size. Mol. Breed. 3:213-217. [Google Scholar]
- 19.Gibson, R. W., J. P. Legg, and G. W. Otim-Nape. 1996. Unusually severe symptoms are a characteristic of the current epidemic of mosaic virus disease of cassava in Uganda. Ann. Appl. Biol. 128:479-490. [Google Scholar]
- 20.Gorbalenya, A. E., and E. V. Koonin. 1993. Helicase: amino acid sequence comparisons and structure-function relationships. Curr. Opin. Struct. Biol. 3:419-429. [Google Scholar]
- 21.Gutierrez, C. 2000. DNA replication and cell cycle in plants: learning from geminiviruses. EMBO J. 19:792-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanley-Bowdoin, L., S. B. Settlage, B. M. Orozco, S. Nagar, and D. Robertson. 1999. Geminiviruses: models for plant DNA replication, transcription, and cell cycle regulation. Crit. Rev. Plant Sci. 18:71-106. [PubMed] [Google Scholar]
- 23.Harrison, B. D., and D. J. Robinson. 1999. Natural genomic and antigenic variation in whitefly-transmitted geminiviruses (begomoviruses). Annu. Rev. Phytopathol. 37:369-398. [DOI] [PubMed] [Google Scholar]
- 24.Hartitz, M. D., G. Sunter, and D. M. Bisaro. 1999. The tomato golden mosaic virus transactivator (TrAP) is a single-stranded DNA and zinc-binding phosphoprotein with an acidic activation domain. Virology 263:1-14. [DOI] [PubMed] [Google Scholar]
- 25.Ilyina, T. V., and E. V. Koonin. 1992. Conserved sequence motifs in the initiator proteins for rolling circle DNA replication encoded by diverse replicons from eubacteria, eucaryotes, and archaebacteria. Nucleic Acids Res. 20:3279-3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeske, H., M. Lütgemeier, and W. Preiss. 2001. Distinct DNA forms indicate rolling circle and recombination-dependent replication of Abutilon mosaic geminivirus. EMBO J. 20:6158-6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang, H., D. Giedroc, and T. Kodadek. 1993. The role of protein-protein interactions in the assembly of the presynaptic filament for T4 homologous recombination. J. Biol. Chem. 268:7904-7911. [PubMed] [Google Scholar]
- 28.Jupin, I., F. De Kouchkovsky, F. Jouanneau, and B. Gronenborn. 1994. Movement of tomato yellow leaf curl geminivirus (TYLCV): involvement of the protein encoded by ORF C4. Virology 204:82-90. [DOI] [PubMed] [Google Scholar]
- 29.Kong, L.-J., and L. Hanley-Bowdoin. 2002. A geminivirus replication protein interacts with a protein kinase and a motor protein that display different expression patterns during plant development and infection. Plant Cell 14:1817-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kong, L.-J., B. M. Orozco, J. L. Roe, S. Nagar, S. Ou, H. S. Feiler, T. Durfee, A. B. Miller, W. Gruissem, D. Robertson, and L. Hanley-Bowdoin. 2000. A geminivirus replication protein interacts with the retinoblastoma protein through a novel domain to determine symptoms and tissue specificity of infection in plants. EMBO J. 19:3485-3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koonin, E. V., and T. V. Ilyina. 1992. Geminivirus replication proteins are related to prokaryotic plasmid rolling circle DNA replication initiator proteins. J. Gen. Virol. 73:2763-2766. [DOI] [PubMed] [Google Scholar]
- 32.Kornberg, A., and T. A. Baker. 1992. DNA replication. W. H. Freeman and Co., New York, N.Y.
- 33.Krake, L. R., M. A. Rezaian, and I. B. Dry. 1998. Expression of the tomato leaf curl geminivirus C4 gene produces viruslike symptoms in transgenic plants. Mol. Plant-Microbe Interact. 11:413-417. [Google Scholar]
- 34.Latham, J. R., K. Saunders, M. S. Pinner, and J. Stanley. 1997. Induction of plant cell division by beet curly top virus gene C4. Plant J. 11:1273-1283. [Google Scholar]
- 35.Moffat, A. 1999. Geminiviruses emerge as serious crop threat. Science 286:1835. [Google Scholar]
- 36.Mosig, G. 1998. Recombination and recombination-dependent DNA replication in bacteriophage T4. Annu. Rev. Genet. 32:379-413. [DOI] [PubMed] [Google Scholar]
- 37.Mosig, G., J. Gewin, A. Luder, N. Colowick, and D. Vo. 2001. Two recombination-dependent DNA replication pathways of bacteriophage T4, and their roles in mutagenesis and horizontal gene transfer. Proc. Natl. Acad. Sci. USA 98:8306-8311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Padidam, M., S. Sawyer, and C. M. Fauquet. 1999. Possible emergence of new geminiviruses by frequent recombination. Virology 285:218-225. [DOI] [PubMed] [Google Scholar]
- 39.Palmer, K. E., and E. P. Rybicki. 1998. The molecular biology of mastreviruses. Adv. Virus Res. 50:183-234. [DOI] [PubMed] [Google Scholar]
- 40.Permana, P. A., C. A. Ferrer, and R. M. Snapka. 1994. Inverse relationship between catenation and superhelicity in newly replicated simian virus 40 daughter chromosomes. Biochem. Biophys. Res. Commun. 201:1510-1517. [DOI] [PubMed] [Google Scholar]
- 41.Permana, P. A., R. M. Snapka, L. L. Shen, D. T. W. Chu, J. Clement, and J. J. Plattner. 1994. Quinobenoxazines: a class of novel antitumor quinolones and potent mammalian DNA topoisomerase II catalytic inhibitors. Biochemistry 33:11333-11339. [DOI] [PubMed] [Google Scholar]
- 42.Pilartz, M., and H. Jeske. 1992. Abutilon mosaic geminivirus double-stranded DNA is packed into minichromosomes. Virology 189:800-802. [DOI] [PubMed] [Google Scholar]
- 43.Pooma, W., and I. T. D. Petty. 1996. Tomato golden mosaic virus open reading frame AL4 is genetically distinct from its C4 analogue in monopartite geminiviruses. J. Gen. Virol. 77:1947-1951. [DOI] [PubMed] [Google Scholar]
- 44.Rigden, J. E., L. R. Krake, M. A. Rezaian, and I. B. Dry. 1994. ORF C4 of tomato leaf curl geminivirus is a determinant of symptom severity. Virology 204:847-850. [DOI] [PubMed] [Google Scholar]
- 45.Rybicki, E. P., R. W. Briddon, J. K. Brown, C. M. Fauquet, D. P. Maxwell, B. D. Harrison, P. G. Markham, D. M. Bisaro, D. Robinson, and J. Stanley. 2000. Family Geminiviridae, p. 285-297. In M. H. V. van Regenmortel, C. M. Fauquet, and D. H. L. Bishop (ed.), Virus taxonomy: classification and nomenclature of viruses. Academic Press, Inc., San Diego, Calif.
- 46.Sanz, A. I., A. Fraile, F. Garcia-Arenal, X. Zhou, D. Robinson, S. Khalid, T. Butt, and B. D. Harrison. 2000. Multiple infection, recombination and genome relationships among begomovirus isolates found in cotton and other plants in Pakistan. J. Gen. Virol. 81:1839-1849. [DOI] [PubMed] [Google Scholar]
- 47.Saunders, K., I. D. Bedford, R. W. Briddon, P. G. Markham, S. M. Wong, and J. Stanley. 2000. A unique virus complex causes Ageratum yellow vein disease. Proc. Natl. Acad. Sci. USA 97:6890-6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saunders, K., A. Lucy, and J. Stanley. 1991. DNA forms of the geminivirus African cassava mosaic virus consistent with a rolling circle mechanism of replication. Nucleic Acids Res. 19:2325-2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saunders, K., A. Lucy, and J. Stanley. 1992. RNA-primed complementary-sense DNA synthesis of the geminivirus African cassava mosaic virus. Nucleic Acids Res. 20:6311-6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saunders, K., and J. Stanley. 1995. Complementation of African cassava mosaic virus AC2 gene function in a mixed bipartite geminivirus infection. J. Gen. Virol. 76:2287-2292. [DOI] [PubMed] [Google Scholar]
- 51.Settlage, S. B., A. B. Miller, W. Gruissem, and L. Hanley-Bowdoin. 2001. Dual interaction of a geminivirus replication accessory factor with a viral replication protein and a plant cell cycle regulator. Virology 279:570-576. [DOI] [PubMed] [Google Scholar]
- 52.Settlage, S. B., A. B. Miller, and L. Hanley-Bowdoin. 1996. Interactions between geminivirus replication proteins. J. Virol. 70:6790-6795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Snapka, R. M., P. A. Permana, G. Marquit, and C.-G. Shin. 1991. Two-dimensional agarose gel analysis of simian virus 40 DNA replication intermediates. Methods Companion Methods Enzymol. 3:73-82. [Google Scholar]
- 54.Stanley, J., R. Townsend, and S. J. Curson. 1985. Pseudorecombinants between cloned DNAs of two isolates of cassava latent virus. J. Gen. Virol. 66:1055-1061. [Google Scholar]
- 55.Sung, Y. K., and R. H. A. Coutts. 1995. Pseudorecombination and complementation between potato yellow mosaic geminivirus and tomato golden mosaic geminivirus. J. Gen. Virol. 76:2809-2815. [DOI] [PubMed] [Google Scholar]
- 56.Sunter, G., and D. M. Bisaro. 1991. Transactivation in a geminivirus: AL2 gene product is needed for coat protein expression. Virology 180:416-419. [DOI] [PubMed] [Google Scholar]
- 57.Sunter, G., and D. M. Bisaro. 1997. Regulation of a geminivirus coat protein promoter by AL2 protein (TrAP): evidence for activation and derepression mechanisms. Virology 232:269-280. [DOI] [PubMed] [Google Scholar]
- 58.Sunter, G., M. D. Hartitz, S. G. Hormuzdi, C. L. Brough, and D. M. Bisaro. 1990. Genetic analysis of tomato golden mosaic virus: ORF AL2 is required for coat protein accumulation while ORF AL3 is necessary for efficient DNA replication. Virology 179:69-77. [DOI] [PubMed] [Google Scholar]
- 59.Sunter, G., D. C. Stenger, and D. M. Bisaro. 1994. Heterologous complementation by geminivirus AL2 and AL3 genes. Virology 203:203-210. [DOI] [PubMed] [Google Scholar]
- 60.Sunter, G., J. L. Sunter, and D. M. Bisaro. 2001. Plants expressing tomato golden mosaic virus AL2 or beet curly top virus L2 transgenes show enhanced susceptibility to infection by DNA and RNA viruses. Virology 285:59-70. [DOI] [PubMed] [Google Scholar]
- 61.Unseld, S., M. Ringel, P. Höfer, M. Höhnle, H. Jeske, I. D. Bedford, P. G. Markham, and T. Frischmuth. 2000. Host range and symptom variation of pseudorecombinant virus produced by two distinct bipartite geminiviruses. Arch. Virol. 145:1449-1454. [DOI] [PubMed] [Google Scholar]
- 62.Unseld, S., M. Ringel, A. Konrad, S. Lauster, and T. Frischmuth. 2000. Virus-specific adaptations for the production of a pseudorecombinant virus formed by two distinct bipartite geminiviruses from Central America. Virology 274:179-188. [DOI] [PubMed] [Google Scholar]
- 63.von Hippel, P. H. 2000. The recombination-replication interface. Trends Biochem. Sci. 25:155.. [DOI] [PubMed] [Google Scholar]
- 64.Wege, C., R.-D. Gotthardt, T. Frischmuth, and H. Jeske. 2000. Fulfilling Koch's postulates for Abutilon mosaic virus. Arch. Virol. 145:2217-2225. [DOI] [PubMed] [Google Scholar]
- 65.Wege, C., K. Saunders, J. Stanley, and H. Jeske. 2001. Comparative analysis of tissue tropism of bipartite geminiviruses. J. Phytopathol. 149:359-368. [Google Scholar]
- 66.Xie, Q., A. P. Sanz-Burgos, G. J. Hannon, and C. Gutierrez. 1996. Plant cells contain a novel member of the retinoblastoma family of growth regulatory proteins. EMBO J. 15:4900-4908. [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang, W., N. H. Olson, T. S. Baker, L. Faulkner, M. Agbandje-McKenna, M. I. Boulton, J. W. Davies, and R. McKenna. 2001. Structure of the maize streak virus geminate particle. Virology 279:471-477. [DOI] [PubMed] [Google Scholar]
- 68.Zhou, X., Y. Liu, L. Calvert, C. Munoz, G. W. Otim-Nape, D. J. Robinson, and B. D. Harrison. 1997. Evidence that DNA: a of a geminivirus associated with severe cassava mosaic disease in Uganda has arisen by interspecific recombination. J. Gen. Virol. 78:2101-2111. [DOI] [PubMed] [Google Scholar]
- 69.Zhou, X. P., Y. L. Liu, D. J. Robinson, and B. D. Harrison. 1998. Four DNA-A variants among Pakistani isolates of cotton leaf curl virus and their affinities to DNA-A of geminivirus isolates from okra. J. Gen. Virol. 79:915-923. [DOI] [PubMed] [Google Scholar]