Abstract
Telomere length is abnormally short in the CD8+ T-cell compartment of human immunodeficiency virus type 1 (HIV-1)-infected persons, likely because of chronic cell turnover. Although clonal exhaustion of CD8+ cytotoxic T lymphocytes (CTL) has been proposed as a mechanism for loss of antigen-specific responses, the functional consequences of exhaustion are poorly understood. Here we used telomerase transduction to evaluate the impact of senescence on CTL effector functions. Constitutive expression of telomerase in an HIV-1-specific CTL clone results in enhanced proliferative capacity, in agreement with prior studies of other human cell types. Whereas the CTL remain phenotypically normal in terms of antigenic specificity and requirements for proliferation, their cytolytic and antiviral capabilities are superior to those of control CTL. In contrast, their ability to produce gamma interferon and RANTES is essentially unchanged. The selective enhancement of cytolytic function in memory CTL by ectopic telomerase expression implies that loss of this function (but not cytokine production) is a specific consequence of replicative senescence. These data suggest a unifying mechanism for the in vivo observations that telomere lengths are shortened in the CD8+ cells of HIV-1-infected persons and that HIV-1-specific CTL are deficient in perforin. Telomerase transduction could therefore be a tool with which to explore a potential therapeutic approach to an important pathophysiologic process of immune dysfunction in chronic viral infection.
Human T lymphocytes have the capacity to undergo a finite number of cell divisions before entering replicative senescence, a state in which cells remain metabolically active but are incapable of further proliferation (14). A major feature of replicative senescence is the erosion of telomeres, the hexameric sequences located at the distal ends of eukaryotic chromosomes, which function to stabilize the chromosome and progressively shorten in length with each cell division (1, 5, 15). Telomeres and their accessory binding proteins are thought to serve as a mitotic counter of the number of divisions a cell has completed, triggering cell cycle arrest and replicative senescence when the telomere length is too short to ensure chromosomal integrity (7, 31). Cell senescence is thus considered to be a protective mechanism that prevents unlimited growth and tumorigenesis (12).
T-cell clonal exhaustion in individuals chronically infected with human immunodeficiency virus type 1 (HIV-1) has been proposed as a mechanism of immune failure (3, 14). In support of this notion, abnormally short telomere length in the CD8+ T-cell compartment of infected individuals has been documented (13, 27, 36), suggesting that infection induces excessive CD8+ T-cell turnover and/or activation, leading to accelerated telomere shortening and premature senescence. This observation has significant implications, because CD8+ cytotoxic T lymphocytes (CTL) are believed to play a pivotal protective role in HIV-1 infection (42). CTL are thought to suppress viremia in acute infection (8, 21) and chronic infection (26) and are potent inhibitors of HIV-1 replication in vitro (11, 40), yet it is unknown why they ultimately fail to control infection in the majority of infected persons (22). During the asymptomatic phase of infection, viral replication in vivo has been estimated at approximately 1010 virions per day (17, 34), and thus, CTL face a continuous antigenic challenge over years of infection. Replicative senescence due to chronic turnover and exhaustion is therefore a potential contributing factor in the eventual failure of cellular immunity in HIV-1 infection (13).
Recent studies have demonstrated that transfection and constitutive expression of the catalytic subunit of human telomerase reverse transcriptase (hTERT) in various primary human cell types results in telomere lengthening and an increased proliferative life span with no apparent phenotype alteration (7, 31, 38). This strategy may therefore be useful in preventing replicative senescence in cells undergoing chronic turnover. Two published studies have examined the effects of constitutive telomerase expression on CD8+ T lymphocytes. Rufer et al. (28) demonstrated that hTERT transduction of naive CD8+ T-cell clones extends their ability to proliferate over time without detectable alteration of phenotype or function, and Hooijberg et al. (18) achieved similar results with a melanoma-specific CD8+ memory T-cell clone. However, it is unclear what effect telomerase has on virus-specific memory effector T cells that have proliferated to near senescence in response to antigen from a pathogen. In the case of HIV-1, persistent exposure to antigen leads to continuous turnover of virus-specific CTL over years. In this study, we evaluated the effects of telomerase transduction on HIV-1-specific CTL and addressed the potential functional impact of telomerase on memory T cells near the end of their replicative life span.
MATERIALS AND METHODS
Polyclonal CD8+ T-cell lines.
CD8+ cell lines were derived from the peripheral blood mononuclear cells (PBMC) of HLA A*0201 HIV-1-seropositive participants in the Los Angeles Multicenter AIDS Cohort Study. These CTL-enriched lines were produced by coculturing 4 × 106 freshly Ficoll-isolated PBMC with 106 autologous peptide-pulsed PBMC (previously labeled with synthetic peptide SLYNTVATL, ILKEPVHGV, or LWVTVYYGV at 10 μg/ml for 90 min) in Yssel's T-cell medium (Gemini Bio-Products, Woodland, Calif.) supplemented with l-glutamine, penicillin and streptomycin (both from Cellgro, Herndon, Va.), and recombinant interleukin-2 (IL-2; Roche Diagnostics, Indianapolis, Ind.). After 10 to 14 days, the CD8+ T cells were purified by magnetic cell sorting (Miltenyi Biotec, Auburn, Calif.) and plated at 4 × 106 cells/well of a 12-well plate in Yssel's T-cell medium supplemented with 150 IU of IL-2 per ml. Cells were transduced with hTERT or the vector control at 4 weeks after primary stimulation and restimulated every 3 to 4 weeks with irradiated autologous peptide-pulsed Epstein-Barr virus-transformed B cells. Population doublings were determined by counting viable cells every 1 to 2 weeks.
HIV-1-specific CTL.
HIV-1 specific CTL clone 68A62 was obtained from the blood of an infected individual by cloning of PBMC at limiting dilution and characterized for specificity and HLA restriction as previously described (33). This clone recognized the A2-restricted epitope ILKEPVHGV (IV9) in reverse transcriptase (amino acids 309 to 317 in HXB2) and was carried in long-term culture by weekly restimulation with anti-CD3 antibody and irradiated allogeneic feeder PBMC with IL-2 as previously described (33). Each restimulation was performed on 106 CTL in a total volume of approximately 15 ml. Concentrations of viable cells were determined manually by light microscopy of trypan blue-exposed cells after each weekly expansion.
Amphotropic viral transduction vector containing hTERT.
The amphotropic PA317 packaging cell line (derived from the ecotropic packaging cell line PE501) containing stably transfected retroviral vector pBABE with hTERT cDNA (cloned from pGRN145) (7) was provided by Geron Corporation (Menlo Park, Calif.). Supernatants containing retrovirus were harvested from plates at 40 to 60% confluence, passed through a 0.45-μm-pore-size filter, and used to infect CTL.
Transduction of CD8+ T cells and CTL with hTERT.
Two days after restimulation, the cells were cultured on RetroNectin (Takara Shuzo Co., Shiga, Japan)-precoated T25 flasks (Falcon) and transduced with hTERT vector, empty control vector, or no vector for 18 h. After transduction, the CTL were passaged in parallel with weekly restimulations. Clone 68A62 was successfully transduced twice in independent experiments.
Calculation of cell doublings.
After restimulations of polyclonal CD8+ T cells and CTL clones, the increase in the number of cells was used to estimate cell doublings in accordance with the following formula: doublings = log2(final cell concentration/initial cell concentration). The sum of all prior doublings in previous restimulations was designated the cumulative doublings of a cell line.
Measurement of telomerase activity (TRAP assay).
A modified telomeric repeat amplification protocol (TRAP) assay was used to detect telomerase activity (20). Measurement of telomerase activity in transduced and control CTL was performed with lysates of 104 cells by using the TRAPeze kit (Oncor, Gaithersburg, Md.) in accordance with the manufacturer's protocol. The resulting PCR products were then electrophoresed on a 10% 19:1 acrylamide/bisacrylamide gel, followed by Phosphorimager analysis (Packard, Downers Grove, Ill.) to visualize and quantitate the products.
Chromium release assays.
Standard chromium release assays were performed by using transduced and control CTL as effector cells (39). In brief, T1 cells (expressing HLA A2) were labeled with 50 μCi of 51Cr in the presence or absence of the IV9 peptide at 10 μg/ml for 1 h. These cells were then washed and plated in a 96-well U-bottom plate at 104 cells/well. CTL were then added at the indicated ratios for a 4-h incubation, after which supernatant was analyzed by scintillation counting for 51Cr (MicroBeta 1450; EG&G Wallac). Spontaneous release was determined in the absence of CTL, and maximal release was determined in the presence of 2.5% Triton X-100 (Sigma). All wells were run in duplicate or triplicate, and specific lysis was calculated as follows: (experimental release − spontaneous release)/(maximal release − spontaneous release).
Peptide-major histocompatibility complex (MHC) tetramer analysis.
Allophycocyanin-labeled HLA A2 tetramer containing the ILKEPVHGV peptide was obtained from the National Institute of Allergy and Infectious Diseases MHC Tetramer Core Facility. Staining was performed by incubating CTL with a pretitrated dilution of tetramer for 30 min at 4°C as previously described (26). Cells were then analyzed by using a FACScalibur flow cytometer and CellQuest software (Becton Dickinson).
Intracellular cytokine measurement.
Standard intracellular cytokine staining for gamma interferon (IFN-γ) or RANTES was performed by using commercial reagents (BDBiosciences, San Diego, Calif.). Briefly, cells were first stimulated for 6 h with the anti-CD3 antibody 12F6 (37) at 1 μg/ml in the presence of 1 μg of monensin per ml. The cells were then surface stained with PerCP-labeled anti-CD8 antibody or an isotype control, fixed and permeabilized in Cytofix/Cytoperm buffer, and washed with Perm/Wash buffer. Intracellular staining was then performed with fluorescein isothiocyanate-labeled anti-IFN-γ antibody or anti-RANTES antibody (BD Pharmingen) and followed by analysis on a FACScalibur flow cytometer with CellQuest software (Becton Dickinson).
Analysis of CTL ability to suppress HIV-1 replication.
A functional assay to evaluate the ability of CTL to suppress viral replication was performed as previously described (40). In brief, T1 cells were acutely infected with HIV-1 IIIB at a multiplicity of 10−2 50% tissue culture infective doses per cell. The T1 cells were then cocultured in 24-well flat-bottom plates at a ratio of 5 × 105 cells to 1.25 × 105 CTL in 2 ml of medium. At the indicated time points, 1 ml of medium was removed for quantitative p24 antigen enzyme-linked immunosorbent assay and replaced with fresh medium.
RESULTS
Telomerase transduction preserves the replicative capacity of polyclonal CD8+ T cells.
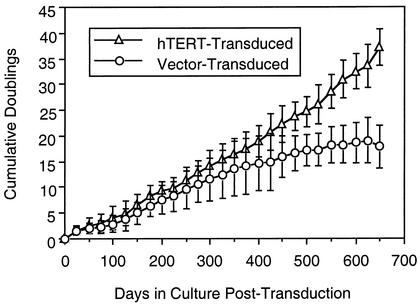
Ectopic telomerase transduction has been reported to prevent replicative senescence in primary CD8+ T cells (18, 28). We tested the effect of hTERT transduction on the proliferation of early-passage polyclonal human CD8+ T-cell lines derived from HIV-1-infected individuals (Fig. 1). Proliferation of the transduced and control (vector-transduced) cells progressively diverged over time, and whereas the doublings of the nontransduced cells plateaued after approximately 400 to 500 days of culture, the transduced cells continued to proliferate at a constant rate. On average, the control cells stopped proliferating after a mean of 20 ± 5 doublings over 589 ± 117 days, whereas the transduced cells continued proliferating beyond 800 days of follow-up (not shown). Telomerase activity, analyzed by the TRAP assay, was maintained in the transduced cell cultures but absent in the control cells (not shown). These results extend findings on the antisenescence effects of telomerase on CD8+ T cells from HIV-1-infected persons.
FIG. 1.
Proliferation of hTERT-transduced polyclonal CD8+ cells from HIV-1-infected individuals. Low-passage peptide-stimulated CD8+ T-cell lines derived from the PBMC of five persons were transduced with hTERT or the control vector. The cells were restimulated approximately every 3 weeks. Mean cumulative cell doublings (the number of twofold increases required to achieve the change in cell numbers after restimulations) over time are plotted for transduced versus nontransduced lines. Error bars represent 1 standard deviation.
Telomerase activity is maintained in hTERT-transduced HIV-1-specific CTL.
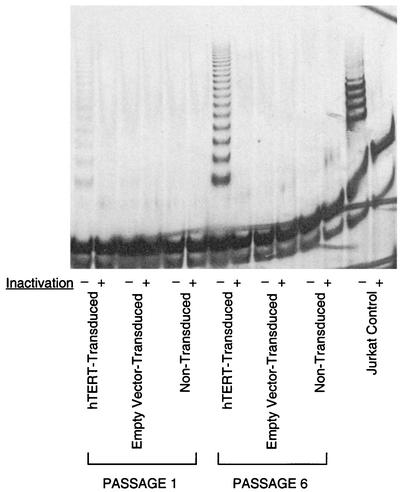
To specifically study the effects of telomerase transduction on HIV-1-specific CD8+ T cells, we then transduced the human CD8+ HIV-1-specific CTL clone 68A62, which recognizes an epitope in reverse transcriptase (39, 40). To verify successful transduction and expression of hTERT, we assayed these cells for telomerase activity at 14 days after restimulation (Fig. 2), a time point when endogenous telomerase activity had declined to undetectable levels. At the first passage after transduction, the hTERT-transduced cells already demonstrated significant activity, as seen by characteristic DNA laddering in a TRAP assay (Fig. 2, lane 1). By the sixth passage after transduction, telomerase activity had increased markedly (Fig. 2, lane 7), suggesting preferential expansion of cells ectopically expressing the transgene. It is worth noting that there was no experimental manipulation to enrich for transduced cells during passaging. Telomerase activity was absent in the negative control nontransduced (Fig. 2, lanes 5 and 11) and empty-vector-transduced (Fig. 2, lanes 3 and 9) cells at both passages analyzed. These results indicate that the CTL were successfully transduced with hTERT and suggest that transduction conferred a selective proliferative advantage.
FIG. 2.
Ectopic telomerase activity in hTERT-transduced CTL. Telomerase activity, as measured by the TRAP assay, was tested in extracts from hTERT-transduced and control CTL at 14 days after restimulation, when endogenous telomerase activity is quiescent. Telomerase activity was reflected by DNA laddering (Jurkat cell positive control). Heat-treated cellular extracts (to inactivate telomerase) served as negative controls. CTL (hTERT transduced, empty vector transduced, and nontransduced) from one passage after transduction (lanes 1 to 6) and six passages after transduction (lanes 7 to 12) were evaluated.
HIV-1-specific CTL transduced with hTERT exhibit enhanced proliferative capacity.
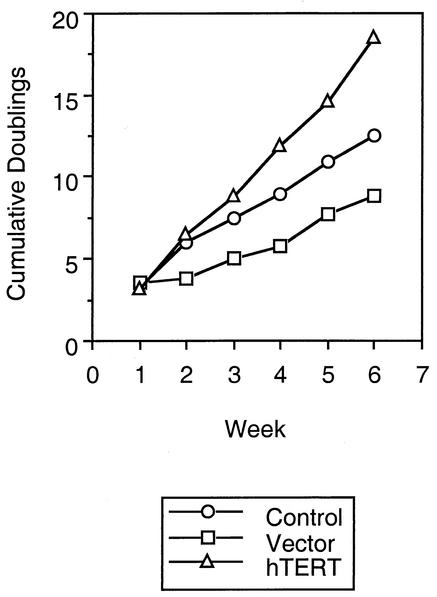
The proliferative capacity of CTL upon repeated stimulation in vitro dramatically decreases as they are serially passaged (Fig. 3 and unpublished observations). To determine whether introduction of telomerase had an effect on the growth of CTL, we followed their rate of expansion for multiple passages after transduction (Fig. 3). Immediately after transduction, hTERT-transduced and control CTL proliferated to similar degrees. However, with further passaging, the control CTL showed blunted levels of proliferation in comparison to the hTERT-expressing cells. The expansion of the cells still remained dependent upon stimulation (by anti-CD3 antibody and irradiated feeder cells) and IL-2, and their growth plateaued about 7 to 10 days after each restimulation (not shown). Thus, ectopic expression of telomerase in the CTL led to relative preservation of proliferative potential over time compared to that of untransduced cells, without evidence of transformation.
FIG. 3.
Growth of hTERT-transduced CTL over repeated passages. After hTERT transduction, cell doublings were calculated after each passage of 1 week. Cumulative cell doublings over time are plotted for nontransduced, vector-transduced, and hTERT-transduced CTL clones.
Antigen-specific lytic function is preserved in hTERT-transduced but not nontransduced CTL.
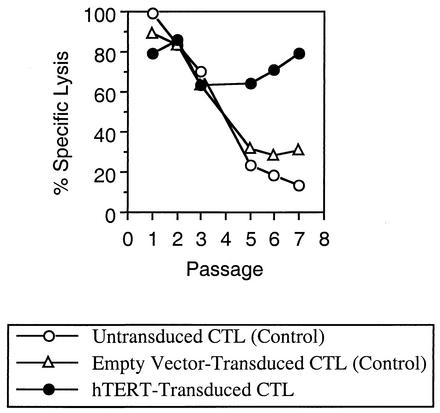
To evaluate the effect of constitutive telomerase expression on the peptide-specific lytic function of CTL, killing of peptide-loaded target cells by chromium release assay was evaluated after each passage (Fig. 4). Whereas the level of killing by control CTL dropped steadily over time, cytolytic activity was maintained by hTERT-transduced CTL for approximately 10 weeks. In agreement with published observations on HIV-1-specific CTL, cytolysis by these CTL was mediated entirely through the calcium-dependent proteolytic pathway and not through apoptosis (not shown). These data suggest that telomerase activity contributed to the preservation of proteolytic cytolytic function in these cells over time.
FIG. 4.
Antigen-specific lytic activity of hTERT-transduced CTL over repeated passages. After each passage, control and hTERT-transduced CTL were evaluated for the ability to kill peptide-loaded target cells (the T1 cell line matched at HLA A2, loaded with the IV9 peptide) as measured by a standard chromium release assay. Results obtained at an effector-to-target cell ratio of 5:1 are shown; similar results were obtained at lower ratios. Target cell killing was peptide specific in all cases (not shown). These data are representative of two independent transduction experiments. In both experiments, cytolytic activity in the hTERT-transduced CTL waned after about 10 weekly passages (not shown).
Loss of lytic function in nontransduced CTL is not due to overgrowth of nonspecific cells.
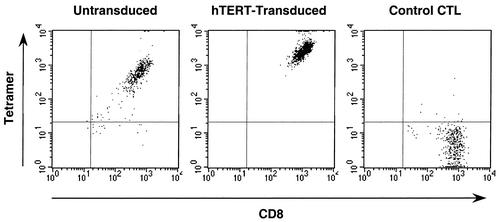
To exclude the possibility that loss of killing activity in the nontransduced cells was due to overgrowth of non-reverse-transcriptase-specific cells lacking the appropriate T-cell receptor (TCR), the cultures were analyzed by peptide-MHC tetramer staining (Fig. 5). This analysis indicated that both hTERT-transduced (100%) and nontransduced (96%) CTL expressed the TCR recognizing the HLA A2-restricted epitope ILKEPVHGV, although the intensity of staining for both CD8 and the TCR was lower in the nontransduced cells. Thus, the waning of cytolytic activity in the control CTL was not due to dilutional loss of CTL with continued passaging.
FIG. 5.
TCR expression by hTERT-transduced CTL. Control and hTERT-transduced CTL passaged for 6 weeks after transduction were assessed for expression of the appropriate TCR by flow cytometric analysis of CD8 and TCR expression by antibody and peptide-MHC tetramer binding. Untransduced CTL were 96% positive for CD8 and TCR, and hTERT-transduced CTL were 100% positive. The mean fluorescence intensities of staining of CD8 were 610 and 1,130, and those of the TCR were 756 and 2,951, respectively. A parallel specificity control (a CTL clone recognizing a different epitope) is also shown; these cells were 97% CD8 positive and TCR negative by tetramer staining, with a CD8 mean fluorescence intensity of 767.
IFN-γ and RANTES production is relatively unaffected by hTERT transduction of CTL.
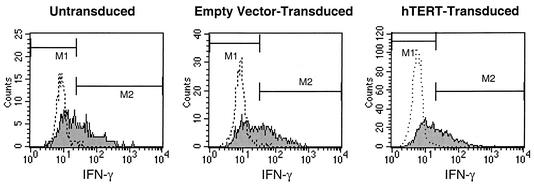
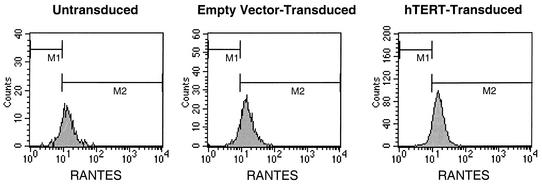
We evaluated the ability of CTL to produce cytokines associated with important effector functions, namely, IFN-γ, whose synthesis is induced by antigenic stimulation (19), and RANTES, which is presynthesized and stored in cytolytic granules of CTL at rest (32). By using intracellular staining, we evaluated telomerase-transduced and control CTL for the ability to generate IFN-γ in response to stimulation (Fig. 6) and RANTES at baseline (Fig. 7). Production of both of these cytokines was similar, regardless of telomerase transduction status. IFN-γ staining intensity was induced 4.3-fold in hTERT-transduced CTL, compared to 4.5- and 5.7-fold in control CTL. RANTES in CTL at rest produced mean fluorescence intensities of 17.0 for hTERT-transduced cells and 13.3 and 15.5 for control cells. Thus, telomerase transduction did not appear to markedly affect production of these cytokines by CTL, in contrast to the effects on cytolytic function.
FIG. 6.
IFN-γ production by hTERT-transduced CTL. Control and hTERT-transduced CTL passaged for 5 weeks after transduction were assessed for production of IFN-γ before and after stimulation with anti-CD3 antibody. Flow cytometric analysis of intracellular IFN-γ was assessed after blocking with monensin. Expression of IFN-γ before stimulation (dotted histograms) and after stimulation (filled histograms) is shown. Mean fluorescence intensity of IFN-γ staining after stimulation increased 4.5-fold for untransduced CTL, 5.7-fold for empty-vector-transduced CTL, and 4.3-fold for hTERT-transduced CTL.
FIG. 7.
RANTES production by hTERT-transduced CTL. Control and hTERT-transduced CTL passaged for 5 weeks after transduction were assessed for intracellular RANTES by flow cytometry. The level of RANTES stored in resting CTL was reflected by mean fluorescence staining intensities of 13.3 for untransduced cells, 15.5 in empty-vector-transduced cells, and 17.0 in hTERT-transduced cells.
Telomerase transduction enhances the antiviral function of HIV-1-specific CTL.
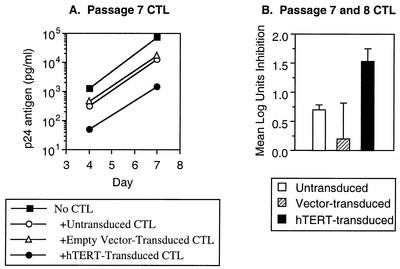
Finally, we evaluated the antiviral function of CTL in coculture with acutely HIV-1-infected cells (Fig. 8). Control nontransduced CTL decreased HIV-1 replication about 10-fold, similar to previously published studies with this clone (40, 41). However, hTERT-transduced CTL were an order of magnitude more efficient, exerting about 100-fold suppression of viral replication. Thus, ectopic telomerase activity appears to preserve or augment the antiviral function of CTL in parallel with its effects on cytolytic function. These observations are consistent with the central role of cytolysis in the ability of CTL to suppress HIV-1 replication (40).
FIG. 8.
Suppression of HIV-1 replication by hTERT-transduced CTL. Control and hTERT-transduced CTL passaged for 7 or 8 weeks after transduction were cocultured with acutely HIV-1-infected T1 cells (1.25 × 105 CTL with 5 × 105 T1 cells infected with HIV-1 IIIB at 10−2 50% tissue culture infective doses per cell), and viral replication was measured by serial quantitative p24 antigen enzyme-linked immunosorbent assays. (A) Viral replication in the absence or presence of CTL after seven passages is plotted. (B) Suppression of HIV-1 replication after 7 days of coculture is plotted. The bars represent the means of independent experiments with CTL after 7 and 8 weeks of passage, and the error bars represent 1 standard deviation.
DISCUSSION
T cells differ from most human somatic cells in that they express telomerase transiently during differentiation and activation (6, 10, 16, 35). Nevertheless, the level of telomerase activity induced in memory T cells declines with repeated stimulation, particularly in CD8+ T cells, where the ability to upregulate telomerase is lost after repeated encounters with antigen (30). Thus, despite physiologic surges of telomerase activity, repeatedly stimulated T cells undergo progressive telomere shortening and replicative senescence (14, 35). This mechanism is believed to have an impact on HIV-1 infection, where the antigen persists for a long time in the face of the CTL response. Here, we report that primary HIV-1-specific CTL can be successfully transduced with the hTERT gene and examine the impact of transduction in terms of several key CTL functions.
Consistent with what had been reported for other cell types (7, 31, 38), stable expression of telomerase in both polyclonal and clonal HIV-1-specific memory T cells extended their proliferative capacity. Transduced cells proliferated more robustly than control cells over many replicative cycles, similar to findings of others using hTERT-transduced human naive CD8+ T cells (28) and a melanoma-specific CTL clone (18). Ectopic expression of telomerase likely conferred a selective growth advantage, as demonstrated by the increasing levels of telomerase activity over time in the hTERT-transduced cell cultures.
Also in agreement with findings of other studies, telomerase transduction did not appear to alter the phenotype of the CTL in terms of functional specificity. The transduced cells still required antigenic stimulation to trigger their effector functions and proliferation. Their light microscopic appearance was that of normal primary T lymphocytes, and interestingly, they appeared larger and healthier than the control CTL, which were smaller and irregular in contour (not shown). These findings are similar to those of a report showing that extensively passaged endothelial cells expressing ectopic telomerase maintained their young primary cell morphology while the parental cells developed a flattened morphology typical of late passage (38).
It is of particular interest that ectopic telomerase expression had effects not only on the proliferative capacity of CTL (as observed by others) but also on specific functions of these cells. These results are consistent with studies on other cell types indicating that replicative senescence induces not only cell cycle arrest but significant changes in cell function as well (12). Here, ectopic telomerase expression augmented the ability of CTL to kill target cells in an antigen-specific manner and also to suppress viral replication. To our knowledge, this is a novel finding, with important implications for the immunopathogenesis of HIV-1 infection. These results imply that replicative senescence due to chronic turnover may specifically impair the cytolytic effector function of CTL and that the defect is at least partially preventable by hTERT transduction. Telomere shortening and replicative senescence may therefore be important contributing factors in the inability of CTL to contain HIV-1 infection in vivo, which is a topic of intense discussion (4, 22, 23). Consistent with this hypothesis, HIV-1-specific CTL in vivo have been found to contain reduced levels of perforin compared to cytomegalovirus-specific CTL in the same persons, suggesting a specific defect in the lytic pathway (2). Intriguingly, a recent study has shown that long-term nonprogressing HIV-1 infection appears to be associated with perforin production and proliferative capacity of HIV-1-specific CTL and that these are linked (25). Finally, the decrease in CD8 and/or TCR expression we noted in the nontransduced CTL could play a role in diminished antiviral function by reducing the sensitivity of CTL for their target cells. It has been noted that some HIV-1 epitopes may be presented by infected cells at a limiting concentration (29, 39), and a decrease in TCR and CD8 expression could therefore have a significant impact on infected cell recognition by CTL. In sum, chronic turnover and shortened telomeres in HIV-1-specific CTL could thus lead to diminished antiviral activity in vivo through one or a combination of these mechanisms.
In contrast to its effects on cytolytic and antiviral functions, telomerase transduction did not markedly affect the ability of CTL to produce two important cytokines. Production of both IFN-γ, which is induced by antigenic exposure (19), and RANTES, which is constitutively produced but stored in the cytolytic granules for release upon antigenic exposure (32), was maintained in the absence of ectopic telomerase activity. This suggests either that telomerase transduction of presenescent CTL cannot prevent defects in cytokine production or, more likely, that production of these cytokines does not wane as dramatically with senescence as lytic activity. Although suppression of HIV-1 replication by CTL in vitro has been shown to occur through cytokine (including chemokine)- and cytolysis-mediated pathways (11, 40), the cytolytic activity of CTL appears to add several orders of magnitude of inhibition over that of cytokines alone (40). A preferential diminution of cytolytic function by senescence would therefore be predicted to result in loss of antiviral activity, consistent with our finding that suppression of HIV-1 by CTL is enhanced by telomerase transduction. Importantly, because the most widely used new technologies for CTL quantitation in vivo (9) rely on detection of IFN-γ responses or direct binding of the TCR, they do not reflect the lytic or antiviral activity of these CTL. As a result, these assays may fail to detect dysfunction induced by senescence.
The lytic ability of the transduced cells did eventually wane after about 10 weeks of continuous passaging. This was in contrast to the observation of Rufer et al., who found that hTERT-transduced CD8+ T cells became essentially immortal (28). The reason for this difference is unclear, but it could be related to the fact that the HIV-1-specific CTL were already nearly senescent at the time of transduction. Moreover, others have found that even telomerase-transduced T cells may still be subject to exhaustion (24). The T cells used in our study were originally cloned from the blood of a chronically HIV-1-infected individual and passaged for a long time in vitro and were therefore likely to be closer to senescence than cells previously studied by other groups. Further study is required to confirm the etiology of this difference.
In conclusion, ectopic expression of hTERT in HIV-1-specific CTL leads not only to enhancement of the replicative capacity of these cells but also to preservation of cytolytic effector function and antiviral activity. These findings have ramifications for our understanding of the consequences of chronic CTL turnover in HIV-1-infected individuals and suggest that cellular immune dysfunction is at least partially preventable by telomerase transduction. In sum, these data suggest that telomerase transduction is a potential novel experimental approach by which to dissect the mechanisms of immune dysfunction induced by chronic HIV-1 infection or other conditions under which chronic turnover leads to T-cell senescence.
Acknowledgments
R.B.E. and O.O.Y. contributed equally to this work.
This work was funded by National Institutes of Health grants RO1 AI43203 (O.O.Y.), R21 AI47665 (R.B.E.), RO1 AI35040 (R.B.E.), and F31 AG05920 (M.D.). IL-2 was provided by the National Institutes of Health AIDS Research and Reference Reagent Repository.
We thank Bruce D. Walker for providing clone 68A62 and Geron Corporation for providing the hTERT construct vector.
REFERENCES
- 1.Allsopp, R. C., H. Vaziri, C. Patterson, S. Goldstein, E. V. Younglai, A. B. Futcher, C. W. Greider, and C. B. Harley. 1992. Telomere length predicts replicative capacity of human fibroblasts. Proc. Natl. Acad. Sci. USA 89:10114-10118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appay, V., D. F. Nixon, S. M. Donahoe, G. M. Gillespie, T. Dong, A. King, G. S. Ogg, H. M. Spiegel, C. Conlon, C. A. Spina, D. V. Havlir, D. D. Richman, A. Waters, P. Easterbrook, A. J. McMichael, and S. L. Rowland-Jones. 2000. HIV-specific CD8+ T cells produce antiviral cytokines but are impaired in cytolytic function. J. Exp. Med. 192:63-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bestilny, L. J., M. J. Gill, C. H. Mody, and K. T. Riabowol. 2000. Accelerated replicative senescence of the peripheral immune system induced by HIV infection. AIDS 14:771-780. [DOI] [PubMed] [Google Scholar]
- 4.Bevan, M. J., and T. J. Braciale. 1995. Why can't cytotoxic T cells handle HIV? Proc. Natl. Acad. Sci. USA 92:5765-5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blackburn, E. H. 1991. Structure and function of telomeres. Nature 350:569-573. [DOI] [PubMed] [Google Scholar]
- 6.Bodnar, A. G., N. W. Kim, R. B. Effros, and C. P. Chiu. 1996. Mechanism of telomerase induction during T cell activation. Exp. Cell Res. 228:58-64. [DOI] [PubMed] [Google Scholar]
- 7.Bodnar, A. G., M. Ouellette, M. Frolkis, S. E. Holt, C. P. Chiu, G. B. Morin, C. B. Harley, J. W. Shay, S. Lichtsteiner, and W. E. Wright. 1998. Extension of life-span by introduction of telomerase into normal human cells. Science 279:349-352. [DOI] [PubMed] [Google Scholar]
- 8.Borrow, P., H. Lewicki, B. H. Hahn, G. M. Shaw, and M. B. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 68:6103-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brander, C., and P. J. R. Goulder. 2000. The evolving field of HIV CTL epitope mapping: new approaches to the identification of novel epitopes, p. I1-I19. In B. T. M. Korber, C. Brander, B. F. Haynes, R. Koup, C. Kuiken, J. P. Moore, B. D. Walker, and D. Watkins (ed.), HIV molecular immunology database, vol. 2000. Los Alamos National Laboratory: Theoretical Biology and Biophysics, Los Alamos, N.Mex.
- 10.Buchkovich, K. J., and C. W. Greider. 1996. Telomerase regulation during entry into the cell cycle in normal human T cells. Mol. Biol. Cell 7:1443-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buseyne, F., M. Fevrier, S. Garcia, M. L. Gougeon, and Y. Riviere. 1996. Dual function of a human immunodeficiency virus (HIV)-specific cytotoxic T-lymphocyte clone: inhibition of HIV replication by noncytolytic mechanisms and lysis of HIV-infected CD4+ cells. Virology 225:248-253. [DOI] [PubMed] [Google Scholar]
- 12.Campisi, J. 2001. From cells to organisms: can we learn about aging from cells in culture? Exp. Gerontol. 36:607-618. [DOI] [PubMed] [Google Scholar]
- 13.Effros, R. B., R. Allsopp, C. P. Chiu, M. A. Hausner, K. Hirji, L. Wang, C. B. Harley, B. Villeponteau, M. D. West, and J. V. Giorgi. 1996. Shortened telomeres in the expanded CD28-CD8+ cell subset in HIV disease implicate replicative senescence in HIV pathogenesis. AIDS 10:F17-F22. [DOI] [PubMed] [Google Scholar]
- 14.Effros, R. B., and G. Pawelec. 1997. Replicative senescence of T cells: does the Hayflick limit lead to immune exhaustion? Immunol. Today 18:450-454. [DOI] [PubMed] [Google Scholar]
- 15.Harley, C. B., A. B. Futcher, and C. W. Greider. 1990. Telomeres shorten during ageing of human fibroblasts. Nature 345:458-460. [DOI] [PubMed] [Google Scholar]
- 16.Hiyama, K., Y. Hirai, S. Kyoizumi, M. Akiyama, E. Hiyama, M. A. Piatyszek, J. W. Shay, S. Ishioka, and M. Yamakido. 1995. Activation of telomerase in human lymphocytes and hematopoietic progenitor cells. J. Immunol. 155:3711-3715. [PubMed] [Google Scholar]
- 17.Ho, D. D., A. U. Neumann, A. S. Perelson, W. Chen, J. M. Leonard, and M. Markowitz. 1995. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature 373:123-126. [DOI] [PubMed] [Google Scholar]
- 18.Hooijberg, E., J. J. Ruizendaal, P. J. Snijders, E. W. Kueter, J. M. Walboomers, and H. Spits. 2000. Immortalization of human CD8+ T cell clones by ectopic expression of telomerase reverse transcriptase. J. Immunol. 165:4239-4245. [DOI] [PubMed] [Google Scholar]
- 19.Jassoy, C., T. Harrer, T. Rosenthal, B. A. Navia, J. Worth, R. P. Johnson, and B. D. Walker. 1993. Human immunodeficiency virus type 1-specific cytotoxic T lymphocytes release gamma interferon, tumor necrosis factor alpha (TNF-α), and TNF-β when they encounter their target antigens. J. Virol. 67:2844-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim, N. W., and F. Wu. 1997. Advances in quantification and characterization of telomerase activity by the telomeric repeat amplification protocol (TRAP). Nucleic Acids Res. 25:2595-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lieberman, J., P. Shankar, N. Manjunath, and J. Andersson. 2001. Dressed to kill? A review of why antiviral CD8 T lymphocytes fail to prevent progressive immunodeficiency in HIV-1 infection. Blood 98:1667-1677. [DOI] [PubMed] [Google Scholar]
- 23.McMichael, A. J., and R. E. Phillips. 1997. Escape of human immunodeficiency virus from immune control. Annu. Rev. Immunol. 15:271-296. [DOI] [PubMed] [Google Scholar]
- 24.Migliaccio, M., M. Amacker, T. Just, P. Reichenbach, D. Valmori, J. C. Cerottini, P. Romero, and M. Nabholz. 2000. Ectopic human telomerase catalytic subunit expression maintains telomere length but is not sufficient for CD8+ T lymphocyte immortalization. J. Immunol. 165:4978-4984. [DOI] [PubMed] [Google Scholar]
- 25.Migueles, S. A., A. C. Laborico, W. L. Shupert, M. S. Sabbaghian, R. Rabin, C. W. Hallahan, D. V. Baarle, S. Kostense, F. Miedema, M. McLaughlin, L. Ehler, J. Metcalf, S. Liu, and M. Connors. 2002. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat. Immunol. 3:1061-1068. [DOI] [PubMed] [Google Scholar]
- 26.Ogg, G. S., X. Jin, S. Bonhoeffer, P. R. Dunbar, M. A. Nowak, S. Monard, J. P. Segal, Y. Cao, S. L. Rowland-Jones, V. Cerundolo, A. Hurley, M. Markowitz, D. D. Ho, D. F. Nixon, and A. J. McMichael. 1998. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science 279:2103-2106. [DOI] [PubMed] [Google Scholar]
- 27.Palmer, L. D., N. Weng, B. L. Levine, C. H. June, H. C. Lane, and R. J. Hodes. 1997. Telomere length, telomerase activity, and replicative potential in HIV infection: analysis of CD4+ and CD8+ T cells from HIV-discordant monozygotic twins. J. Exp. Med. 185:1381-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rufer, N., M. Migliaccio, J. Antonchuk, R. K. Humphries, E. Roosnek, and P. M. Lansdorp. 2001. Transfer of the human telomerase reverse transcriptase (TERT) gene into T lymphocytes results in extension of replicative potential. Blood 98:597-603. [DOI] [PubMed] [Google Scholar]
- 29.Tsomides, T. J., A. Aldovini, R. P. Johnson, B. D. Walker, R. A. Young, and H. N. Eisen. 1994. Naturally processed viral peptides recognized by cytotoxic T lymphocytes on cells chronically infected by human immunodeficiency virus type 1. J. Exp. Med. 180:1283-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valenzuela, H. F., and R. B. Effros. 2002.. Divergent telomerase patterns in human CD4 and CD8 T cells following repeated encounters with the same antigenic stimulus. Clin. Immunol., 105:117-125. [DOI] [PubMed]
- 31.Vaziri, H., and S. Benchimol. 1998. Reconstitution of telomerase activity in normal human cells leads to elongation of telomeres and extended replicative life span. Curr. Biol. 8:279-282. [DOI] [PubMed] [Google Scholar]
- 32.Wagner, L., O. O. Yang, E. A. Garcia-Zepeda, Y. Ge, S. A. Kalams, B. D. Walker, M. S. Pasternack, and A. D. Luster. 1998. Beta-chemokines are released from HIV-1-specific cytolytic T-cell granules complexed to proteoglycans. Nature 391:908-911. [DOI] [PubMed] [Google Scholar]
- 33.Walker, B. D., C. Flexner, K. Birch-Limberger, L. Fisher, T. J. Paradis, A. Aldovini, R. Young, B. Moss, and R. T. Schooley. 1989. Long-term culture and fine specificity of human cytotoxic T-lymphocyte clones reactive with human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA 86:9514-9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei, X., S. K. Ghosh, M. E. Taylor, V. A. Johnson, E. A. Emini, P. Deutsch, J. D. Lifson, S. Bonhoeffer, M. A. Nowak, B. H. Hahn, et al. 1995. Viral dynamics in human immunodeficiency virus type 1 infection. Nature 373:117-122. [DOI] [PubMed] [Google Scholar]
- 35.Weng, N. P., B. L. Levine, C. H. June, and R. J. Hodes. 1996. Regulated expression of telomerase activity in human T lymphocyte development and activation. J. Exp. Med. 183:2471-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolthers, K. C., G. Bea, A. Wisman, S. A. Otto, A. M. de Roda Husman, N. Schaft, F. de Wolf, J. Goudsmit, R. A. Coutinho, A. G. van der Zee, L. Meyaard, and F. Miedema. 1996. T cell telomere length in HIV-1 infection: no evidence for increased CD4+ T cell turnover. Science 274:1543-1547. [DOI] [PubMed] [Google Scholar]
- 37.Wong, J. T., and R. B. Colvin. 1987. Bi-specific monoclonal antibodies: selective binding and complement fixation to cells that express two different surface antigens. J. Immunol. 139:1369-1374. [PubMed] [Google Scholar]
- 38.Yang, J., E. Chang, A. M. Cherry, C. D. Bangs, Y. Oei, A. Bodnar, A. Bronstein, C. P. Chiu, and G. S. Herron. 1999. Human endothelial cell life extension by telomerase expression. J. Biol. Chem. 274:26141-26148. [DOI] [PubMed] [Google Scholar]
- 39.Yang, O. O., S. A. Kalams, M. Rosenzweig, A. Trocha, N. Jones, M. Koziel, B. D. Walker, and R. P. Johnson. 1996. Efficient lysis of human immunodeficiency virus type 1-infected cells by cytotoxic T lymphocytes. J. Virol. 70:5799-5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang, O. O., S. A. Kalams, A. Trocha, H. Cao, A. Luster, R. P. Johnson, and B. D. Walker. 1997. Suppression of human immunodeficiency virus type 1 replication by CD8+ cells: evidence for HLA class I-restricted triggering of cytolytic and noncytolytic mechanisms. J. Virol. 71:3120-3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang, O. O., A. C. Tran, S. A. Kalams, R. P. Johnson, M. R. Roberts, and B. D. Walker. 1997. Lysis of HIV-1-infected cells and inhibition of viral replication by universal receptor T cells. Proc. Natl. Acad. Sci. USA 94:11478-11483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang, O. O., and B. D. Walker. 1997. CD8+ cells in human immunodeficiency virus type I pathogenesis: cytolytic and noncytolytic inhibition of viral replication. Adv. Immunol. 66:273-311. [DOI] [PubMed] [Google Scholar]