Abstract
Checkpoint pathways inhibit cyclin-dependent kinases (Cdks) to arrest cell cycles when DNA is damaged or unreplicated. Early embryonic cell cycles of Xenopus laevis lack these checkpoints. Completion of 12 divisions marks the midblastula transition (MBT), when the cell cycle lengthens, acquiring gap phases and checkpoints of a somatic cell cycle. Although Xenopus embryos lack checkpoints prior to the MBT, checkpoints are observed in cell-free egg extracts supplemented with sperm nuclei. These checkpoints depend upon the Xenopus Chk1 (XChk1)-signaling pathway. To understand why Xenopus embryos lack checkpoints, xchk1 was cloned, and its expression was examined and manipulated in Xenopus embryos. Although XChk1 mRNA is degraded at the MBT, XChk1 protein persists throughout development, including pre-MBT cell cycles that lack checkpoints. However, when DNA replication is blocked, XChk1 is activated only after stage 7, two cell cycles prior to the MBT. Likewise, DNA damage activates XChk1 only after the MBT. Furthermore, overexpression of XChk1 in Xenopus embryos creates a checkpoint in which cell division arrests, and both Cdc2 and Cdk2 are phosphorylated on tyrosine 15 and inhibited in catalytic activity. These data indicate that XChk1 signaling is intact but blocked upstream of XChk1 until the MBT.
INTRODUCTION
Checkpoints that arrest the cell cycle in the presence of unreplicated or damaged DNA are conserved features among eukaryotes, and mutations that incapacitate these checkpoints are frequent in cancers (Elledge, 1996). The early, embryonic cell cycles of Xenopus laevis provide rare examples of nonpathological cell divisions that lack cell cycle checkpoints. Following fertilization, the Xenopus egg divides after 90 min. It then begins rapid and synchronous cleavage cycles 2–12. These cell divisions are simplified cell cycles that alternate between DNA replication and mitosis without cell growth or gap phases. Cell divisions proceed “unchecked” when DNA replication is blocked (Kimelman et al., 1987; Newport and Dasso, 1989; Clute and Masui, 1997) or DNA is damaged (Anderson et al., 1997; Hensey and Gautier, 1997). These simplified cell cycles are characterized by oscillations in the activity of Cdc2 (due to periodic synthesis and degradation of cyclins A and B) and in the activity of Cdk2 (due to an unresolved regulatory mechanism).
Completion of the 12th cleavage marks the midblastula transition (MBT) when zygotic transcription initiates, cells gain motility, an apoptotic program becomes functional, and embryonic cell cycles lengthen as they acquire gap phases between DNA replication and mitosis (Newport and Kirschner, 1982a; Frederick and Andrews, 1994; Sible et al., 1997). Cell cycle checkpoints become operative after the MBT (Newport and Dasso, 1989; Clute and Masui, 1997), but the relationship between checkpoint acquisition and other events of the MBT is not well understood. To better understand the molecular basis for checkpoint acquisition, our laboratory has investigated checkpoint-signaling pathways during early Xenopus development.
Although Xenopus embryos lack checkpoint controls prior to the MBT, artificial checkpoints can be created in cell-free extracts derived from Xenopus eggs. When DNA replication is blocked with aphidicolin (Dasso and Newport, 1990) or DNA is damaged by UV-irradiation (Kumagai et al., 1998b), extracts supplemented with sperm nuclei arrest with low Cdc2 activity that is insufficient to induce mitosis. However, neither aphidicolin nor irradiation arrests cell cycles of the intact Xenopus embryo prior to the MBT (Kimelman et al., 1987; Newport and Dasso, 1989; Anderson et al., 1997; Clute and Masui, 1997; Hensey and Gautier, 1997).
The engagement of cell cycle checkpoints in Xenopus egg extracts involves biochemical signaling events that are conserved among eukaryotes (Paulovich et al., 1997; Westphal, 1997) and are mediated by the checkpoint kinase Chk1. Chk1 was first identified in the fission yeast Schizosaccharomyces pombe as a gene encoding a protein kinase that interacts genetically with cdc2 and is required for the DNA damage checkpoint (Walworth et al., 1993). In fission yeast and mammals, Chk1 is activated by a phosphorylation event in response to damaged DNA (Walworth and Bernards, 1996; Sanchez et al., 1997). Chk1 regulates Cdk activity indirectly by phosphorylating Cdc25 (Furnari et al., 1997; Sanchez et al., 1997; Zeng et al., 1998), generating a binding site for the cytoskeletal protein 14-3-3, which sequesters Cdc25 in the cytoplasm (Peng et al., 1997; Zeng et al., 1998). In addition to mediating a DNA damage checkpoint, Chk1 plays a role in the checkpoint signal that responds to unreplicated DNA in yeast. However, its function in the DNA replication checkpoint is redundant with the Cds1 (Chk2) checkpoint kinase (Zeng et al., 1998).
Many features of Chk1 signaling are reconstituted in cell-free egg extracts from Xenopus. In response to damaged or unreplicated DNA in these extracts, Cdc2 is maintained in its tyrosine-phosphorylated inactive state because Cdc25 becomes phosphorylated on serine 287 and associates with 14-3-3 (Kumagai et al., 1998b). As in yeast, a Xenopus homolog of Chk1 (XChk1) is phosphorylated in egg extracts in the presence of damaged or unreplicated DNA (Kumagai et al., 1998a). XChk1 phosphorylates Cdc25C in vitro, and XChk1 signaling is required for complete engagement of a caffeine-sensitive DNA replication checkpoint in egg extracts (Kumagai et al., 1998a).
These studies indicate that the XChk1 pathway is functional prior to the MBT in egg extracts, yet cell cycle checkpoints are not operational in the intact pre-MBT embryo. An investigation of XChk1 signaling in the intact Xenopus embryo was performed to resolve these apparent paradoxes in checkpoint controls between the in vitro and in vivo systems. The goal of the following experiments was to address four questions: 1) When is XChk1 expressed during Xenopus embryonic development? 2) When is XChk1 signaling first activated in response to damaged or unreplicated DNA? 3) Can a checkpoint be created prior to the MBT by manipulating XChk1 signaling? and 4) Which cyclin-dependent kinases (Cdks) are the targets of XChk1 signaling in the intact Xenopus embryo?
MATERIALS AND METHODS
Cloning Xenopus Chk1 (XChk1)
To identify a Chk1 homolog in the Xenopus genome, the following three pairs of degenerate oligodeoxyribonucleotide primers were designed based on conserved regions within the human, mouse, Drosophila, and Caenorhabditis elegans chk1 sequences (GenBank accession numbers AF016582, AF016583, AF057041, and U44902, respectively): primer pair 1, 5′-GGIGA(G/A) GGIGCIT(A/T)(T/C)GGIGA(G/A)-3′ and 5′-(T/C)TCIGGIGCI(G/A)C(G/A)TAIGGIAIIGTICC(G/A)CA-3′; primer pair 2, 5′-GGIGA(G/A)GGIGCIT(A/T)(T/C)GGIGA(G/A)-3′ and 5′-(G/A)-TCCCAIGGIA(G/A)(T/C)TCICCIG(T/C)IA(G/A)CAT-3′; and primer pair 3, 5′-ATG(T/C)TI(G/A)CIGGIGA(G/A)(T/C)TICCITGGGA(T/C)-3′ and 5′-IC(T/G)(T/C)TT(G/A)AA(T/C)TCIA(G/A)ICC(G/A)(A/C)ICC(T/C)TT-3′.
Using these primer pairs and total RNA derived from Xenopus oocytes or stage 28–30 embryos as template for a reverse transcription-polymerase chain reaction, several regions of the Xenopus chk1 gene were amplified. These PCR products were subcloned into pGEMT-Easy vectors (Promega, Madison, WI), and the inserts were labeled with [α-32P]dCTP by random priming and used in combination as probes to screen an oocyte cDNA library in a λZAP Express phagemid vector. The sequence of the longest clone was determined on both strands by a combination of manual and automated sequencing techniques. Based on homology with human and mouse genes, the clone encodes a full-length chk1 homolog that was named xchk1. The GenBank accession number is AF117816.
Maintenance and Manipulation of Embryos
Eggs from wild-type X. laevis (Xenopus Express) were fertilized in vitro, dejellied in 2% cysteine, 0.1× MMR (0.5 mM HEPES, pH 7.8, 10 mM NaCl, 0.2 mM KCl, 0.1 mM MgSO4, 0.2 mM CaCl2, 0.01 mM EDTA,), and maintained in 0.1× MMR. Embryos were staged according to Nieuwkoop and Faber (1975). For some experiments, embryos were microinjected at the one-cell stage with the indicated quantities of mRNA dissolved in TE buffer (10 mM Tris, pH 8.0, 1 mM EDTA) and then maintained in 5% Ficoll, 0.1× MMR. To block DNA replication, embryos were incubated at the indicated stages in 0.1× MMR containing 100 μg/ml aphidicolin (Calbiochem, La Jolla, CA) and 1% dimethyl sulfoxide. Control embryos were maintained in 0.1× MMR, 1% dimethyl sulfoxide. To inflict DNA damage, embryos were treated at the indicated stage with 60-Gy ionizing radiation (IR) emitted from a TFI Mini Shot X-ray chamber. Embryos were observed with an Olympus SZX12 stereo microscope and photographed with an Olympus DP10 digital camera.
Northern Analysis of XChk1 Expression
Northern analysis of XChk1 mRNA expression during Xenopus development was performed essentially as described previously (Sible et al., 1997). Total RNA was isolated from embryos by using TriReagent (Molecular Research Center, Inc., Cincinnati, OH). Twenty micrograms of each RNA was resolved by denaturing gel electrophoresis, transferred to a 0.2-μm Nytran membrane with a TurboBlotter apparatus (Schleicher & Schuell, Keene, NH), and then cross-linked to the membrane with a Stratagene UV cross-linker. A fragment of the xchk1 gene, derived from the PCR amplification of cDNA from stage 28–30 embryos by using primer pair 2, was labeled with [α-32P]dCTP by random priming. This probe was hybridized with the RNA blot in QuickHyb solution (Stratagene, La Jolla, CA). After washing, the blot was exposed to Kodak XAR5 film.
Generation of a Polyclonal XChk1 Antibody
A recombinant His-tagged XChk1 fusion protein was generated by subcloning the xchk1 open reading frame into the pET30 expression vector (Novagen, Madison, WI). BL21DE3 cells were transformed with this plasmid. Bacteria were grown to an OD600 of 0.6 and then induced to express recombinant XChk1 protein with 1 mM isopropyl β-d-thiogalactoside and cultured at 37°C for 4 h. The recombinant protein was purified under denaturing conditions on TALON cobalt beads (Clontech, Palo Alto, CA) according to the manufacturer's protocol. Polyclonal antibodies were generated by immunizing rabbits with recombinant His-tagged XChk1 on TALON beads. To purify XChk1 antibodies, the antiserum was diluted 1:10 in 10% nonfat dry milk in phosphate-buffered saline (PBS) (100 mM Na2HPO4, pH 7.5, 100 mM NaCl) and incubated with nitrocellulose strips containing ∼100 μg of antigen. Strips were washed with PBS, 0.05% Tween, antibodies were eluted from the strips with 100 mM glycine, pH 2.8, and the purified antibodies were neutralized with 1/10 volume 1 M Tris, pH 8.0, and 1/10 volume 10× PBS. Sodium azide was added to 0.002%, and the antibodies were stored at 4°C.
Western Analysis of XChk1
Embryos were collected at the times indicated, snap frozen on dry ice, and homogenized in EB buffer (20 mM HEPES, pH 7.5, 80 mM β-glycerophosphate, 15 mM MgCl2, 20 mM EGTA, 50 mM NaF, 1 mM sodium orthovanadate, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl flouride, 20 μg/ml leupeptin, 1 mM microcystin), resolved by SDS-PAGE, transferred to nylon or nitrocellulose membranes, and blotted essentially as described previously (Hartley et al., 1996; Sible et al., 1997). For Figure 1, modified Laemmli SDS-polyacrylamide gels were used (separating gel = 12.2% acrylamide, 0.4% bis-acrylamide, 0.3 M Tris, pH 8.8, 0.1% SDS, 10% glycerol; running buffer = 0.025 M Tris, pH 8.3, 0.192 M glycine, 0.1% SDS). For Figures 2–5, modified Anderson SDS-polyacrylamide gels were used (separating gel = 10–12% acrylamide, 0.10–0.13% bis-acrylamide, 0.37 M Tris, pH 8.7, 0.1% SDS; running buffer = 0.05 M Tris, pH 8.3, 0.384 M glycine, 0.2% SDS). The XChk1 antibody was generated as described above, and the antibody against tyrosine-phosphorylated Cdc2 and Cdk2 was purchased from New England Biolabs (Beverly, MA; catalog number 9111). Western analysis of XChk1 was performed by diluting antibodies in 10% nonfat dry milk in PBS and washing membranes in PBS, 0.1% Tween. Western analysis of phosphotyrosine on Cdks was performed by diluting antibodies in 5% bovine serum albumin in TBS (20 mM Tris, pH 7.6, 137 mM NaCl), 0.1% Tween and washing membranes with TBS, 0.1% Tween. To visualize immunoreactive proteins, a horseradish peroxidase-conjugated secondary antibody was hybridized, and chemiluminescence from the secondary antibody was detected with the ECL Plus kit (Amersham, Arlington Heights, IL). Densitometry was performed by using the Alpha Innotech imaging system.
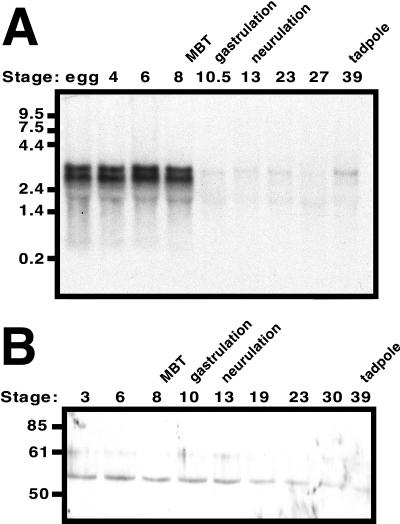
Figure 1.
Developmental expression of Xenopus chk1 (xchk1). (A) Northern analysis of xchk1 gene expression during Xenopus development. Total RNA was isolated from Xenopus embryos at the indicated stages of development. The RNA blot was probed with a random-primed 588-base pair fragment of xchk1 that was generated by RT-PCR and encodes amino acids 13–209. The migration of molecular-weight markers in kilobases is shown on the left. Twenty micrograms total RNA was loaded per lane. (B) Western analysis of XChk1 protein during Xenopus development. Extracts were prepared from embryos at the indicated stages of development. The migration of molecular-weight markers in kDa is shown. One embryo equivalent was loaded per lane.
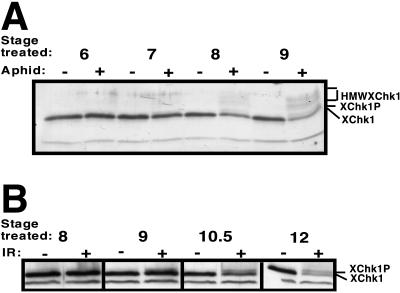
Figure 2.
Altered electrophoretic mobility of XChk1 in response to aphidicolin and IR. Embryos were treated with 100 μg/ml aphidicolin (A) or 60-Gy IR (B), collected after sibling controls had completed 1–2 cell cycles, and Western analysis of XChk1 was performed. XChk1-P indicates the band of retarded electrophoretic mobility, previously shown to be the phosphorylated form of XChk1 (Kumagai et al., 1998a). HMWXCHK1 indicates high-molecular-weight species of XChk1 that migrate more slowly than XChk1 and XChk1P. The immunoreactive band that migrates below XChk1 is observed in some experiments and is nonspecific. One embryo equivalent was loaded per lane.
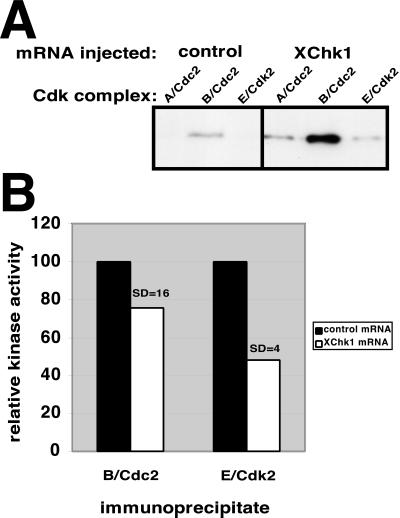
Figure 5.
Both Cdc2 and Cdk2 are targets of XChk1. Embryos were microinjected with 15 ng of FLAG-tagged luciferase (control) or FLAG-tagged XChk1 mRNA at the one-cell stage. At stage 6.5 (3.5 h postfertilization), embryos were collected, and cyclin A/Cdc2, cyclin B/Cdc2 and cyclin E/Cdk2 complexes were immunoprecipitated with antibodies against cyclins A, B, and E, respectively. (A) Immunoprecipitates were probed with an antibody that recognizes phosphorylated tyrosine 15 on Cdc2 and Cdk2. (B) Cyclin B/Cdc2 and cyclin E/Cdk2 immunoprecipitates were assayed for ability to phosphorylate histone H1. Ten embryo equivalents were loaded per lane.
Expression of Exogenous XChk1 in Xenopus Embryos
The PCR was used to subclone N-terminal FLAG-tagged xchk1 open reading frame into the pSP64polyA vector (Promega). This plasmid construction was linearized and used as template for the Ambion SP6 mMessage mMachine in vitro transcription kit to synthesize polyadenylated XChk1 mRNA containing a 5′ 7-methyl-guanosine cap. FLAG-tagged XChk1 N135A mRNA (in which asparagine 135 was mutationally altered to alanine to generate catalytically inactive protein) and FLAG-tagged luciferase mRNA were prepared similarly. (A luciferase cDNA clone was provided by Dr. Charles Rutherford, Virginia Polytechnic Institute and State University, Blacksburg, VA) For the experiments in Figure 4, XChk1 mRNA was transcribed directly from the original phagemid clone by using T3 polymerase. The control mRNA for this experiment was a polyadenylated cyclin E mRNA. All mRNAs were injected into one-cell fertilized eggs in a volume of <30 nl.
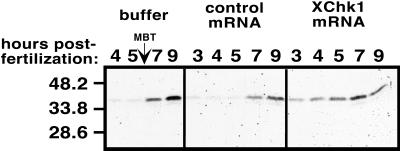
Figure 4.
Premature phosphorylation of Cdks on tyrosine 15 in embryos expressing exogenous XChk1. Embryos were injected at the one-cell stage with buffer, 15 ng of control mRNA, or 15-ng XChk1 mRNA. Western blots were reacted with an antibody that recognizes the phosphorylated tyrosine 15 epitope of Cdc2 and Cdk2. The equivalent of one embryo is loaded per lane. These results were reproducible in multiple experiments by using different control RNAs. The migration of molecular-weight markers in kDa is shown at the left.
Immunoprecipitation and Kinase Assays of Cyclin–Cdk Complexes
Embryos injected at the one-cell stage with 15 ng of luciferase or wild-type XChk1 mRNA were collected at 3.5 h postfertilization (stage 6.5), and antibodies against cyclins A, B, and E were used to immunoprecipitate cyclin A/Cdc2, cyclin B/Cdc2, and cyclin E/Cdk2, respectively. (Cyclin antibodies were kindly provided by Dr. James L. Maller, University of Colorado Health Sciences Center, Denver, CO.) Embryos were lysed in EB buffer, lysates were precleared with protein G Sepharose beads (Sigma, St. Louis, MO) for 30 min, mixed with appropriate antiserum, and incubated overnight on ice. Protein G Sepharose beads were added the next day and incubated with the immunoprecipitates for 1 h with rotation. Beads were then washed twice in low-salt buffer (20 mM Tris, pH 7.4, 5 mM EDTA, 0.1% Triton X-100, 100 mM NaCl) and once or twice in high-salt buffer (20 mM Tris, pH 7.4, 5 mM EDTA, 0.1% Triton X-100, 1 M NaCl). For Western blotting, beads were then mixed with 12.5 μl 2× gel loading buffer (1× = 0.6 mM Tris base, 2% glycerol, 3% SDS, 0.002% bromphenol blue) containing 10 mM n-ethylmaleimide, and resolved by electrophoresis on a polyacrylamide gel.
For kinase assays, immunoprecipitates were washed twice in kinase buffer (20 mM HEPES, pH 7.5, 15 mM MgCl2, 5 mM EGTA, 1 mM dithiothreitol), and then incubated 20 min at room temperature with 25 μl of kinase buffer containing 0.2 mg/ml bovine serum albumin, 0.5 mg/ml histone H1, 200 μM [γ-32P]ATP [2 cpm/fmol]). Reactions were terminated with 8 μl of 5× gel loading buffer containing 25% β-mercaptoethanol, heated at 95°C for 2 min, and resolved by PAGE. The gels were stained with Coomassie Blue and dried. Incorporation of [γ-32P]ATP was determined by Cerenkov scintillation counting of the excised histone H1 band.
RESULTS
Xchk1 Is Expressed during pre-MBT Embryonic Cell Cycles That Lack Checkpoints
To investigate XChk1 signaling in Xenopus, a clone containing an xchk1 cDNA of 2377 base pairs was isolated from a Xenopus oocyte cDNA library. The xchk1 gene encodes a protein of 473 amino acids with a predicted molecular weight of 54 kDa. The GenBank accession number is AF117816. XChk1 exhibits 77 and 74% amino acid identity to the human and mouse Chk1 proteins, respectively. Two other laboratories have recently cloned Xenopus chk1 genes (GenBank accession numbers AF053120 [Kumagai et al., 1998a] and AB019218 [Nakajo et al., 1999]). All three sequences are 98% identical in predicted amino acid sequence, although considerable differences in the nucleotide sequences of the 3′-untranslated regions exist (Sible and Hartley, unpublished observations).
To characterize the expression of xchk1 in the Xenopus embryo, Northern analysis with a 32P-labeled fragment of xchk1 cDNA as probe was performed with total RNA isolated from embryos at several stages of development (Figure 1A). XChk1 mRNA is ∼3 kilobases in length and migrates as a doublet, implying the existence of two distinct genes or alternatively spliced variants. Both species of xchk1 RNA are abundant in the unfertilized egg and persist in the embryo until the MBT (stage 8), at which point they are degraded. The larger species of XChk1 mRNA remains at low levels in post-MBT embryos and then increases in abundance in the early tadpole (stage 39). This pattern of expression is identical to that observed for the grp1 gene (a homolog of xchk1) in the Drosophila embryo (Fogarty et al., 1997) and suggests a role for XChk1 during early development or at the MBT.
It appears paradoxical that XChk1 mRNA level is high in the pre-MBT embryo, which lacks checkpoints, and then decreases at the MBT when checkpoints are acquired. To determine whether this pattern of mRNA expression was reflected at the protein level, polyclonal antiserum was generated in rabbits inoculated with recombinant, bacterially expressed XChk1. Western analysis with the purified antibody indicates that XChk1 protein level remains constant during early Xenopus development (Figure 1B). These data are consistent with recently reported results (Nakajo et al., 1999). Because the level of XChk1 protein is constant prior to and after the MBT, the absence of cell cycle checkpoints in the pre-MBT Xenopus embryo cannot be explained by a lack of XChk1.
XChk1 Signaling Pathway Can Be Induced by DNA Damage and by Inhibition of DNA Replication
To determine when endogenous XChk1 signaling becomes operational in the intact Xenopus embryo, embryos were treated at various stages with 100 μg/ml aphidicolin to block DNA replication or 60-Gy IR to damage DNA. Administration of either treatment prior to the MBT induces a rapid and synchronous embryonic death at a time corresponding to the onset of gastrulation in sibling control embryos (Anderson et al., 1997; Hensey and Gautier, 1997; our unpublished results). However, neither treatment disrupts development prior to the MBT. To allow time for checkpoint engagement, control and treated embryos were collected after sibling controls had completed 1–2 additional cell cycles. Western analysis with purified antibody against XChk1 indicated a shift in the electrophoretic mobility of XChk1 (Figure 2). This shift was recently demonstrated to result from phosphorylation and correlates with checkpoint engagement (Kumagai et al., 1998a). A mobility shift was detected in a small fraction of the XChk1 when embryos were treated with aphidicolin as early as stage 7 (pre-MBT), whereas additional XChk1 was phosphorylated when treatment was performed at stage 9 (post-MBT) (Figure 2A). The appearance of XChk1P correlates with the ability of aphidicolin to slow the embryonic cell cycles that precede the MBT (Kimelman et al., 1987; Clute and Masui, 1997; Hartley et al., 1997) and its ability to arrest cell cycles only after the MBT (Newport and Dasso, 1989; Clute and Masui, 1997).
IR also induced a shift in the electrophoretic mobility of XChk1, but this shift was only observed in embryos treated after the MBT (Figure 2B, stages 10.5 and beyond). Correspondingly, IR has no discernable effect on pre-MBT cell cycles. These data indicate that XChk1 is activated in response to aphidicolin and IR at distinct developmental times that correlate with observed effects on the embryonic cell cycles (Anderson et al., 1997; Hensey and Gautier, 1997; Sible et al., 1997). Furthermore, these data suggest that early embryos do not arrest in response to damaged and unreplicated DNA prior to the MBT because sufficient quantities of XChk1 do not get phosphorylated. Therefore, the checkpoint-signaling pathway is blocked upstream of XChk1 in the early embryo.
Upon treatment of embryos with aphidicolin or IR, multiple high-molecular-weight species that reacted with the XChk1 antibody (HMWXChk1) were observed, and the level of XChk1 + XChk1P decreased (Figure 2A). The significance of this observation is unknown but may suggest that upon activation, XChk1 subsequently becomes phosphorylated and/or polyubiquitinated and targeted for degradation. These events have not been described in other species or in egg extracts.
Overexpression of Xenopus Chk1 Disrupts Cleavage Cell Cycles
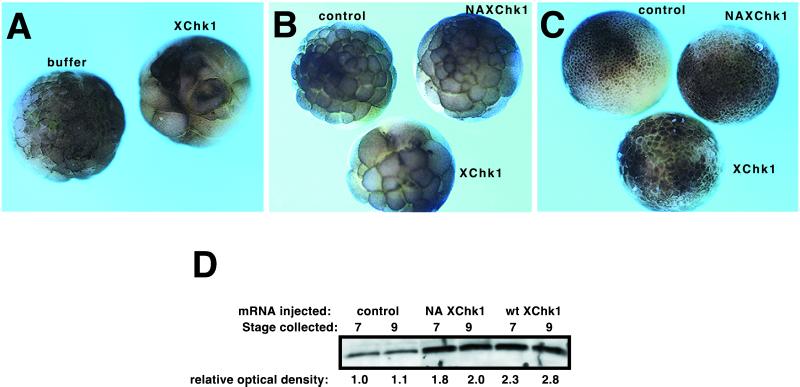
The experiments in Figure 2 define the developmental time at which XChk1 signaling is first engaged in response to damaged or unreplicated DNA and indicate a block in signaling upstream of XChk1 in the pre-MBT embryo. To determine whether signaling downstream of XChk1 could be engaged prior to the MBT, XChk1 was overexpressed in Xenopus embryos. mRNA encoding FLAG-tagged XChk1 was microinjected into fertilized eggs at the one-cell stage (Figure 3). XChk1 mRNA was efficiently translated as determined by Western analysis with a polyclonal FLAG antibody (our unpublished results) or the XChk1 antibody (Figure 3D). Exogenous XChk1 mRNA induced a dose-dependent, reproducible delay or arrest of the early cleavage cycles (Figure 3). Embryos injected with 2 ng of XChk1 RNA expressed XChk1 protein ∼2- to 3-fold over endogenous levels (Figure 3D). These embryos exhibited slower cleavage cycles and were delayed by approximately one cell cycle at the MBT (Figure 3B). This dose of XChk1 was not lethal, and many embryos developed normally, although delayed (Figure 3C). However, embryos injected with 12 ng of XChk1 mRNA failed to gastrulate and died after the MBT (Figure 3A). No delay was induced by injection with buffer or with mRNAs encoding luciferase, catalytically inactive XChk1 (Figure 3), or a variety of other proteins (our unpublished results). Therefore, Xenopus embryos are sensitive to exogenous XChk1, and overexpression of XChk1 disrupts development and arrests the cell cycle. These results indicate that even prior to checkpoints are normally triggered in response to damaged or unreplicated DNA, the signaling pathway downstream of XChk1 is intact in the pre-MBT embryo.
Figure 3.
Exogenous XChk1 lengthens cleavage cycles. (A) Embryos were microinjected at the one-cell stage with buffer or 12 ng of FLAG-tagged XChk1 mRNA and photographed when controls reached stage 8 (the MBT). (B and C) Embryos were microinjected at the one-cell stage with 2 ng of FLAG-tagged luciferase (control) mRNA, FLAG-tagged catalytically inactive XChk1 mRNA (NA XChk1), or FLAG-tagged wild-type XChk1 mRNA (XChk1). Embryos were photographed when controls reached stage 7 (B; pre-MBT) and stage 9 (C; post-MBT). (D) Western analysis of XChk1 protein in embryos treated and collected as in B and C. NA XChk1, inactive XChk1 mRNA; wt XChk1, wild-type XChk1 mRNA. Numeric values represent relative band intensity as determined by densitometry. The intensity of the XChk1 band in control embryos collected at stage 7 was arbitrarily set to 1. One embryo equivalent was loaded per lane.
Both Cdc2 and Cdk2 Are Targets of XChk1 Signaling in Xenopus Embryos
If the cell cycle arrest induced by expression of exogenous XChk1 results from engagement of a genuine cell cycle checkpoint, then embryonic Cdks should be phosphorylated and their catalytic activity should be inhibited. To test this prediction, extracts from control embryos and embryos expressing exogenous XChk1 were immunoblotted with an antibody specific for the phosphorylated tyrosine 15 epitope of Cdks (Figure 4). Embryos injected with buffer or control mRNAs exhibited the developmentally regulated pattern previously described (Ferrell et al., 1991; Hartley et al., 1997): a striking increase in the level of phosphorylated tyrosine 15 epitope on Cdks after the MBT. Although previous studies failed to detect phosphorylation of Cdks prior to the MBT (Ferrell et al., 1991; Hartley et al., 1997), low levels of phosphorylation were detected in pre-MBT embryos in our study (Figure 4). This observation may reflect the greater sensitivity of the antibody used in these studies compared with those described previously (Hartley et al., 1997) and has been confirmed in recent experiments with the same antibody (Kim et al., 1999). Compared with controls, embryos microinjected with 15 ng of mRNA encoding XChk1 exhibited enhanced phosphorylation of tyrosine 15 on Cdks prior to the MBT (3–4 h postfertilization, stages 6 and 7; Figure 4), correlating with the observed cell cycle arrest (Figure 3A). Phosphorylation of tyrosine 15 appeared within 1 h in embryos injected with as little as 2 ng of XChk1 mRNA (our unpublished results), correlating with cell cycle delay (Figure 3B). These results indicate that overexpression of XChk1 in early embryos lengthens cleavage cycles by tyrosine phosphorylation and inhibition of one or more Cdks.
These results do not distinguish whether the substrate of the inhibitory phosphorylation is Cdc2, Cdk2, or both, because the antibody used recognizes the phosphorylated tyrosine 15 epitope on both Cdks. To identify which Cdks are targets of the XChk1 pathway, embryos were injected with control or XChk1 mRNA, and specific cyclin/Cdk complexes were immunoprecipitated with antisera against cyclins A, B, and E. In the pre-MBT embryo, cyclins A and B are exclusively complexed with Cdc2 and cyclin E with Cdk2 (Gabrielli et al., 1992; Jackson et al., 1995; Rempel et al., 1995). Control experiments confirmed that Cdc2 does not coimmunoprecipitate with cyclin E (our unpublished results). Immunoprecipitates were analyzed for phosphorylation of tyrosine 15 on each Cdk (Figure 5A). All three complexes exhibited enhanced phosphorylation on tyrosine 15 relative to controls. These results identify Cdk2 in addition to Cdc2 as a target of the XChk1 pathway, something not previously demonstrated in any model system. These data are in agreement with the observation that Cdk2 is subject to multiple phosphorylation events in Xenopus egg extracts and can be activated by Cdc25 (Gabrielli et al., 1992).
To determine whether the tyrosine phosphorylation of Cdc2 and Cdk2 in response to exogenous XChk1 altered the catalytic activity of these enzymes, cyclin B–Cdc2 and cyclin E–Cdk2 complexes were immunoprecipitated and analyzed for the ability to phosphorylate histone H1 (Figure 5B). In embryos injected with XChk1 mRNA, both Cdc2 and Cdk2 exhibit a decrease in kinase activity relative to controls. Cdc2 complexes demonstrated an average 25% decrease in kinase activity, and Cdk2 complexes exhibited an average 52% decrease in kinase activity. The effect of XChk1 on Cdc2 activity may actually be larger than our measurement because Cdc2 activity oscillates more dramatically than Cdk2 activity during the pre-MBT cell cycles (Hartley et al., 1996), and it is likely that embryos were not always collected during the peak in Cdc2 kinase activity at mitosis. This explanation also can account for the larger SD in the effect of exogenous XChk1 on Cdc2 activity.
DISCUSSION
Cell Cycle Checkpoints in the Early Xenopus Embryo
The first 12 cell divisions of the developing Xenopus embryo present unique features for the study of cell cycle control. Most eukaryotic cell cycles arrest when the fidelity of the genome is threatened by damaged or unreplicated DNA. When checkpoints fail, mutations result, which may render the cell inviable or occasionally, malignant. The cell cycles of early Xenopus embryos sacrifice checkpoint surveillance in exchange for a period of rapid cell divisions, resulting in the formation of the 4096-cell blastula 5 h after fertilization. Xenopus embryos may compensate for the lack of checkpoints during early development by engaging a program of apoptosis in damaged cells after the MBT (Anderson et al., 1997; Hensey and Gautier, 1997; Sible et al., 1997; Stack and Newport, 1997).
The molecular basis for the absence of checkpoints during early Xenopus development is not known. The lack of p53-mediated checkpoints, which rely in part upon transcription of p21 (for review, see Levine, 1997), may be explained by the fact that transcription is not activated in Xenopus embryos until the MBT (Newport and Kirschner, 1982b). An alternative explanation is needed for the absence of checkpoints that use the Chk1-signaling pathway, which does not require transcription. Our laboratory has dissected the XChk1-signaling pathway in the intact Xenopus embryo prior to the MBT. The results of this study suggest that the XChk1 program is functional in the early embryo but awaits an appropriate signal to trigger activation of XChk1, most likely threshold levels of damaged or unreplicated DNA.
XChk1 Signaling In Vitro and In Vivo
This study investigates differences regarding checkpoint engagement in Xenopus egg extracts versus intact embryos. Cell-free extracts engage DNA damage and DNA replication checkpoints that involve the XChk1-signaling pathway (Kumagai et al., 1998a). Consistent with these results, maternally supplied XChk1 protein is detected in the intact embryo during pre-MBT development (Nakajo et al., 1999; Figure 1B). However, checkpoints are not operational in embryos until the MBT, and there is good correlation between the extent of XChk1 phosphorylation and the engagement of checkpoints (Figure 2). Inhibition of DNA replication has no effect on embryonic cell cycles and does not alter the electrophoretic mobility of XChk1 until 4 h postfertilization (stage 7, Figure 2A). Likewise, cell cycle arrest in response to aphidicolin correlates with increased phosphorylation of XChk1 after the MBT (stage 9). Similarly, high doses of IR alter the mobility of XChk1 only when administered after the MBT, when the checkpoint is operative (Figure 2B).
The DNA content in an egg extract and an embryo may explain the difference in XChk1 signaling. At fertilization, the egg possesses 1 diploid nucleus/μl (Hausen and Riebesell, 1991), and by the MBT, the concentration reaches 4096 nuclei/μl. This is the critical nuclear/cytoplasmic ratio that triggers the onset of zygotic transcription (Newport and Kirschner, 1982b). Although egg extracts represent the first mitotic cell cycles, these extracts are supplemented with 1000–3000 sperm nuclei/μl to induce a checkpoint and phosphorylation of XChk1 when DNA replication is blocked (Kumagai et al., 1998a). A similar concentration of nuclei is found in the stage 7 embryo (1024/embryo) when a mobility shift of XChk1 and cell cycle delay are first detected in response to unreplicated DNA (Figure 2A). In extracts, XChk1 phosphorylation in response to DNA damage is observed at a concentration of 3000 sperm nuclei/μl, approximating the concentration of nuclei required to activate XChk1 in the intact embryo (>4096 diploid nuclei/embryo; Figure 2B). Overall, the concentrations of nuclei required to engage checkpoints in extracts and embryos are in close enough agreement to suggest that a threshold concentration of unreplicated or damaged DNA is required to trigger XChk1 signaling.
Cdk2 Is a Target of XChk1 Signaling
In the present study, Cdk2 was phosphorylated and its catalytic activity was inhibited when the XChk1-signaling pathway was engaged by overexpression of XChk1 (Figure 5A). Cdk2 has not been reported previously as a target of Chk1 in any model system. Our results suggest tyrosine phosphorylation as a candidate regulator of Cdk2 activity prior to the MBT. Hartley et al. (1997) discovered that cyclin E/Cdk2 oscillates twice per cell cycle during the first 12 cleavages despite constant levels of cyclin E. Furthermore, cyclin E is degraded at the MBT in a maternally programmed manner (Howe and Newport, 1996; Hartley et al., 1997). Discovery of how Cdk2 activity is controlled may elucidate the mechanism that governs the cyclin E developmental timer (Hartley et al., 1997). A role for XChk1 in modifying the tyrosine phosphorylation of Cdks at the MBT would parallel the role that Grp1 plays in timing the maternal-zygotic transition in Drosophila (Fogarty et al., 1997; Sibon et al., 1997). Experiments in other model organisms such as Drosophila are required to determine whether Cdk2 is a universal target of Chk1, which would imply a role in G1/S checkpoints, or whether this observation represents a unique aspect of molecular physiology in the Xenopus embryo.
CONCLUSION
XChk1 protein is present throughout early development of X. laevis (Figure 1B). However, phosphorylation of XChk1 is first detected at stage 7 when DNA replication is blocked and at stage 9 when DNA is damaged (Figure 2). The timing of this phosphorylation event correlates well with the delay or arrest of embryonic cell cycles in response to aphidicolin and IR (Figure 3). Therefore, the activation of XChk1 corresponds temporally with the engagement of cell cycle checkpoints in Xenopus embryos. Furthermore, overexpression of XChk1 prior to the MBT induces a checkpoint, indicating that the downstream pathway is operational (Figures 3 and 4). We hypothesize that the XChk1 pathway is normally inactive prior to the MBT because of subthreshold levels of DNA. Both Cdc2 and Cdk2 are targets of XChk1 signaling (Figure 5). These data indicate that Cdk2 activity may be modulated by phosphorylation prior to the MBT. Future studies in Xenopus will explore whether Chk1 and related proteins function as regulators of the timing of the MBT by participating in the disengagement of the cyclin E/Cdk2 developmental timer.
ACKNOWLEDGMENTS
We thank Rebecca Hartley, John Tyson, and James Maller for comments and suggestions on this manuscript. XChk1 was cloned in the laboratory of Dr. James L. Maller, Howard Hughes Medical Institute, University of Colorado Health Sciences Center. This research was supported by grants from the Jeffress Trust, Oak Ridge Associated Universities, and the American Cancer Society (IRG-99-225-01) to J.S. and by grants from the Virginia Tech Biological Sciences Initiative and Pfizer Summer Undergraduate Research Fellowship Program to N.K.
Abbreviations used:
- Cdks
cyclin-dependent kinases
- IR
ionizing radiation
- MBT
midblastula transition
- XChk1
Xenopus Chk1
REFERENCES
- Anderson JA, Lewellyn AL, Maller JL. Ionizing radiation induces apoptosis and elevates cyclin A1-Cdk2 activity prior to but not after the midblastula transition in Xenopus. Mol Biol Cell. 1997;8:1195–1206. doi: 10.1091/mbc.8.7.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clute P, Masui Y. Microtubule dependence of chromosome cycles in Xenopus laevis blastomeres under the influence of a DNA synthesis inhibitor, aphidicolin. Dev Biol. 1997;185:1–13. doi: 10.1006/dbio.1997.8540. [DOI] [PubMed] [Google Scholar]
- Dasso M, Newport JW. Completion of DNA replication is monitored by a feedback system that controls the initiation of mitosis in vitro: studies in Xenopus. Cell. 1990;61:811–823. doi: 10.1016/0092-8674(90)90191-g. [DOI] [PubMed] [Google Scholar]
- Elledge SJ. Cell cycle checkpoints: preventing an identity crisis. Science. 1996;274:1664–1671. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- Ferrell JE, Wu M, Gerhart JC, Martin GS. Cell cycle tyrosine phosphorylation of p34cdc2 and a microtubule-associated protein kinase homolog in Xenopus oocytes and eggs. Mol Cell Biol. 1991;11:1965–1971. doi: 10.1128/mcb.11.4.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty P, Campbell SD, Abu-Shumays R, de Saint Phalle B, Yu KR, Uy GL, Goldberg ML, Sullivan W. The Drosophila grapes gene is related to checkpoint gene chk1/rad27 and is required for late syncytial division fidelity. Curr Biol. 1997;7:418–426. doi: 10.1016/s0960-9822(06)00189-8. [DOI] [PubMed] [Google Scholar]
- Frederick DL, Andrews MT. Cell cycle remodeling requires cell-cell interactions in developing Xenopus embryos. J Exp Zool. 1994;270:410–416. doi: 10.1002/jez.1402700411. [DOI] [PubMed] [Google Scholar]
- Furnari B, Rhind N, Russell P. Cdc25 mitotic inducer targeted by Chk1 DNA damage checkpoint kinase. Science. 1997;277:1495–1497. doi: 10.1126/science.277.5331.1495. [DOI] [PubMed] [Google Scholar]
- Gabrielli BG, Lee MS, Walker DH, Piwnica-Worms H, Maller JL. Cdc25 regulates the phosphorylation and activity of the Xenopus cdk2 protein kinase. J Biol Chem. 1992;26:18040–18046. [PubMed] [Google Scholar]
- Hartley RS, Rempel RE, Maller JL. In vivo regulation of the early embryonic cell cycles in Xenopus. Dev Biol. 1996;173:408–419. doi: 10.1006/dbio.1996.0036. [DOI] [PubMed] [Google Scholar]
- Hartley RS, Sible JC, Lewellyn AL, Maller JL. A role for cyclin E/Cdk2 in the timing of the midblastula transition in Xenopus embryos. Dev Biol. 1997;188:312–321. doi: 10.1006/dbio.1997.8647. [DOI] [PubMed] [Google Scholar]
- Hausen P, Riebesell M. The Early Development of Xenopus laevis. New York: Springer Verlag; 1991. [Google Scholar]
- Hensey C, Gautier J. A developmental timer that regulates apoptosis at the onset of gastrulation. Mech Dev. 1997;69:183–195. doi: 10.1016/s0925-4773(97)00191-3. [DOI] [PubMed] [Google Scholar]
- Howe JA, Newport JW. A developmental timer regulates degradation of cyclin E1 at the midblastula transition during Xenopus embryogenesis. Proc Natl Acad Sci USA. 1996;93:2060–2064. doi: 10.1073/pnas.93.5.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson PK, Chevalier S, Philippe M, Kirschner MW. Early events in DNA replication require cyclin E and are blocked by p21CIP1. J Cell Biol. 1995;130:755–769. doi: 10.1083/jcb.130.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Li C, Maller J. A maternal form of the phosphatase Cdc25A regulates early embryonic cell cycles in Xenopus laevis. Dev Biol. 1999;212:381–391. doi: 10.1006/dbio.1999.9361. [DOI] [PubMed] [Google Scholar]
- Kimelman D, Kirschner M, Scherson T. The events of the midblastula transition in Xenopus are regulated by changes in the cell cycle. Cell. 1987;48:399–407. doi: 10.1016/0092-8674(87)90191-7. [DOI] [PubMed] [Google Scholar]
- Kumagai A, Guo Z, Emami KH, Wang SX, Dunphy WG. The Xenopus Chk1 protein kinase mediates a caffeine-sensitive pathway of checkpoint control in cell-free extracts. J Cell Biol. 1998a;142:1559–1569. doi: 10.1083/jcb.142.6.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai A, Yakowec PS, Dunphy WG. 14-3-3 Proteins act as negative regulators of the mitotic inducer Cdc25 in Xenopus egg extracts. Mol Biol Cell. 1998b;9:345–354. doi: 10.1091/mbc.9.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- Nakajo N, Oe T, Uto K, Sagata N. Involvement of Chk1 kinase in prophase I arrest of Xenopus embryos. Dev Biol. 1999;207:432–444. doi: 10.1006/dbio.1998.9178. [DOI] [PubMed] [Google Scholar]
- Newport J, Dasso M. On the coupling between DNA replication and mitosis. J Cell Sci Suppl. 1989;12:149–160. doi: 10.1242/jcs.1989.supplement_12.13. [DOI] [PubMed] [Google Scholar]
- Newport J, Kirschner M. A major developmental transition in early Xenopus embryos: I. Characterization and timing of cellular changes at the midblastula stage. Cell. 1982a;30:675–686. doi: 10.1016/0092-8674(82)90272-0. [DOI] [PubMed] [Google Scholar]
- Newport J, Kirschner M. A major developmental transition in early Xenopus embryos: II. Control of the onset of transcription. Cell. 1982b;30:687–696. doi: 10.1016/0092-8674(82)90273-2. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal Table of Xenopus laevis. Amsterdam: North Holland Publishing Company; 1975. [Google Scholar]
- Paulovich AG, Toczyski DP, Hartwell LH. When checkpoints fail. Cell. 1997;88:315–321. doi: 10.1016/s0092-8674(00)81870-x. [DOI] [PubMed] [Google Scholar]
- Peng C-Y, Graves PR, Thoma RS, Wu Z, Shaw AS, Piwnica-Worms H. Mitotic and G2 checkpoint control: regulation of 14-3-3 protein binding by phosphorylation of Cdc25 on serine-216. Science. 1997;277:1501–1505. doi: 10.1126/science.277.5331.1501. [DOI] [PubMed] [Google Scholar]
- Rempel RE, Sleight SB, Maller JL. Maternal Xenopus Cdk2-cyclin E complexes function during meiotic and early embryonic cell cycles that lack a G1 phase. J Biol Chem. 1995;270:6843–6855. doi: 10.1074/jbc.270.12.6843. [DOI] [PubMed] [Google Scholar]
- Sanchez Y, Wong C, Thoma RS, Richman R, Wu Z, Piwnica-Worms H, Elledge SJ. Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science. 1997;277:1497–1501. doi: 10.1126/science.277.5331.1497. [DOI] [PubMed] [Google Scholar]
- Sible JC, Anderson JA, Lewellyn AL, Maller JL. Zygotic transcription is required to block a maternal program of apoptosis in Xenopus embryos. Dev Biol. 1997;189:335–346. doi: 10.1006/dbio.1997.8683. [DOI] [PubMed] [Google Scholar]
- Sibon OCM, Stevenson VA, Theurkauf WE. DNA-replication checkpoint control at the Drosophila midblastula transition. Nature. 1997;388:93–97. doi: 10.1038/40439. [DOI] [PubMed] [Google Scholar]
- Stack JH, Newport JW. Developmentally regulated activation of apoptosis early in Xenopus gastrulation results in cyclin A degradation during interphase of the cell cycle. Development. 1997;124:3185–3195. doi: 10.1242/dev.124.16.3185. [DOI] [PubMed] [Google Scholar]
- Walworth N, Davey S, Beach D. Fission yeast chk1 protein kinase links the rad checkpoint pathway to cdc2. Nature. 1993;363:368–371. doi: 10.1038/363368a0. [DOI] [PubMed] [Google Scholar]
- Walworth NC, Bernards R. rad-Dependent response of the chk1-encoded protein kinase at the DNA damage checkpoint. Science. 1996;271:353–356. doi: 10.1126/science.271.5247.353. [DOI] [PubMed] [Google Scholar]
- Westphal CH. Cell-cycle signaling: Atm displays its many talents. Curr Biol. 1997;7:R787–R792. doi: 10.1016/s0960-9822(06)00406-4. [DOI] [PubMed] [Google Scholar]
- Zeng Y, Forbes KC, Wu Z, Moreno S, Piwnica-Worms H, Enoch T. Replication checkpoint requires phosphorylation of the phosphatase Cdc25 by Cds1 or Chk1. Nature. 1998;395:507–510. doi: 10.1038/26766. [DOI] [PubMed] [Google Scholar]