SYNOPSIS
Although chronic hepatitis B and chronic hepatitis C are diseases of public health importance, only a few health departments nationally have chronic viral hepatitis under surveillance; these programs rely primarily on direct reporting by medical laboratories. We conducted an evaluation to determine if lessons from these programs can guide other health departments.
Between December 2002 and February 2003, we visited the Connecticut Department of Public Health, the Multnomah County Health Department in Portland, Oregon, and the Minnesota Department of Health to determine the capacity of their chronic hepatitis registries to monitor trends and provide case management.
We found that the registries facilitated investigations of potentially acute cases by identifying previously known infections, and aided prevention planning by pinpointing areas where viral hepatitis was being diagnosed. For chronic cases, case management (defined as the process of ensuring that infected individuals and their partners receive medical evaluation, counseling, vaccination, and referral to specialists for treatment when indicated) was provided for hepatitis B in Multnomah County, but was limited in other programs; barriers included resource constraints, difficulties confirming chronic infection, and privacy concerns. Finding innovative ways to overcome these barriers and improve case management is important if chronic hepatitis surveillance is to realize its full potential.
Public health surveillance for viral hepatitis has traditionally focused on acute cases. However, serosurveys have estimated that 1.25 million Americans are chronically infected with hepatitis B virus (HBV),1 and 2.7 million persons are infected with hepatitis C virus (HCV).2–4 Long-term liver damage may occur during the chronic phases of both these infections, including cirrhosis, liver failure, and hepatic carcinoma.1,5 A minority of health departments conduct surveillance for chronic hepatitis. Thus, many health departments lack the data needed to plan prevention programs and improve provision of services such as counseling, immunization, and treatment for chronically infected individuals.
At the national level, efforts to improve chronic viral hepatitis surveillance are underway.6–8 In 2002, the Council of State and Territorial Epidemiologists (CSTE) approved surveillance case definitions for chronic HBV and chronic HCV infections and recommended placing confirmed cases under national surveillance through the National Notifiable Disease Surveillance System (Figure 1).9,10 In the same year, the Centers for Disease Control and Prevention (CDC) published guidelines on viral hepatitis surveillance and case management,11 and by January 2003, began receiving reports of chronic HBV and HCV infections from some state health departments. In addition, CDC has supported several types of demonstration projects to develop and refine models for surveillance of chronic viral hepatitis infections in state and county health departments.
Figure 1.
Laboratory case definitions for chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infection, approved by the Council of State and Territorial Epidemiologists
SOURCES: Council of State and Territorial Epidemiologists. Chronic hepatitis B case definition. Document 02-ID-03 [cited 2004 Mar 30]. Available from: URL: http://www.cste.org/pdffiles/02-ID-03.Pdf.
Council of State and Territorial Epidemiologists. Chronic hepatitis C case definition. Document 02-ID-01 [cited 2004 Mar 30]. Available from: URL: http://www.cste.org/pdffiles/02-ID-01.Pdf
We conducted an evaluation to describe how surveillance programs for viral hepatitis operate and to determine their capacity to (1) describe the population with chronic hepatitis and monitor changes in detection of disease; and (2) provide case management, defined as the process of ensuring that infected individuals and their partners receive medical evaluation, counseling, vaccination, and referral to specialists for treatment as appropriate. The evaluation also aimed to identify the barriers to, and resources needed for, developing and implementing effective programs.
METHODS
The CDC's Updated Guidelines for Evaluating Public Health Surveillance Systems was used to guide the evaluation.12
Site selection
Sites were selected for evaluation from among state and county health departments that had received CDC support for chronic viral hepatitis surveillance and from other health departments with established chronic viral hepatitis surveillance. The programs were grouped into three approximate levels of size and complexity by number of staff members, funding, and special features such as electronic laboratory reporting. From each of these three levels, one program was chosen that exemplified qualities and practices of potential interest to other localities developing hepatitis surveillance programs. The three programs included the Connecticut Department of Public Health (a simpler program), the Multnomah County Health Department in Portland, Oregon (a program of medium complexity), and the Minnesota Department of Health (a complex program).
Data collection
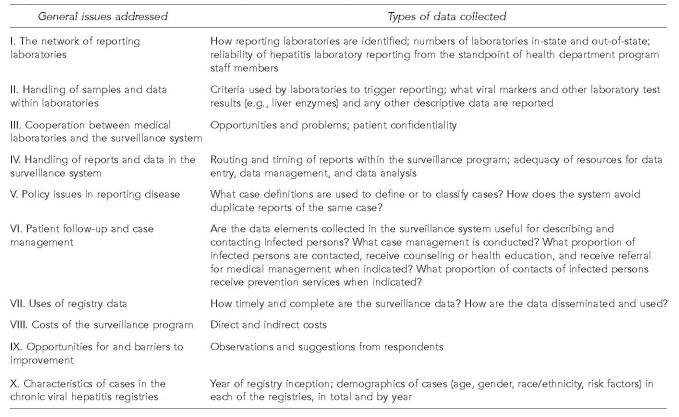
We began by discussing the basic activities of hepatitis surveillance programs with CDC staff members, then described these activities in the form of a flow diagram (Figure 2). We then used the flow diagram to formulate the common data collection instrument (Figure 3) to be used during site visits.
Figure 2.
Surveillance for chronic viral hepatitis
SOURCE: Discussions with CDC staff about the general operations of surveillance programs for chronic viral surveillance.
Figure 3.
Data elements collected during the surveillance system evaluation
Two of us (DF and AZ) visited the three programs between December 2002 and February 2003. We interviewed all program staff directly involved with the chronic hepatitis surveillance program, as well as data managers, data entry personnel, outreach workers, nurse epidemiologists, and staff members of perinatal hepatitis B prevention programs. We also reviewed materials and reports generated by the surveillance programs, as well as relevant public health reporting laws and statutes.
Data analysis
Program staff members at each health department provided de-duplicated and de-identified data drawn from their databases (referred to here as “registries”) of hepatitis B and hepatitis C cases. We analyzed the data using SAS, Version 8.2.13
We analyzed six registries (two from each health department). The date of first entry for each individual in the registry was defined by the specimen collection date (or the date of the laboratory report) of the first positive hepatitis test result for that individual. The analysis included data from each registry's year of inception (defined as the first year for which there were 20 or more new entries to the registry) through December 31, 2002. The analysis excluded cases missing both specimen collection date and date of laboratory report, and cases found to be acute during the health department investigation. For Multnomah County's hepatitis B database, the analysis excluded cases classified as “suspected” that did not have a documented positive hepatitis B surface antigen (HBsAg). Data on race/ethnicity and behavioral risk factors for hepatitis were categorized into a simplified set of categories common across the three sites.
RESULTS
Reporting laboratories
The chronic hepatitis surveillance programs collected data under the authority of pre-existing public health reporting laws for laboratory reporting of hepatitis markers (see Figure 3). Two chronic hepatitis surveillance programs also undertook additional initiatives. First, as part of a larger electronic laboratory reporting initiative, Minnesota's chronic hepatitis surveillance program had been working with two large laboratories to electronically report hepatitis B and C results, accounting for roughly 10% of all hepatitis reports at the health department. Second, in Multnomah County, where state law had mandated reporting of positive hepatitis C results only for cases in which paired sera showed recent seroconversion, program staff made a special request to encourage laboratories to report all positive hepatitis C results.14
The amount of information received from laboratories varied substantially. Some laboratories reported only the one or two positive serologic tests that specifically indicated viral hepatitis, but did not provide results of liver enzyme tests or other supporting information (such as IgM anti-HBc, or pregnancy tests) needed to gauge how the program should respond. Further, state reporting laws did not require laboratories to report negative confirmatory test results. Thus, to identify potentially acute cases, the programs needed to conduct further follow-up with laboratories, clinicians, or patients. Some program staff members noted that increasing patient privacy concerns leading up to the enforcement of the Health Insurance Portability and Accountability Act (HIPAA) in April 2003 had increased the caution shown by some laboratory staff members about releasing any patient information not specifically required by public health reporting laws.
Staff members reported good cooperation from laboratory staff members, and did not know of any laboratories of significant size that did not report positive hepatitis results. In many cases one laboratory would perform the screening tests on a blood sample, then send the residual sample to a reference laboratory for additional or confirmatory testing. Thus, results on a given patient often arrived at different times, or from different sources.
Handling of reports within the surveillance programs
Once a report indicating viral hepatitis reached the health department, the department's first task was to determine whether the infection was acute, in which case immediate attention to case investigation was required. In addition, cases of hepatitis B among pregnant women required a special response, and were referred to the perinatal hepatitis B prevention program.
The great majority of reports arrived at the health departments in paper form, usually by fax. Connecticut and Minnesota conducted the subsequent investigation of hepatitis reports using paper-based systems. In Connecticut, a feature in the EpiInfo registry software produced “tickler” reports to alert staff to unfinished tasks in hepatitis B investigations.15
In Multnomah County, investigation of hepatitis reports was coordinated by a special software program (in Microsoft Access), which included features to track the various steps in an investigation, record results obtained by various staff members, document actions taken, and produce tickler reports to alert staff members about unfinished tasks.16 The software had been designed for the Multnomah County Health Department's use with a variety of communicable diseases, and had special screens for HBV and HCV infections. Chronic hepatitis C cases were transferred to a separate database used as the hepatitis C registry.
Each of the three health departments had relatively separate surveillance programs for chronic hepatitis B and for chronic hepatitis C, each with its own hepatitis registry, although staff members from both programs overlapped or worked closely together.
Hepatitis B
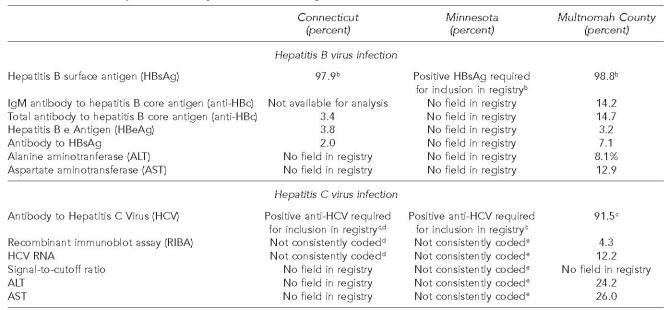
At all three health departments, laboratory reports of positive HBsAg were entered into the registry, usually by hand. Program staff members reported that other data elements (such as IgM anti-HBc, liver enzymes, or repeat HBsAg results) were available only occasionally, and were not always entered into the registry if they were available (Table 1).
Table 1.
Availability of information on selected markers for hepatitis B virus infection and hepatitis C virus infection: percent of cases in each of the chronic hepatitis registries with a result (either positive or negative) recorded, by markera
All results for each registry are from the registry's inception to December 31, 2002.
Since an initial positive result for HBsAg was usually required for inclusion in the hepatitis B registry, it is assumed that the great majority of registry cases had a positive HBsAg result, even if this result was not recorded.
Similarly, since an initial positive result for anti-HCV was usually required for inclusion in the hepatitis C registry, it is assumed that the great majority of registry cases had a positive anti-HCV result, even if this result was not recorded.
In Connecticut, staff members entered only the first HCV test result received. 13.0% of tests were EIA anti-HCV, 1.7% were RIBA, and 1.0% were PCR. 83.3% were coded “unknown” but most were likely EIA.
In Minnesota, the registry had the capacity to record one of a number of HCV “test types,” but 99.1% of cases' test type was anti-HCV. RIBA, HCV PCR, ALT, and AST accounted 0.1% or less each. There were no entries for signal-to-cutoff ratio.
EIA = enzyme-linked immunoassay
RIBA = recombinant immunoblot assay
PCR = polymerase chair reaction
ALT = alanine aminotransferase
AST = aspartate aminotransferase
In Connecticut, program staff members investigated all HBsAg-positive reports among females, and some HBsAg-positive reports among males 18 years old or younger (cases among older males were not investigated at all). If information on pregnancy status or clinical situation (such as liver enzyme testing results) was not initially available, staff contacted laboratories or clinicians to obtain this information. Cases with no available evidence for acute infection were entered into the registry as “prevalent” cases. Later, case status could be changed to “carrier” (chronic case) if a second HBsAg drawn six or more months later was received (34.4% of all cases were coded as “carrier”). No further action was taken at the state health department to provide follow-up or case management. Rather, local health departments throughout Connecticut received their own copies of reports directly from laboratories, and were responsible for investigating and managing possible acute cases of hepatitis B among non-pregnant patients. If needed, local health officials could contact the state chronic hepatitis B registry to request information on a particular case.
In Minnesota, with each laboratory report of hepatitis B, program staff contacted laboratories, clinicians, and/or patients as needed to obtain additional laboratory results and determine the patient's clinical situation, pregnancy status, and risk factors. Staff members referred patients with acute infections to an epidemiologist in the state or selected local health departments. Staff members entered as chronic cases all those that either (1) had laboratory evidence for chronic infection, such as HBsAg that was positive in two tests more than six months apart; (2) had a history of “chronic hepatitis” reported by the clinician or patient; or (3) had no further information available after investigation. The Minnesota hepatitis program worked with staff members from counties covering Minneapolis and St. Paul, who conducted the investigations of possible acute cases in their jurisdictions, maintained their own databases of hepatitis cases, and shared case-specific data with the state hepatitis program through telephone calls or other communication.
In Multnomah County, nurse epidemiologists contacted laboratories, clinicians, or patients as needed to obtain additional laboratory results and determine the patients' clinical situations and pregnancy status. Staff entered all nonacute cases as “confirmed” or “presumptive” chronic infections based on criteria established by the Oregon Department of Human Services; prior to 2000, a “suspect” case definition was also included. To be classified as “confirmed,” two HBsAg results checked six or more months apart, or a single positive HBsAg result and a “patient history of a previous diagnosis” of hepatitis were adequate.17,18
Hepatitis C
With laboratory results indicating HCV infection, first consideration was given to determining whether the infection was acute. In Connecticut and in Multnomah County, if available evidence did not indicate acute disease, no further investigation was conducted. In Minnesota, the hepatitis program sent out a “supplemental report” to request information from the clinician who had ordered the test; approximately 65% of clinicians returned the report. The program relied at least in part on the clinicians' judgment as to whether the case was “chronic.” Although the supplemental report contained case definitions, it was not known how clinicians applied them. In all three health departments, hepatitis program staff members reported that few confirmatory laboratory results—such as recombinant immunoblot assay (RIBA) or HCV ribonucleic acid (RNA)—were available to confirm infection or document chronic infection, and were not always entered into the registry when they were (Table 1).
Data entry and software
Connecticut used EpiInfo software for its hepatitis B and hepatitis C registries.15 Minnesota used Visual FoxPro for hepatitis B and FoxPro 2.6 for hepatitis C.19,20 Multnomah County used two Microsoft Access 2000 databases: one that had been designed for use by the Communicable Disease Program for a variety of communicable diseases, with special screens for hepatitis B and acute hepatitis C, and the other designed for chronic hepatitis only.16
Program staff members reported that three software features were important in increasing efficiency and reducing errors: (1) “de-duplicating” cases during data entry to ensure that staff could link new laboratory results to those of the same patients already listed in the database; (2) logic checks to prevent entry of erroneous data (such as laboratory dates that occurred before the patient's birth date); and (3) generating standard summary reports about new entries to the registries and tickler reports to remind staff of unfinished tasks in patient investigations.
Entry of paper laboratory results was time-consuming at all three sites. In Connecticut, a staff member was not always available to enter results into the registry, which led to gaps in data, especially in 1997 and 1998.
Uses of registry data: reports generated, and who used them
The health departments used hepatitis surveillance data for annual reports, grant writing, funding advocacy, and answering inquiries. The registries helped identify geographic areas and populations in which hepatitis was being diagnosed, such as large urban areas, Native American reservations and communities, and corrections facilities.
Case management
Hepatitis B
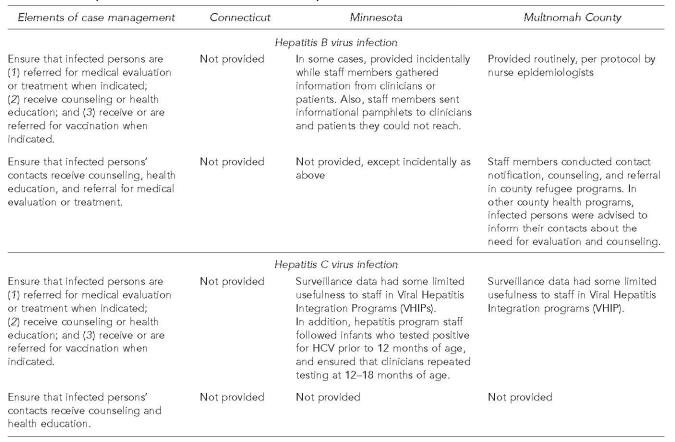
In Multnomah County, as part of their work with a variety of communicable diseases, a team of nurse epidemiologists followed up each new case of chronic hepatitis B. As part of their case management, nurse epidemiologists (1) counseled patients on transmission of the virus; (2) referred them for confirmatory testing and medical care; (3) advised them how to avoid further damage to their livers by avoiding alcohol and other hepatotoxic substances; and (4) recommended they inform their contacts about the need for testing, counseling, and vaccination. In Multnomah County's refugee programs, health department staff members called or visited contacts of hepatitis B patients and provided counseling and referral (Figure 5).
Figure 5.
Case management provided at the three health departments for patients with chronic hepatitis B virus infection and chronic hepatitis C virus infection
In Minnesota, hepatitis B program staff members incidentally provided some information on chronic hepatitis B, depending on the situation, to clinicians and patients they had contacted in the course of investigating hepatitis B laboratory reports. In addition, staff members sent informational pamphlets to some patients or clinicians whom they could not reach. However, program staff members indicated they did not have sufficient personnel or funding to support extensive, systematic case management for chronically infected individuals.
In Connecticut, available personnel and funding did not support case management activities.
Hepatitis C
For hepatitis C, the three health departments used surveillance data to improve case management in two situations. First, Minnesota hepatitis program staff members followed infants who tested positive for HCV (antibody, RNA, or both) prior to 12 months of age and ensured that providers repeated the testing at 12–18 months of age. Second, in Minnesota and in Multnomah County, demonstration programs called Viral Hepatitis Integration Programs (VHIPs) reached out to persons at high risk in the community and counseled them about ways to reduce their risk and get tested for HCV. If a VHIP staff member wanted to ascertain whether a particular patient had been tested previously for hepatitis C, the staff member could check the result in the hepatitis C registry while at the health department offices, or could telephone from the field. However, this was a relatively time-consuming process, mostly because the data management person at the health department was busy and not always at her desk. For this reason, VHIP staff kept their own, separate records of HCV results in their offices.
Costs of maintaining the program
Program staff members provided a very rough estimate of the proportion of their personnel time devoted to investigating cases, counseling patients, entering data, and maintaining the chronic hepatitis registries each year. Overall, estimates of total full-time equivalents (FTEs) among the various staff members at each of the six chronic hepatitis registries ranged from one to two FTEs. The exception was the hepatitis B program in Connecticut, which maintained its registry with approximately 0.2 FTE. Connecticut program staff members reported that lack of staff made the registry difficult to maintain.
Registry data: description of cases in the chronic hepatitis registries
Table 2 describes the cases in each registry from its year of inception to the end of 2002. The gender distribution of cases shows a relative male predominance for both hepatitis B and hepatitis C. Age at first entry into the registries was greater for hepatitis C than for hepatitis B. Among chronic hepatitis B registry cases with information on race, Asian/Pacific Islanders comprised the largest proportion (44% to 57%) at all three sites. In contrast, for hepatitis C, information on race and risk factors was available only in the Minnesota registry.
Table 2.
Selected characteristics of chronic hepatitis registries at three health departments
For percentages above, denominators included all records with data on the characteristic, excluding missing entries and those coded “unknown.”
Data on gender and age were available for the great majority of cases.
Data on race/ethnicity were available at the following rates: for chronic hepatitis B, 61% of entries in Connecticut, 84% in Minnesota, and 76% in Multnomah County; for hepatitis C, 67% of cases in Minnesota, but,<10% of cases in Connecticut and Multnomah County.
For comparison of race/ethnicity figures, general population in year 2000 at each site was as follows: for white persons, African Americans, and Asian/Pacific Islanders (respectively): Connecticut (81.6%, 9.1%, 2.4%); Minnesota (89.4%, 3.5%, 2.9%); Multnomah County (79.2%, 5.7%, 6.1%). Census Bureau (US). Census 2000 data from “state and county quickfacts” [cited 2004 Mar 30]. Available from: URL: http://quickfacts.census.gov/qfd/
Data on behavioral risk factors were sparse: For chronic hepatitis B, data were available for 18% of entries in Connecticut, 62% in Minnesota, and 16% in Multnomah County. For hepatitis C, data were available for no entries in Connecticut, 28% in Minnesota, and none in Multnomah County. In Connecticut, only one main risk factor was recorded; in Minnesota and Multnomah County, each record could have more than one risk factor recorded, thus percentages do not add to 100%. The six registries coded sexual risk factors (such as commercial sex work, homosexual sex, men who have sex with men, heterosexual risk) in different ways, so these were aggregated under the category “sexual.”
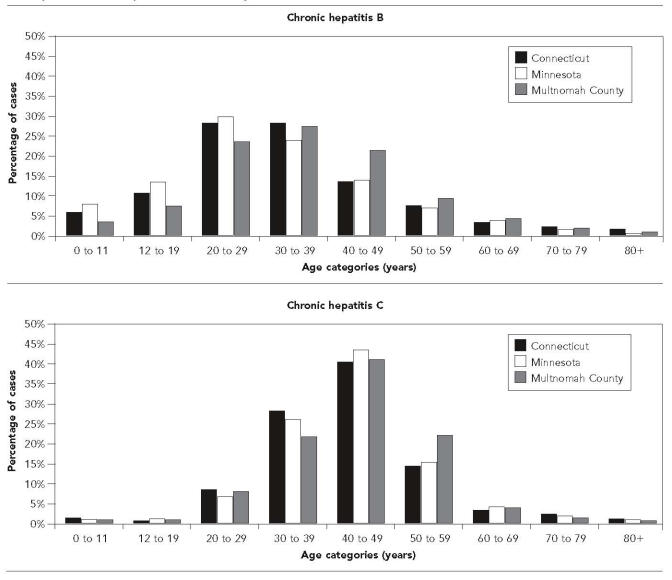
Figure 6 shows the distribution of ages at first entry into the registries for hepatitis B and hepatitis C overall. Figure 7 charts over time the mean age at entry and the gender mix at each of the registries. Mean age at entry for both hepatitis B and C increased slightly over time in Connecticut and Minnesota, but remained relatively stable in Multnomah County. The ratio of male to female registry entrants decreased somewhat over time for hepatitis B (at the two sites where this information was available), but showed no clear trends for hepatitis C.
Figure 6.
Distribution of ages at first entry into chronic hepatitis registries at three health departments, for hepatitis B and hepatitis C, since inception
Figure 7.
Mean age at first entry into chronic hepatitis registries, and ratio of male entries to female entries, for hepatitis B and hepatitis C, by health department
DISCUSSION
All three health departments had developed relatively complex systems to manage the flow of data from laboratories and to gather additional or confirmatory information on potential cases. Given the substantial effort required, it is essential to ensure that surveillance data are used effectively, not only to guide population-based prevention programs, but also to improve case management for individual patients.
How surveillance data were used
Excluding acute infection
The chronic hepatitis registries had an immediate utility to the health departments in the investigation of potential acute infections. During investigations, program staff members first checked to see if a given patient was already listed in the hepatitis registry. If so, staff members could be reassured that the infection was previously known, and the investigation of the case as potentially acute could be ended.
Planning population-based prevention programs
The chronic hepatitis registries helped to measure the burden of disease and to identify geographic areas particularly affected by hepatitis, such as some urban areas and Native American reservations. However, in other respects, the surveillance programs appeared to use chronic hepatitis surveillance data to guide their prevention activities in only a relatively limited way.
Improving case management
In five of the six chronic hepatitis registries, the surveillance program used surveillance data in only a limited way to improve case management for nonpregnant infected individuals or their partners. Reasons included lack of resources and staff, uncertainty over confirming chronic hepatitis in individual patients, and privacy concerns. In Multnomah County, a team of nurse epidemiologists provided extensive case management services for hepatitis B. However, these resources would not be available at many state departments of health, especially in states where local health authorities are responsible for handling medical care issues in individual patients. This represents a missed opportunity for ensuring that infected individuals and their partners were counseled and referred for effective therapy or vaccination, especially when the individuals were tested by clinicians with whom they had no regular contact.
Detecting chronic viral hepatitis in the community
Uncertainty about the accuracy of hepatitis test results in the registries could limit their usefulness in improving case management for some individuals with chronic infection. At the three health departments, the chronic hepatitis registries contained an unknown number of cases with false-positive results, as well as cases in individuals whose infections never became chronic. Laboratory test results confirming chronic infection were often unavailable or difficult to obtain. Furthermore, reporting laws did not clearly require laboratories to report negative confirmatory test results, thus allowing false-positive results to remain uncorrected. The three health departments used different sets of criteria for including cases in their registries, but all of these criteria pre-dated the strictly laboratory-based 2002 CSTE case definition.9,10 For at least some cases in each of the registries, an initial positive laboratory test result was confirmed only by a history of chronic hepatitis related by clinicians or patients themselves.
It could not be determined how well the cases identified by passive surveillance refiected chronic hepatitis in the community. Most individuals in the community with chronic hepatitis are asymptomatic, and clinicians may not screen patients at risk. Thus, changes in the numbers or composition of detected cases may refiect changes in the medical system or in screening practices at least as much as they refiect changes in incidence of infection.
Characteristics of chronic hepatitis cases in the registries
The registries identified risk groups known from other studies.21,22 Male registry cases for both chronic hepatitis B and chronic hepatitis C outnumbered females, consistent with the higher seroprevalences found for males in the third National Health and Nutrition Examination Survey (NHANES III, 1988–1994).2–4 In all three hepatitis B registries, Asian/Pacific Islanders were disproportionately represented, presumably refiecting the high seroprevalence in individuals from areas of the world where HBV infection is endemic, or the presence of hepatitis screening programs for refugees from these areas. For hepatitis C, data on race/ethnicity were available only in Minnesota, where whites were the largest single racial-ethnic group in the registry. However, racial/ethnic minorities were still disproportionately represented in the hepatitis registries when compared to Minnesota's general population.
The distribution of ages at entry generally corresponded to national seroprevalences measured in NHANES III. One exception, however, was that older individuals with chronic hepatitis B were sparsely represented in the registries, compared to their high seroprevalence in NHANES III, suggesting that older patients were less likely to be tested for hepatitis B than younger patients, or that they were tested before the registries were developed.
RECOMMENDATIONS
We offer the following general recommendations to those initiating or enhancing chronic hepatitis surveillance programs.
Strengthen case management
To improve case management, we recommend that hepatitis surveillance programs:
Contact the clinician who ordered each test, and provide guidelines for confirmatory testing and resources for referral and management of partners. Software could automatically generate a letter to the clinician using contact information already available with the laboratory results.
Contact some patients directly, especially those who may have no regular clinician, such as patients tested in emergency departments, prisons, drug treatment programs, or blood donation sites. Registry software could automatically generate a letter to newly detected patients, recommending confirmatory testing and counseling with a clinician. The potential for false-positive results, and for violations of patient privacy, must be considered.
Make registry data directly available in a confidential manner to local health authorities and hepatitis prevention programs to facilitate patient follow-up and avoid duplicate record-keeping systems.
Conduct special studies of a sample of registry entrants to determine how many have been informed and counseled about their hepatitis status; have access to health care for their chronic viral hepatitis; have been referred to specialists for potential treatment; and have received treatment.
Ensure that registry data are actively used to inform and guide hepatitis prevention programs
To ensure that registry data are used optimally, we recommend programs:
Generate reports about numbers of new reports and risk groups identified, and review them regularly within the chronic viral hepatitis surveillance program.
Conduct an inventory of other health department programs that could make use of surveillance data, such as STD clinics, drug abuse prevention programs, or refugee health programs, and discuss results with these programs regularly.
Make an effort to gather supplementary data necessary to confirm chronic hepatitis C in persons with positive anti-HCV.
Improve data entry and management
To improve data entry and management practices, surveillance programs should:
Ensure that the registry software de-duplicates cases and uses logic checks during data entry, rather than later on. Accurate de-duplication of cases is pivotal, as results on a given patient may arrive at different times and from different sources.
Link or combine hepatitis B and hepatitis C databases to avoid duplication of effort.
Automate the production of “tickler reports” to list cases for which further follow-up is needed.
Program software to generate standard epidemiological reports for easy review of trends.
Consider automated electronic laboratory reporting. Keep in mind, however, that this can be a time-consuming process, and may be superseded eventually by the implementation of the National Electronic Disease Surveillance System (NEDSS).
Note that most of these recommendations can be accomplished through enhancements to programs within the software applications already being used, rather than through transfer of the data to a different application.
If chronic hepatitis surveillance programs are to reach their full potential, funding must be adequate not only to provide for data management and investigation of reports, but also to strengthen case management.
Figure 4.
Laboratory reporting laws for hepatitis B and C in the jurisdictions of the three health departments
SOURCES: Interviews with staff members at each site and review of public health reporting laws and regulations.
Oregon Department of Human Services. Hepatitis C: case definitions, diagnosis, and laboratory services. Investigative guidelines for notifiable diseases (12/2003) [cited 2004 Mar 30]. 2004. Available from: URL: http://oregon.gov/DHS/ph/acd/reporting/guideln/hepc.pdf
Connecticut Department of Public Health. Public health code. Reportable diseases and laboratory findings (current through 8/26/2003) [cited 2004 Mar 30]. Available from: URL: http://www.dph.state.ct.us/phc/docs/73_Reportable_Diseases_and_.doc
Minnesota Department of Health. Reporting hepatitis [cited 2004 Mar 30]. Available from: URL: http://www.health.state.mn.us/divs/idepc/dtopics/reportable/hepatitis.html
Acknowledgments
The authors gratefully acknowledge Ronette Briefel of Mathematica Policy Research and Kris Ehresmann of the Minnesota Department of Health for their review of the manuscript and for their helpful comments. The authors also thank Jigar Bhatt and Stephanie Chin of MPR for their expert assistance in project management.
This work was sponsored by a grant from the Centers for Disease Control and Prevention.
References
- 1.Centers for Disease Control and Prevention (US); National Center for Infectious Diseases; Viral Hepatitis Surveillance. Disease burden from viral hepatitis A, B, and C in the United States. [cited 2004 Jul 28]. Available from: URL: http://www.cdc.gov/ncidod/diseases/hepatitis/resource/dz_burden02.htm.
- 2.McQuillan GM, Coleman PJ, Kruszon-Moran D, Moyer LA, Lambert SB, Margolis HS. Prevalence of hepatitis B virus infection in the United States: the National Health and Nutrition Examination Surveys, 1976 through 1994. Am J Public Health. 1999;89:14–8. doi: 10.2105/ajph.89.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alter MJ, Kruszon-Moran D, Nainan OV, McQuillan GM, Gao F, Moyer LA, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. New Engl J Med. 1999;341:556–62. doi: 10.1056/NEJM199908193410802. [DOI] [PubMed] [Google Scholar]
- 4.Coleman PJ, McQuillan GM, Moyer LA, Lambert SB, Margolis HS. Incidence of hepatitis B virus infection in the United States, 1976-1994: estimates from the National Health and Nutrition Examination Surveys. J Infect Dis. 1998;178:954–9. doi: 10.1086/515696. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (US); National Center for Infectious Diseases. National hepatitis C prevention strategy. A comprehensive strategy for the prevention and control of hepatitis C virus infection and its consequences. [cited 2004 Feb 19]. Available from: URL: http://www.cdc.gov/ncidod/diseases/hepatitis/c/plan/index.htm.
- 6.Centers for Disease Control and Prevention (US) Guidelines for laboratory testing and result reporting of antibody to hepatitis C virus. MMWR Recomm Rep. 2003;52(RR03):1–16. [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention (US) Achievements in public health: hepatitis B vaccination—United States, 1982–2002. MMWR Morb Mortal Wkly Rep. 2002;51(25):549–552. 563. [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (US) Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep. 1998;47(RR19):1–39. [PubMed] [Google Scholar]
- 9.Council of State and Territorial Epidemiologists. Chronic hepatitis B [case definition]. Document 02-ID-03. [cited 2004 Mar 30]. Available from: URL: http://www.cste.org/pdffiles/02-ID-03.pdf.
- 10.Council of State and Territorial Epidemiologists. Chronic hepatitis C [case definition]. Document 02-ID-01. [cited 2004 Mar 30]. Available from: URL: http://www.cste.org/pdffiles/02-ID-01.pdf.
- 11.Centers for Disease Control and Prevention (US) Guidelines for viral hepatitis surveillance and case management; June 2002. [cited 2004 Mar 30]. Available from: URL: http://www.cdc.gov/ncidod/diseases/hepatitis/resource/PDFs/revised%20GUIDELINES%20 formatted4.pdf.
- 12.Centers for Disease Control and Prevention (US) Updated guidelines for evaluation of public health surveillance systems. MMWR Recomm Rep. 2001;50(RR13):1–35. [PubMed] [Google Scholar]
- 13.SAS Institute Inc. SAS, Release 8.2. Cary (NC): SAS Institute Inc; 2001. [Google Scholar]
- 14.Oregon Department of Human Services. Investigation and control of diseases. Chapter 333, Divisions 17, 18, 19. Oregon Administrative Rules (OARS); 2004. [cited 2004 May 25]. Available from: URL: http://www.dhs.state.or.us/publichealth/acd/oars/labsfaq.cfm.
- 15.Dean AG, Dean JA, Coulombier D, Brendel KA, Smith DC, Burton AH, et al. EpiInfo™, Version 6.0, a word processing, database, and statistics program for public health on IBM-compatible microcomputers. Atlanta: Centers for Disease Control and Prevention; 1994. [Google Scholar]
- 16.Microsoft Corporation. Access 2000. Redmond (WA): Microsoft Corporation; 2002. [Google Scholar]
- 17.Oregon Department of Human Services. Hepatitis B: case definitions, diagnosis, and laboratory services. Investigative guidelines for notifiable diseases (12/2003) 2004. [cited 2004 Mar 30]. pp. 4–5. Available from: URL: http://oregon.gov/DHS/ph/acd/reporting/guideln/hepb.pdf.
- 18.Oregon Department of Human Services. Hepatitis C: case definitions, diagnosis, and laboratory services. Investigative guidelines for notifiable diseases (12/2003) 2004. [cited 2004 Mar 30]. Available from: URL: http://oregon.gov/DHS/ph/acd/reporting/guideln/hepc.pdf)
- 19.Microsoft Corporation. FoxPro: Version 2.6. Redmond (WA): Microsoft Corporation; 1994. [Google Scholar]
- 20.Microsoft Corporation. Visual FoxPro: Version 6. Redmond (WA): Microsoft Corporation; 2000. [Google Scholar]
- 21.Alter MJ, Hadler SC, Judson FN, Mares A, Alexander WJ, Hu PY, et al. Risk factors for acute non-A, non-B hepatitis in the United States and association with hepatitis C virus infection. JAMA. 1990;264:2231–5. [PubMed] [Google Scholar]
- 22.Goldstein ST, Alter MJ, Williams IT, et al. Incidence and risk factors for acute hepatitis B in the United States, 1982–1998: implications for vaccination programs. J Infect Dis. 2002;185:713–9. doi: 10.1086/339192. [DOI] [PubMed] [Google Scholar]